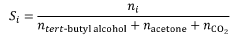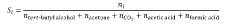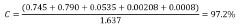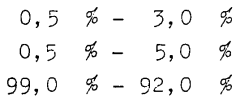Safe Usage Protocols for Isobutane in Pharmaceutical Manufacturing
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane in Pharma: Background and Objectives
Isobutane, a highly flammable hydrocarbon, has gained significant attention in the pharmaceutical manufacturing industry due to its unique properties and potential applications. The evolution of isobutane usage in pharmaceutical processes can be traced back to the early 2000s when researchers began exploring alternative solvents and propellants for various drug formulations. As environmental concerns and regulatory pressures increased, isobutane emerged as a promising candidate due to its low global warming potential and ozone depletion potential compared to traditional chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs).
The pharmaceutical industry's interest in isobutane has been driven by its versatility in drug delivery systems, particularly in aerosol-based formulations. Its low boiling point and high vapor pressure make it an excellent propellant for metered-dose inhalers (MDIs) and other pressurized drug delivery devices. Additionally, isobutane's solvent properties have been explored for potential use in extraction processes and as a reaction medium for certain pharmaceutical syntheses.
However, the widespread adoption of isobutane in pharmaceutical manufacturing has been hindered by safety concerns associated with its high flammability and potential for forming explosive mixtures with air. These challenges have necessitated the development of robust safety protocols and specialized handling equipment to mitigate risks in industrial settings. The pharmaceutical industry has been working closely with regulatory bodies, such as the FDA and EMA, to establish guidelines for the safe use of isobutane in drug manufacturing processes.
The primary objective of implementing safe usage protocols for isobutane in pharmaceutical manufacturing is to harness its beneficial properties while minimizing associated risks. This involves developing comprehensive safety measures, including proper storage and handling procedures, explosion-proof equipment design, and advanced ventilation systems. Additionally, there is a focus on training personnel in hazard recognition and emergency response procedures specific to isobutane-related incidents.
Another key goal is to optimize isobutane utilization in various pharmaceutical applications, exploring its potential beyond propellants to include areas such as extraction processes, reaction solvents, and cooling systems. This requires a deep understanding of isobutane's physicochemical properties and how they can be leveraged in different pharmaceutical manufacturing processes.
As the industry moves towards more sustainable practices, there is also an emphasis on developing technologies that allow for the efficient recovery and recycling of isobutane in pharmaceutical processes. This aligns with broader sustainability goals and helps address concerns about the environmental impact of hydrocarbon usage in industrial settings.
The pharmaceutical industry's interest in isobutane has been driven by its versatility in drug delivery systems, particularly in aerosol-based formulations. Its low boiling point and high vapor pressure make it an excellent propellant for metered-dose inhalers (MDIs) and other pressurized drug delivery devices. Additionally, isobutane's solvent properties have been explored for potential use in extraction processes and as a reaction medium for certain pharmaceutical syntheses.
However, the widespread adoption of isobutane in pharmaceutical manufacturing has been hindered by safety concerns associated with its high flammability and potential for forming explosive mixtures with air. These challenges have necessitated the development of robust safety protocols and specialized handling equipment to mitigate risks in industrial settings. The pharmaceutical industry has been working closely with regulatory bodies, such as the FDA and EMA, to establish guidelines for the safe use of isobutane in drug manufacturing processes.
The primary objective of implementing safe usage protocols for isobutane in pharmaceutical manufacturing is to harness its beneficial properties while minimizing associated risks. This involves developing comprehensive safety measures, including proper storage and handling procedures, explosion-proof equipment design, and advanced ventilation systems. Additionally, there is a focus on training personnel in hazard recognition and emergency response procedures specific to isobutane-related incidents.
Another key goal is to optimize isobutane utilization in various pharmaceutical applications, exploring its potential beyond propellants to include areas such as extraction processes, reaction solvents, and cooling systems. This requires a deep understanding of isobutane's physicochemical properties and how they can be leveraged in different pharmaceutical manufacturing processes.
As the industry moves towards more sustainable practices, there is also an emphasis on developing technologies that allow for the efficient recovery and recycling of isobutane in pharmaceutical processes. This aligns with broader sustainability goals and helps address concerns about the environmental impact of hydrocarbon usage in industrial settings.
Market Demand Analysis for Isobutane-Based Pharmaceuticals
The pharmaceutical industry has shown a growing interest in isobutane-based products, driven by the increasing demand for more efficient and cost-effective manufacturing processes. Market analysis indicates a steady rise in the adoption of isobutane as a key component in various pharmaceutical applications, particularly in aerosol-based drug delivery systems and as a propellant in metered-dose inhalers (MDIs).
The global market for isobutane in pharmaceutical manufacturing is projected to expand significantly over the next five years, with a compound annual growth rate (CAGR) outpacing the overall pharmaceutical industry growth. This surge is attributed to the versatility of isobutane in formulation development and its favorable physicochemical properties, which contribute to improved drug stability and delivery efficiency.
Respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD), are major drivers of the demand for isobutane-based pharmaceuticals. The prevalence of these conditions is on the rise globally, fueling the need for more advanced and user-friendly inhalation devices. Isobutane's role as a propellant in these devices has become increasingly critical, as it offers superior dispersion characteristics and consistent dose delivery.
Furthermore, the shift towards environmentally friendly propellants has positioned isobutane as a preferred alternative to chlorofluorocarbons (CFCs) and hydrofluoroalkanes (HFAs) in pharmaceutical applications. This transition aligns with stringent regulatory requirements and growing consumer awareness of environmental issues, further boosting the market demand for isobutane-based products.
The cosmeceutical sector, a rapidly growing segment within the pharmaceutical industry, has also contributed to the increased demand for isobutane. Its use in dermatological formulations and topical drug delivery systems has expanded, driven by consumer preferences for convenient and effective application methods.
Geographically, North America and Europe currently dominate the market for isobutane-based pharmaceuticals, owing to their advanced healthcare infrastructure and higher adoption rates of innovative drug delivery technologies. However, emerging markets in Asia-Pacific and Latin America are expected to witness the fastest growth in demand, fueled by improving healthcare access, rising disposable incomes, and increasing prevalence of respiratory diseases in these regions.
Despite the positive market outlook, challenges such as safety concerns and regulatory scrutiny regarding the use of flammable propellants in pharmaceutical products persist. This has led to ongoing research and development efforts focused on enhancing the safety profiles of isobutane-based formulations and developing robust manufacturing protocols to mitigate risks associated with its use.
The global market for isobutane in pharmaceutical manufacturing is projected to expand significantly over the next five years, with a compound annual growth rate (CAGR) outpacing the overall pharmaceutical industry growth. This surge is attributed to the versatility of isobutane in formulation development and its favorable physicochemical properties, which contribute to improved drug stability and delivery efficiency.
Respiratory diseases, including asthma and chronic obstructive pulmonary disease (COPD), are major drivers of the demand for isobutane-based pharmaceuticals. The prevalence of these conditions is on the rise globally, fueling the need for more advanced and user-friendly inhalation devices. Isobutane's role as a propellant in these devices has become increasingly critical, as it offers superior dispersion characteristics and consistent dose delivery.
Furthermore, the shift towards environmentally friendly propellants has positioned isobutane as a preferred alternative to chlorofluorocarbons (CFCs) and hydrofluoroalkanes (HFAs) in pharmaceutical applications. This transition aligns with stringent regulatory requirements and growing consumer awareness of environmental issues, further boosting the market demand for isobutane-based products.
The cosmeceutical sector, a rapidly growing segment within the pharmaceutical industry, has also contributed to the increased demand for isobutane. Its use in dermatological formulations and topical drug delivery systems has expanded, driven by consumer preferences for convenient and effective application methods.
Geographically, North America and Europe currently dominate the market for isobutane-based pharmaceuticals, owing to their advanced healthcare infrastructure and higher adoption rates of innovative drug delivery technologies. However, emerging markets in Asia-Pacific and Latin America are expected to witness the fastest growth in demand, fueled by improving healthcare access, rising disposable incomes, and increasing prevalence of respiratory diseases in these regions.
Despite the positive market outlook, challenges such as safety concerns and regulatory scrutiny regarding the use of flammable propellants in pharmaceutical products persist. This has led to ongoing research and development efforts focused on enhancing the safety profiles of isobutane-based formulations and developing robust manufacturing protocols to mitigate risks associated with its use.
Current Challenges in Isobutane Handling
The handling of isobutane in pharmaceutical manufacturing presents several significant challenges due to its inherent physical and chemical properties. One of the primary concerns is its high flammability and explosiveness. Isobutane has a low flash point and a wide flammability range, making it susceptible to ignition from various sources, including static electricity, sparks, and open flames. This necessitates stringent safety measures and specialized equipment to prevent accidental ignition and potential explosions.
Another challenge lies in the storage and transportation of isobutane. As a liquefied petroleum gas, it requires pressurized containers to maintain its liquid state. Ensuring the integrity of these containers and preventing leaks is crucial, as even small releases can create explosive atmospheres. The risk of Boiling Liquid Expanding Vapor Explosion (BLEVE) is also a significant concern, particularly in the event of fire or excessive heat exposure to storage tanks.
The volatility of isobutane poses challenges in its handling during manufacturing processes. Its low boiling point means it readily vaporizes at room temperature, potentially leading to the formation of explosive mixtures with air. This necessitates careful control of temperature and pressure throughout the manufacturing process, as well as the implementation of robust ventilation systems to prevent the accumulation of vapors.
Occupational health and safety is another critical challenge in isobutane handling. Exposure to high concentrations can lead to asphyxiation by displacing oxygen in confined spaces. Additionally, direct contact with the liquid form can cause severe frostbite due to its rapid evaporation and cooling effect. These risks require comprehensive training programs for personnel and the use of appropriate personal protective equipment.
Environmental concerns also present challenges in isobutane handling. As a volatile organic compound (VOC), isobutane can contribute to air pollution and the formation of ground-level ozone. Strict emission control measures are necessary to comply with environmental regulations and minimize the ecological impact of pharmaceutical manufacturing processes using isobutane.
The regulatory landscape surrounding the use of isobutane in pharmaceutical manufacturing adds another layer of complexity. Compliance with various safety standards, such as those set by OSHA, EPA, and industry-specific guidelines, requires continuous monitoring and adaptation of handling protocols. This often involves significant investments in safety equipment, monitoring systems, and documentation processes to meet regulatory requirements.
Lastly, the integration of isobutane handling into existing pharmaceutical manufacturing processes poses technical challenges. Ensuring compatibility with other materials and processes, maintaining product purity, and validating manufacturing methods that involve isobutane require extensive research and development efforts. This often necessitates modifications to existing equipment and processes, which can be both costly and time-consuming.
Another challenge lies in the storage and transportation of isobutane. As a liquefied petroleum gas, it requires pressurized containers to maintain its liquid state. Ensuring the integrity of these containers and preventing leaks is crucial, as even small releases can create explosive atmospheres. The risk of Boiling Liquid Expanding Vapor Explosion (BLEVE) is also a significant concern, particularly in the event of fire or excessive heat exposure to storage tanks.
The volatility of isobutane poses challenges in its handling during manufacturing processes. Its low boiling point means it readily vaporizes at room temperature, potentially leading to the formation of explosive mixtures with air. This necessitates careful control of temperature and pressure throughout the manufacturing process, as well as the implementation of robust ventilation systems to prevent the accumulation of vapors.
Occupational health and safety is another critical challenge in isobutane handling. Exposure to high concentrations can lead to asphyxiation by displacing oxygen in confined spaces. Additionally, direct contact with the liquid form can cause severe frostbite due to its rapid evaporation and cooling effect. These risks require comprehensive training programs for personnel and the use of appropriate personal protective equipment.
Environmental concerns also present challenges in isobutane handling. As a volatile organic compound (VOC), isobutane can contribute to air pollution and the formation of ground-level ozone. Strict emission control measures are necessary to comply with environmental regulations and minimize the ecological impact of pharmaceutical manufacturing processes using isobutane.
The regulatory landscape surrounding the use of isobutane in pharmaceutical manufacturing adds another layer of complexity. Compliance with various safety standards, such as those set by OSHA, EPA, and industry-specific guidelines, requires continuous monitoring and adaptation of handling protocols. This often involves significant investments in safety equipment, monitoring systems, and documentation processes to meet regulatory requirements.
Lastly, the integration of isobutane handling into existing pharmaceutical manufacturing processes poses technical challenges. Ensuring compatibility with other materials and processes, maintaining product purity, and validating manufacturing methods that involve isobutane require extensive research and development efforts. This often necessitates modifications to existing equipment and processes, which can be both costly and time-consuming.
Existing Safety Measures for Isobutane Usage
01 Safe handling and storage of isobutane
Proper handling and storage procedures are crucial for isobutane safety. This includes using appropriate containers, maintaining proper ventilation, and implementing safety measures to prevent leaks and explosions. Specialized equipment and storage facilities may be required to ensure the safe containment of isobutane.- Safe handling and storage of isobutane: Proper handling and storage procedures are crucial for isobutane safety. This includes using appropriate containers, maintaining proper ventilation, and implementing safety measures to prevent leaks and explosions. Specialized equipment and storage facilities may be required to ensure safe handling of isobutane in industrial settings.
- Isobutane purification and quality control: Purification processes and quality control measures are essential for ensuring the safety of isobutane. These may include distillation, adsorption, or other separation techniques to remove impurities and contaminants that could affect the safety or performance of the product.
- Safety in isobutane production processes: Safety considerations in the production of isobutane are critical. This includes the design of production facilities, implementation of process safety management systems, and the use of safety equipment such as pressure relief valves and emergency shutdown systems to prevent accidents and minimize risks.
- Environmental and health impact of isobutane: Understanding and mitigating the environmental and health impacts of isobutane is crucial for safety. This includes assessing its potential as a greenhouse gas, its effects on air quality, and any potential health risks associated with exposure. Proper handling and disposal methods are essential to minimize environmental and health risks.
- Isobutane as a refrigerant and propellant: When used as a refrigerant or propellant, specific safety measures for isobutane are necessary. This includes proper system design, leak detection, and ventilation to prevent the accumulation of flammable concentrations. Safety standards and regulations for the use of isobutane in these applications must be strictly followed to ensure safe operation.
02 Isobutane purification and quality control
Purification processes and quality control measures are essential for ensuring the safety of isobutane. These may include distillation, adsorption, or other separation techniques to remove impurities and contaminants that could affect the stability or reactivity of isobutane.Expand Specific Solutions03 Safety in isobutane production processes
Safety considerations in the production of isobutane are critical. This involves designing and implementing safe manufacturing processes, using appropriate catalysts and reaction conditions, and incorporating safety features to prevent accidents during production.Expand Specific Solutions04 Isobutane detection and monitoring systems
Developing and implementing effective detection and monitoring systems is crucial for isobutane safety. This includes gas sensors, alarms, and automated shutdown systems to quickly identify and respond to potential leaks or hazardous conditions.Expand Specific Solutions05 Risk assessment and safety protocols for isobutane use
Conducting thorough risk assessments and establishing comprehensive safety protocols are essential for the safe use of isobutane. This includes developing emergency response plans, providing proper training for personnel, and implementing safety measures specific to the applications and industries where isobutane is used.Expand Specific Solutions
Key Players in Isobutane-Based Pharmaceutical Production
The competitive landscape for safe usage protocols of isobutane in pharmaceutical manufacturing is evolving, with the industry in a growth phase. The market size is expanding as pharmaceutical companies seek safer and more efficient manufacturing processes. Technologically, the field is advancing rapidly, with varying levels of maturity among key players. Companies like Eli Lilly, Janssen Sciences Ireland, and Esperion Therapeutics are at the forefront, leveraging their pharmaceutical expertise. PetroChina and Sinopec, with their petrochemical background, are also making strides. Emerging players such as C4 Therapeutics and Gevo are introducing innovative approaches, while established firms like UOP LLC provide specialized technical solutions, creating a diverse and competitive environment.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced safe usage protocols for isobutane in pharmaceutical manufacturing. Their approach includes a multi-layered safety system incorporating real-time monitoring, automated shut-off valves, and specialized containment vessels. Sinopec's protocol emphasizes proper ventilation systems with explosion-proof designs, maintaining isobutane concentrations below 10% of the lower explosive limit[1]. They've implemented advanced leak detection systems using infrared sensors and gas chromatography for continuous monitoring. Sinopec also focuses on employee training, with regular safety drills and certification programs. Their protocol includes detailed emergency response plans and risk assessment methodologies specific to isobutane handling in pharmaceutical settings[3].
Strengths: Comprehensive safety measures, advanced monitoring systems, and extensive employee training. Weaknesses: Potentially higher implementation costs and complexity in smaller-scale operations.
Eli Lilly & Co.
Technical Solution: Eli Lilly has developed a proprietary safe usage protocol for isobutane in pharmaceutical manufacturing, focusing on process optimization and risk mitigation. Their approach integrates advanced process analytical technology (PAT) to monitor isobutane levels in real-time during manufacturing processes[2]. Lilly's protocol emphasizes the use of closed-system transfer devices to minimize exposure risks. They've implemented a sophisticated pressure management system that maintains optimal isobutane pressure throughout the manufacturing process, reducing the risk of leaks or over-pressurization. Lilly's protocol also includes a comprehensive hazard and operability (HAZOP) study specific to isobutane use, which informs their risk assessment and mitigation strategies[4]. Additionally, they've developed specialized handling procedures for isobutane storage and transport within their facilities.
Strengths: Advanced real-time monitoring, closed-system approach, and thorough risk assessment. Weaknesses: May require significant investment in specialized equipment and training.
Innovative Safety Technologies for Isobutane Handling
Hydroxylation of alkanes using ozone
PatentWO2022192866A1
Innovation
- Combining an alkane with ozone in a liquid phase medium containing a protic additive, such as water or alcohols, at mild temperatures and pressures, which stabilizes hydrotrioxide intermediates and maximizes ozone utilization, thereby enhancing the selectivity of hydroxylate products like tert-butyl alcohol.
Propellant for aerosols and dosing aerosols
PatentWO1993006185A1
Innovation
- Isobutane is used as a propellant that can dissolve surfactants, combined with a special pressure filling process, allowing for the production of environmentally neutral aerosols with improved nebulization and distribution of active ingredients, even when partially replaced by propane.
Regulatory Framework for Hazardous Materials in Pharma
The regulatory framework for hazardous materials in pharmaceutical manufacturing is a complex and critical aspect of ensuring safety and compliance in the industry. For isobutane usage, several key regulations and guidelines must be adhered to. The Occupational Safety and Health Administration (OSHA) sets forth standards for handling flammable gases, including isobutane, under 29 CFR 1910.106. This regulation outlines requirements for storage, handling, and use of flammable liquids and gases in the workplace.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating hazardous materials through the Resource Conservation and Recovery Act (RCRA). Under this act, pharmaceutical manufacturers must properly manage and dispose of hazardous waste, including any waste products containing isobutane.
In addition to federal regulations, state and local authorities may impose additional requirements for the use of hazardous materials in pharmaceutical manufacturing. These can include specific permitting processes, reporting requirements, and safety protocols that must be followed.
The Food and Drug Administration (FDA) oversees pharmaceutical manufacturing processes and product quality. While not directly regulating isobutane usage, the FDA's Current Good Manufacturing Practice (cGMP) regulations indirectly impact how hazardous materials are handled in pharmaceutical production to ensure product safety and quality.
International standards also influence the regulatory framework. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that are often adopted by regulatory agencies worldwide. These guidelines address quality, safety, and efficacy aspects of pharmaceutical production, including the use of hazardous materials.
Industry best practices, such as those outlined by the American Society for Testing and Materials (ASTM) and the National Fire Protection Association (NFPA), provide additional guidance for the safe handling of flammable gases like isobutane. These standards often inform regulatory requirements and are frequently referenced in compliance documentation.
Compliance with these regulations requires pharmaceutical manufacturers to implement comprehensive safety management systems, conduct regular risk assessments, provide employee training, and maintain detailed documentation of hazardous material handling procedures. Failure to adhere to these regulations can result in severe penalties, including fines, production shutdowns, and potential legal liabilities.
As regulations evolve to address new safety concerns and technological advancements, pharmaceutical manufacturers must stay informed and adapt their practices accordingly. This ongoing process ensures the continued safe use of hazardous materials like isobutane in pharmaceutical manufacturing while protecting workers, the environment, and public health.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating hazardous materials through the Resource Conservation and Recovery Act (RCRA). Under this act, pharmaceutical manufacturers must properly manage and dispose of hazardous waste, including any waste products containing isobutane.
In addition to federal regulations, state and local authorities may impose additional requirements for the use of hazardous materials in pharmaceutical manufacturing. These can include specific permitting processes, reporting requirements, and safety protocols that must be followed.
The Food and Drug Administration (FDA) oversees pharmaceutical manufacturing processes and product quality. While not directly regulating isobutane usage, the FDA's Current Good Manufacturing Practice (cGMP) regulations indirectly impact how hazardous materials are handled in pharmaceutical production to ensure product safety and quality.
International standards also influence the regulatory framework. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that are often adopted by regulatory agencies worldwide. These guidelines address quality, safety, and efficacy aspects of pharmaceutical production, including the use of hazardous materials.
Industry best practices, such as those outlined by the American Society for Testing and Materials (ASTM) and the National Fire Protection Association (NFPA), provide additional guidance for the safe handling of flammable gases like isobutane. These standards often inform regulatory requirements and are frequently referenced in compliance documentation.
Compliance with these regulations requires pharmaceutical manufacturers to implement comprehensive safety management systems, conduct regular risk assessments, provide employee training, and maintain detailed documentation of hazardous material handling procedures. Failure to adhere to these regulations can result in severe penalties, including fines, production shutdowns, and potential legal liabilities.
As regulations evolve to address new safety concerns and technological advancements, pharmaceutical manufacturers must stay informed and adapt their practices accordingly. This ongoing process ensures the continued safe use of hazardous materials like isobutane in pharmaceutical manufacturing while protecting workers, the environment, and public health.
Environmental Impact of Isobutane in Pharmaceutical Production
The use of isobutane in pharmaceutical manufacturing processes has significant environmental implications that require careful consideration. As a volatile organic compound (VOC), isobutane can contribute to air pollution and the formation of ground-level ozone when released into the atmosphere. This poses potential risks to both human health and ecosystems in the vicinity of pharmaceutical production facilities.
One of the primary environmental concerns associated with isobutane is its global warming potential. Although it has a relatively short atmospheric lifetime compared to other greenhouse gases, isobutane still contributes to climate change when emitted in large quantities. The pharmaceutical industry must therefore implement stringent emission control measures to minimize the release of isobutane during manufacturing processes.
Water pollution is another potential environmental impact of isobutane usage in pharmaceutical production. If not properly contained or disposed of, isobutane can contaminate water sources, affecting aquatic ecosystems and potentially entering the food chain. This necessitates robust waste management protocols and the implementation of advanced water treatment systems in pharmaceutical facilities.
The production and transportation of isobutane also carry environmental risks. Extraction and refining processes contribute to carbon emissions and may lead to habitat disruption in oil and gas production areas. Additionally, the transportation of isobutane to pharmaceutical manufacturing sites increases the carbon footprint of the industry and poses risks of accidental releases during transit.
To mitigate these environmental impacts, pharmaceutical companies are increasingly adopting cleaner production technologies and implementing closed-loop systems to minimize isobutane emissions. Some facilities are exploring the use of alternative, more environmentally friendly solvents or process modifications that reduce or eliminate the need for isobutane altogether.
Regulatory bodies worldwide are tightening environmental standards for pharmaceutical manufacturing, including stricter limits on VOC emissions and requirements for best available techniques to minimize environmental impact. This regulatory pressure is driving innovation in emission control technologies and encouraging the industry to invest in more sustainable manufacturing practices.
Life cycle assessments of pharmaceutical products are becoming more common, taking into account the environmental impact of isobutane usage from cradle to grave. These assessments help identify hotspots in the production process where environmental improvements can be made, leading to more sustainable pharmaceutical manufacturing practices overall.
One of the primary environmental concerns associated with isobutane is its global warming potential. Although it has a relatively short atmospheric lifetime compared to other greenhouse gases, isobutane still contributes to climate change when emitted in large quantities. The pharmaceutical industry must therefore implement stringent emission control measures to minimize the release of isobutane during manufacturing processes.
Water pollution is another potential environmental impact of isobutane usage in pharmaceutical production. If not properly contained or disposed of, isobutane can contaminate water sources, affecting aquatic ecosystems and potentially entering the food chain. This necessitates robust waste management protocols and the implementation of advanced water treatment systems in pharmaceutical facilities.
The production and transportation of isobutane also carry environmental risks. Extraction and refining processes contribute to carbon emissions and may lead to habitat disruption in oil and gas production areas. Additionally, the transportation of isobutane to pharmaceutical manufacturing sites increases the carbon footprint of the industry and poses risks of accidental releases during transit.
To mitigate these environmental impacts, pharmaceutical companies are increasingly adopting cleaner production technologies and implementing closed-loop systems to minimize isobutane emissions. Some facilities are exploring the use of alternative, more environmentally friendly solvents or process modifications that reduce or eliminate the need for isobutane altogether.
Regulatory bodies worldwide are tightening environmental standards for pharmaceutical manufacturing, including stricter limits on VOC emissions and requirements for best available techniques to minimize environmental impact. This regulatory pressure is driving innovation in emission control technologies and encouraging the industry to invest in more sustainable manufacturing practices.
Life cycle assessments of pharmaceutical products are becoming more common, taking into account the environmental impact of isobutane usage from cradle to grave. These assessments help identify hotspots in the production process where environmental improvements can be made, leading to more sustainable pharmaceutical manufacturing practices overall.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!