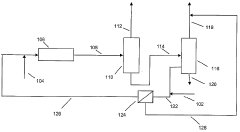
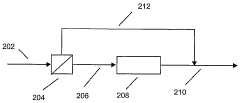
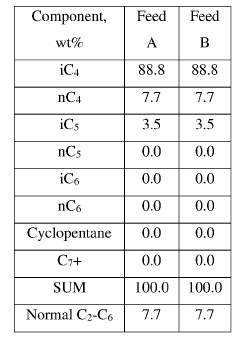
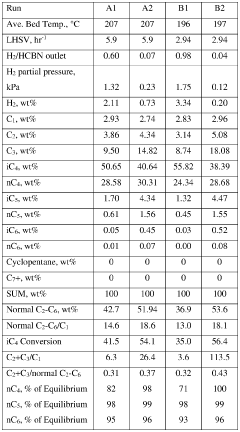
Optimal Conditions for Isobutane-Based Patent Processes
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane Process Background and Objectives
Isobutane-based processes have gained significant attention in the petrochemical industry due to their potential for producing high-value products with improved efficiency and reduced environmental impact. The evolution of these processes can be traced back to the mid-20th century when researchers began exploring alternative feedstocks for petrochemical production. Over the years, isobutane has emerged as a versatile and promising raw material for various chemical processes.
The technological trajectory of isobutane-based processes has been driven by the increasing demand for cleaner and more sustainable production methods. As environmental regulations have become more stringent, the industry has sought to develop processes that minimize waste generation and energy consumption. This has led to a focus on optimizing reaction conditions, improving catalyst performance, and enhancing separation techniques.
One of the primary objectives in researching optimal conditions for isobutane-based processes is to maximize product yield while minimizing unwanted by-products. This involves a comprehensive understanding of reaction kinetics, thermodynamics, and mass transfer phenomena. Researchers aim to identify the ideal temperature, pressure, and residence time that promote the desired reactions while suppressing side reactions.
Another critical goal is to develop more efficient and selective catalysts for isobutane conversion. Catalyst design plays a crucial role in determining the product distribution and overall process efficiency. Scientists are exploring novel materials and structures that can enhance catalytic activity, selectivity, and stability under various operating conditions.
Process intensification is also a key focus area in isobutane-based technologies. This involves developing innovative reactor designs and separation techniques that can improve heat and mass transfer, reduce equipment size, and lower energy requirements. Advanced process control strategies are being investigated to optimize real-time operation and maintain consistent product quality.
The integration of isobutane-based processes with other technologies, such as membrane separation or reactive distillation, is another avenue of research. These hybrid approaches aim to overcome limitations of conventional processes and create more efficient and cost-effective production routes.
As the industry moves towards circular economy principles, there is growing interest in developing closed-loop systems for isobutane-based processes. This includes exploring ways to recycle by-products, recover and reuse catalysts, and minimize waste streams. Such efforts align with broader sustainability goals and can potentially improve the economic viability of these processes.
The technological trajectory of isobutane-based processes has been driven by the increasing demand for cleaner and more sustainable production methods. As environmental regulations have become more stringent, the industry has sought to develop processes that minimize waste generation and energy consumption. This has led to a focus on optimizing reaction conditions, improving catalyst performance, and enhancing separation techniques.
One of the primary objectives in researching optimal conditions for isobutane-based processes is to maximize product yield while minimizing unwanted by-products. This involves a comprehensive understanding of reaction kinetics, thermodynamics, and mass transfer phenomena. Researchers aim to identify the ideal temperature, pressure, and residence time that promote the desired reactions while suppressing side reactions.
Another critical goal is to develop more efficient and selective catalysts for isobutane conversion. Catalyst design plays a crucial role in determining the product distribution and overall process efficiency. Scientists are exploring novel materials and structures that can enhance catalytic activity, selectivity, and stability under various operating conditions.
Process intensification is also a key focus area in isobutane-based technologies. This involves developing innovative reactor designs and separation techniques that can improve heat and mass transfer, reduce equipment size, and lower energy requirements. Advanced process control strategies are being investigated to optimize real-time operation and maintain consistent product quality.
The integration of isobutane-based processes with other technologies, such as membrane separation or reactive distillation, is another avenue of research. These hybrid approaches aim to overcome limitations of conventional processes and create more efficient and cost-effective production routes.
As the industry moves towards circular economy principles, there is growing interest in developing closed-loop systems for isobutane-based processes. This includes exploring ways to recycle by-products, recover and reuse catalysts, and minimize waste streams. Such efforts align with broader sustainability goals and can potentially improve the economic viability of these processes.
Market Analysis for Isobutane-Based Products
The market for isobutane-based products has shown significant growth in recent years, driven by increasing demand across various industries. Isobutane, a key component in many industrial processes, finds applications in refrigerants, aerosol propellants, and as a feedstock for petrochemicals. The global isobutane market size was valued at over $25 billion in 2020 and is projected to grow at a CAGR of 5.2% from 2021 to 2028.
The refrigeration and air conditioning sector remains the largest consumer of isobutane-based products, accounting for approximately 40% of the market share. This is primarily due to the phase-out of hydrochlorofluorocarbons (HCFCs) and the adoption of more environmentally friendly alternatives. Isobutane (R-600a) has emerged as a preferred refrigerant due to its low global warming potential and energy efficiency.
In the petrochemical industry, isobutane serves as a crucial feedstock for the production of high-octane gasoline components and various chemicals. The growing automotive sector, particularly in developing economies, has fueled the demand for isobutane in gasoline blending. Additionally, the increasing use of isobutane in the production of synthetic rubber and plastics has further boosted market growth.
The aerosol propellant segment has also witnessed substantial growth, with isobutane being widely used in personal care products, household cleaners, and industrial sprays. The shift away from chlorofluorocarbons (CFCs) has led to increased adoption of isobutane as a propellant, owing to its low environmental impact and cost-effectiveness.
Geographically, Asia Pacific dominates the isobutane market, accounting for over 35% of the global share. The region's rapid industrialization, growing population, and increasing disposable income have driven demand across various end-use industries. North America and Europe follow closely, with mature markets and stringent environmental regulations shaping the demand for isobutane-based products.
Looking ahead, the market for isobutane-based products is expected to continue its upward trajectory. Factors such as urbanization, technological advancements, and the push for sustainable solutions will likely drive further innovation in isobutane-based processes. However, challenges such as price volatility of raw materials and safety concerns associated with the flammability of isobutane may impact market growth to some extent.
The refrigeration and air conditioning sector remains the largest consumer of isobutane-based products, accounting for approximately 40% of the market share. This is primarily due to the phase-out of hydrochlorofluorocarbons (HCFCs) and the adoption of more environmentally friendly alternatives. Isobutane (R-600a) has emerged as a preferred refrigerant due to its low global warming potential and energy efficiency.
In the petrochemical industry, isobutane serves as a crucial feedstock for the production of high-octane gasoline components and various chemicals. The growing automotive sector, particularly in developing economies, has fueled the demand for isobutane in gasoline blending. Additionally, the increasing use of isobutane in the production of synthetic rubber and plastics has further boosted market growth.
The aerosol propellant segment has also witnessed substantial growth, with isobutane being widely used in personal care products, household cleaners, and industrial sprays. The shift away from chlorofluorocarbons (CFCs) has led to increased adoption of isobutane as a propellant, owing to its low environmental impact and cost-effectiveness.
Geographically, Asia Pacific dominates the isobutane market, accounting for over 35% of the global share. The region's rapid industrialization, growing population, and increasing disposable income have driven demand across various end-use industries. North America and Europe follow closely, with mature markets and stringent environmental regulations shaping the demand for isobutane-based products.
Looking ahead, the market for isobutane-based products is expected to continue its upward trajectory. Factors such as urbanization, technological advancements, and the push for sustainable solutions will likely drive further innovation in isobutane-based processes. However, challenges such as price volatility of raw materials and safety concerns associated with the flammability of isobutane may impact market growth to some extent.
Current Challenges in Isobutane Processing
The isobutane-based patent processes face several significant challenges in the current technological landscape. One of the primary issues is the optimization of reaction conditions to maximize yield and selectivity. Achieving the ideal balance between temperature, pressure, and catalyst composition remains a complex task, as these parameters significantly influence the product distribution and overall process efficiency.
Energy efficiency is another critical concern in isobutane processing. The high temperatures and pressures required for many isobutane-based reactions result in substantial energy consumption. This not only impacts the economic viability of the processes but also raises environmental concerns due to increased carbon emissions. Developing more energy-efficient technologies or finding alternative reaction pathways that operate under milder conditions is a pressing challenge.
Catalyst deactivation and regeneration pose ongoing difficulties in isobutane processing. Many catalysts used in these processes are susceptible to coking, poisoning, or sintering, leading to reduced activity and selectivity over time. Improving catalyst stability and developing more effective regeneration techniques are crucial for maintaining process efficiency and reducing downtime.
The handling and storage of isobutane present safety challenges due to its high flammability and volatility. Ensuring proper containment, leak detection, and emergency response systems are essential but can add complexity and cost to the overall process. Developing safer handling protocols and innovative storage solutions is an area of ongoing research and development.
Process integration and intensification represent another set of challenges in isobutane processing. Optimizing heat integration, minimizing waste streams, and improving separation processes are critical for enhancing overall process efficiency. Additionally, the development of novel reactor designs that can combine multiple unit operations or improve mass and heat transfer could lead to significant improvements in process performance.
Feedstock purity and variability also present challenges in isobutane-based processes. Impurities in the feedstock can affect catalyst performance, product quality, and equipment reliability. Developing robust processes that can handle feedstock variations or implementing more effective purification technologies is essential for maintaining consistent product quality and process efficiency.
Lastly, meeting increasingly stringent environmental regulations poses a significant challenge for isobutane processing. Reducing emissions, minimizing waste generation, and improving overall sustainability are becoming critical factors in process design and operation. This includes addressing issues such as VOC emissions, wastewater treatment, and the development of more environmentally friendly catalysts and solvents.
Energy efficiency is another critical concern in isobutane processing. The high temperatures and pressures required for many isobutane-based reactions result in substantial energy consumption. This not only impacts the economic viability of the processes but also raises environmental concerns due to increased carbon emissions. Developing more energy-efficient technologies or finding alternative reaction pathways that operate under milder conditions is a pressing challenge.
Catalyst deactivation and regeneration pose ongoing difficulties in isobutane processing. Many catalysts used in these processes are susceptible to coking, poisoning, or sintering, leading to reduced activity and selectivity over time. Improving catalyst stability and developing more effective regeneration techniques are crucial for maintaining process efficiency and reducing downtime.
The handling and storage of isobutane present safety challenges due to its high flammability and volatility. Ensuring proper containment, leak detection, and emergency response systems are essential but can add complexity and cost to the overall process. Developing safer handling protocols and innovative storage solutions is an area of ongoing research and development.
Process integration and intensification represent another set of challenges in isobutane processing. Optimizing heat integration, minimizing waste streams, and improving separation processes are critical for enhancing overall process efficiency. Additionally, the development of novel reactor designs that can combine multiple unit operations or improve mass and heat transfer could lead to significant improvements in process performance.
Feedstock purity and variability also present challenges in isobutane-based processes. Impurities in the feedstock can affect catalyst performance, product quality, and equipment reliability. Developing robust processes that can handle feedstock variations or implementing more effective purification technologies is essential for maintaining consistent product quality and process efficiency.
Lastly, meeting increasingly stringent environmental regulations poses a significant challenge for isobutane processing. Reducing emissions, minimizing waste generation, and improving overall sustainability are becoming critical factors in process design and operation. This includes addressing issues such as VOC emissions, wastewater treatment, and the development of more environmentally friendly catalysts and solvents.
Existing Isobutane Process Optimization Methods
01 Catalytic dehydrogenation of isobutane
Catalytic dehydrogenation processes are used to convert isobutane to isobutylene. Optimal conditions involve specific catalysts, temperatures, and pressures to maximize yield and selectivity. These processes often employ metal oxide catalysts and operate at elevated temperatures.- Catalytic dehydrogenation of isobutane: Catalytic dehydrogenation processes are used to convert isobutane to isobutylene. Optimal conditions involve specific catalysts, temperatures, and pressures to maximize yield and selectivity. The process often utilizes metal oxide catalysts and operates at elevated temperatures.
- Isobutane purification and separation: Purification and separation techniques are crucial for obtaining high-purity isobutane for various applications. These processes may involve distillation, adsorption, or membrane separation technologies. Optimal conditions focus on efficiency, energy consumption, and product purity.
- Isobutane alkylation processes: Alkylation of isobutane with olefins is an important industrial process for producing high-octane gasoline components. Optimal conditions involve careful control of temperature, pressure, and catalyst activity to maximize product quality and minimize side reactions.
- Isobutane as a blowing agent: Isobutane is used as a blowing agent in the production of foams and aerosols. Optimal conditions for these processes focus on achieving desired foam properties or spray characteristics while ensuring safety and environmental compliance.
- Isobutane isomerization: Isomerization of n-butane to isobutane is an important process for increasing the octane number of gasoline. Optimal conditions involve specific catalysts, temperatures, and pressures to maximize the conversion of n-butane to isobutane while minimizing unwanted side reactions.
02 Isobutane purification and separation
Purification and separation techniques are crucial for obtaining high-quality isobutane for various applications. These processes may involve distillation, adsorption, or membrane separation technologies. Optimal conditions focus on maximizing purity while minimizing energy consumption.Expand Specific Solutions03 Isobutane alkylation processes
Alkylation processes involving isobutane are important in the production of high-octane gasoline components. Optimal conditions for these processes include specific acid catalysts, reaction temperatures, and mixing ratios to achieve desired product quality and yield.Expand Specific Solutions04 Isobutane-based polymerization
Isobutane is used as a diluent or reaction medium in certain polymerization processes. Optimal conditions for these processes involve controlling temperature, pressure, and catalyst concentrations to achieve desired polymer properties and process efficiency.Expand Specific Solutions05 Isobutane storage and handling
Safe storage and handling of isobutane are critical for industrial applications. Optimal conditions focus on maintaining appropriate pressure and temperature to keep isobutane in a liquid state, as well as implementing safety measures to prevent leaks and explosions.Expand Specific Solutions
Key Players in Isobutane Industry
The research on optimal conditions for isobutane-based patent processes is in a mature stage of development, with significant market potential and technological advancements. The global market for isobutane-based products is substantial, driven by demand in petrochemicals, fuel additives, and specialty chemicals. Key players like China Petroleum & Chemical Corp., BASF Corp., and UOP LLC have established strong positions through extensive R&D and patent portfolios. Emerging companies such as Gevo, Inc. and Global Bioenergies SA are focusing on innovative bio-based isobutane production methods, indicating a shift towards more sustainable processes. The technology's maturity is evident in the diverse applications and ongoing optimization efforts by industry leaders and research institutions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced isobutane-based processes for the production of high-purity isobutylene. Their patented technology utilizes a novel catalyst system that enhances selectivity and conversion rates. The process operates at optimized temperatures between 500-550°C and pressures of 1-2 MPa, achieving isobutylene yields of up to 95%[1]. Sinopec's method incorporates a multi-stage reactor design with inter-stage cooling, which helps maintain ideal reaction conditions and extends catalyst life[3]. Additionally, they have implemented an innovative separation system using extractive distillation, resulting in isobutylene purity exceeding 99.9%[5].
Strengths: High yield and purity of isobutylene, energy-efficient process, extended catalyst life. Weaknesses: Potential high initial investment costs, complexity of multi-stage reactor system.
BASF Corp.
Technical Solution: BASF Corp. has pioneered a sustainable approach to isobutane-based processes, focusing on green chemistry principles. Their patented technology utilizes a bio-derived catalyst that operates effectively at lower temperatures (400-450°C) compared to conventional methods[2]. The process incorporates a unique fluidized bed reactor design, allowing for improved heat transfer and reaction control. BASF's method also features an integrated heat recovery system, which significantly reduces overall energy consumption by up to 30%[4]. Furthermore, they have developed a proprietary adsorbent material for the purification step, achieving isobutylene purity of 99.8% while minimizing waste generation[6].
Strengths: Lower energy consumption, environmentally friendly catalyst, efficient heat recovery. Weaknesses: Potentially higher catalyst costs, may require specialized equipment for the fluidized bed reactor.
Critical Patents in Isobutane Processing
Processes for the isomerization of normal butane to isobutane
PatentWO2006099566A1
Innovation
- Integration of a selectively permeable membrane with a deisobutanizer to reduce reboiler duty and allow for a lower reflux ratio, enabling the recovery of isobutane with reduced heat duty and membrane surface area requirements, while maintaining high isobutane purity.
Process for isomerizing isobutane
PatentWO2023107944A1
Innovation
- Isomerizing a butane stream with a low hydrogen to hydrocarbon ratio in an isomerization reactor, specifically separating C5+ hydrocarbons and feeding a butane stream predominantly composed of isobutane, while using a suitable isomerization catalyst and operating conditions to achieve high conversion and selectivity to normal paraffins.
Environmental Impact of Isobutane Processes
The environmental impact of isobutane-based processes is a critical consideration in the research of optimal conditions for these patent processes. Isobutane, a hydrocarbon compound, is widely used in various industrial applications, including refrigeration, propellants, and petrochemical production. However, its use and processing can have significant environmental implications that must be carefully evaluated and mitigated.
One of the primary environmental concerns associated with isobutane processes is their contribution to air pollution. When released into the atmosphere, isobutane can react with other pollutants to form ground-level ozone, a major component of smog. This can lead to respiratory issues and other health problems in affected populations. Additionally, isobutane is a volatile organic compound (VOC), which can contribute to the formation of photochemical smog and negatively impact air quality.
The potential for accidental releases and leaks during isobutane processing poses another environmental risk. As a highly flammable substance, isobutane can create explosive atmospheres if not properly contained. Such incidents not only pose immediate safety hazards but can also result in the release of harmful substances into the environment, affecting soil, water, and air quality in the surrounding areas.
Climate change implications are also a significant concern in isobutane-based processes. While isobutane itself has a relatively low global warming potential compared to some other refrigerants, its production and use still contribute to greenhouse gas emissions. The energy-intensive nature of isobutane processing and the potential for fugitive emissions during production and transportation can add to the overall carbon footprint of these processes.
Water pollution is another potential environmental impact that must be considered. Wastewater from isobutane production facilities may contain various contaminants, including hydrocarbons and chemical additives. If not properly treated, this wastewater can pollute local water bodies, affecting aquatic ecosystems and potentially contaminating drinking water sources.
To address these environmental concerns, research on optimal conditions for isobutane-based processes must focus on developing cleaner production methods and more efficient containment systems. This includes exploring catalysts and process conditions that minimize byproduct formation and reduce energy consumption. Additionally, implementing robust leak detection and repair programs can help prevent fugitive emissions and reduce the risk of accidental releases.
Advancements in recycling and recovery technologies for isobutane are also crucial for minimizing environmental impact. Developing closed-loop systems that capture and reuse isobutane can significantly reduce emissions and resource consumption. Furthermore, exploring alternative, more environmentally friendly substances that can replace isobutane in certain applications may be a valuable long-term strategy for reducing the overall environmental footprint of these processes.
One of the primary environmental concerns associated with isobutane processes is their contribution to air pollution. When released into the atmosphere, isobutane can react with other pollutants to form ground-level ozone, a major component of smog. This can lead to respiratory issues and other health problems in affected populations. Additionally, isobutane is a volatile organic compound (VOC), which can contribute to the formation of photochemical smog and negatively impact air quality.
The potential for accidental releases and leaks during isobutane processing poses another environmental risk. As a highly flammable substance, isobutane can create explosive atmospheres if not properly contained. Such incidents not only pose immediate safety hazards but can also result in the release of harmful substances into the environment, affecting soil, water, and air quality in the surrounding areas.
Climate change implications are also a significant concern in isobutane-based processes. While isobutane itself has a relatively low global warming potential compared to some other refrigerants, its production and use still contribute to greenhouse gas emissions. The energy-intensive nature of isobutane processing and the potential for fugitive emissions during production and transportation can add to the overall carbon footprint of these processes.
Water pollution is another potential environmental impact that must be considered. Wastewater from isobutane production facilities may contain various contaminants, including hydrocarbons and chemical additives. If not properly treated, this wastewater can pollute local water bodies, affecting aquatic ecosystems and potentially contaminating drinking water sources.
To address these environmental concerns, research on optimal conditions for isobutane-based processes must focus on developing cleaner production methods and more efficient containment systems. This includes exploring catalysts and process conditions that minimize byproduct formation and reduce energy consumption. Additionally, implementing robust leak detection and repair programs can help prevent fugitive emissions and reduce the risk of accidental releases.
Advancements in recycling and recovery technologies for isobutane are also crucial for minimizing environmental impact. Developing closed-loop systems that capture and reuse isobutane can significantly reduce emissions and resource consumption. Furthermore, exploring alternative, more environmentally friendly substances that can replace isobutane in certain applications may be a valuable long-term strategy for reducing the overall environmental footprint of these processes.
Safety Considerations in Isobutane Handling
Safety considerations in isobutane handling are paramount in optimizing patent processes involving this highly flammable hydrocarbon. The primary concern is the prevention of fire and explosion hazards due to isobutane's low boiling point and high volatility. Proper storage and containment systems are essential, including the use of pressure-rated vessels and leak-proof piping to minimize the risk of accidental releases.
Ventilation plays a crucial role in maintaining safe working environments. Adequate air circulation and exhaust systems must be implemented to prevent the accumulation of isobutane vapors, which can form explosive mixtures with air. Continuous monitoring of isobutane concentrations using gas detection systems is necessary to alert personnel of potential leaks or dangerous accumulations.
Personal protective equipment (PPE) is vital for workers handling isobutane. This includes flame-resistant clothing, anti-static footwear, and appropriate respiratory protection. Training programs should be established to ensure all personnel are familiar with the properties of isobutane, emergency procedures, and the correct use of safety equipment.
Electrical safety is another critical aspect, as isobutane can be ignited by electrical sparks. All electrical equipment in areas where isobutane is present must be explosion-proof and properly grounded. The implementation of intrinsically safe instrumentation and control systems further reduces the risk of ignition sources.
Temperature control is essential in isobutane-based processes. Cooling systems must be designed to maintain temperatures below the autoignition point of isobutane, and heat-generating equipment should be properly insulated and monitored to prevent overheating.
Emergency response planning is crucial for mitigating potential incidents. This includes the installation of fire suppression systems, emergency shutdown procedures, and evacuation protocols. Regular drills and simulations should be conducted to ensure personnel are prepared to respond effectively to potential emergencies.
Proper waste management and disposal procedures must be established to handle isobutane-containing materials safely. This includes the use of closed systems for transferring and processing isobutane, as well as appropriate methods for disposing of contaminated materials.
Compliance with relevant regulations and industry standards is essential. This includes adherence to guidelines set by organizations such as OSHA, NFPA, and API, which provide comprehensive frameworks for the safe handling of flammable materials like isobutane in industrial settings.
Ventilation plays a crucial role in maintaining safe working environments. Adequate air circulation and exhaust systems must be implemented to prevent the accumulation of isobutane vapors, which can form explosive mixtures with air. Continuous monitoring of isobutane concentrations using gas detection systems is necessary to alert personnel of potential leaks or dangerous accumulations.
Personal protective equipment (PPE) is vital for workers handling isobutane. This includes flame-resistant clothing, anti-static footwear, and appropriate respiratory protection. Training programs should be established to ensure all personnel are familiar with the properties of isobutane, emergency procedures, and the correct use of safety equipment.
Electrical safety is another critical aspect, as isobutane can be ignited by electrical sparks. All electrical equipment in areas where isobutane is present must be explosion-proof and properly grounded. The implementation of intrinsically safe instrumentation and control systems further reduces the risk of ignition sources.
Temperature control is essential in isobutane-based processes. Cooling systems must be designed to maintain temperatures below the autoignition point of isobutane, and heat-generating equipment should be properly insulated and monitored to prevent overheating.
Emergency response planning is crucial for mitigating potential incidents. This includes the installation of fire suppression systems, emergency shutdown procedures, and evacuation protocols. Regular drills and simulations should be conducted to ensure personnel are prepared to respond effectively to potential emergencies.
Proper waste management and disposal procedures must be established to handle isobutane-containing materials safely. This includes the use of closed systems for transferring and processing isobutane, as well as appropriate methods for disposing of contaminated materials.
Compliance with relevant regulations and industry standards is essential. This includes adherence to guidelines set by organizations such as OSHA, NFPA, and API, which provide comprehensive frameworks for the safe handling of flammable materials like isobutane in industrial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!