Efficiency Comparisons of Isobutane in Distillation Columns
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane Distillation Background and Objectives
Isobutane distillation has been a critical process in the petrochemical industry for decades, playing a vital role in the production of high-purity isobutane for various applications. The technology has evolved significantly since its inception, driven by the increasing demand for more efficient and environmentally friendly separation processes.
The development of isobutane distillation can be traced back to the early 20th century when the petrochemical industry began to expand rapidly. Initially, the process was relatively inefficient, consuming large amounts of energy and producing lower purity products. However, as the understanding of thermodynamics and mass transfer principles improved, so did the design and operation of distillation columns.
In recent years, the focus has shifted towards enhancing the efficiency of isobutane distillation columns. This trend is primarily driven by the need to reduce energy consumption, minimize environmental impact, and improve product quality. The industry has witnessed significant advancements in column design, packing materials, and control systems, all aimed at optimizing the separation process.
The current technological landscape of isobutane distillation is characterized by a diverse range of column configurations and operational strategies. These include conventional tray columns, packed columns, and more advanced designs such as dividing wall columns and heat-integrated distillation columns. Each of these technologies offers unique advantages in terms of separation efficiency, energy consumption, and capital costs.
The primary objective of efficiency comparisons in isobutane distillation columns is to identify the most effective and economical separation methods. This involves evaluating various parameters such as energy consumption, separation efficiency, product purity, and operational flexibility. By conducting comprehensive comparisons, researchers and industry professionals aim to develop innovative solutions that can significantly improve the overall performance of isobutane distillation processes.
Furthermore, these efficiency comparisons are crucial for addressing the growing environmental concerns associated with industrial processes. As regulatory pressures increase and sustainability becomes a key focus for many organizations, there is a pressing need to develop distillation technologies that minimize carbon footprint and reduce waste generation.
Looking ahead, the future of isobutane distillation is likely to be shaped by emerging technologies such as process intensification, advanced process control, and the integration of renewable energy sources. These developments promise to further enhance the efficiency and sustainability of isobutane separation processes, paving the way for more cost-effective and environmentally friendly industrial operations.
The development of isobutane distillation can be traced back to the early 20th century when the petrochemical industry began to expand rapidly. Initially, the process was relatively inefficient, consuming large amounts of energy and producing lower purity products. However, as the understanding of thermodynamics and mass transfer principles improved, so did the design and operation of distillation columns.
In recent years, the focus has shifted towards enhancing the efficiency of isobutane distillation columns. This trend is primarily driven by the need to reduce energy consumption, minimize environmental impact, and improve product quality. The industry has witnessed significant advancements in column design, packing materials, and control systems, all aimed at optimizing the separation process.
The current technological landscape of isobutane distillation is characterized by a diverse range of column configurations and operational strategies. These include conventional tray columns, packed columns, and more advanced designs such as dividing wall columns and heat-integrated distillation columns. Each of these technologies offers unique advantages in terms of separation efficiency, energy consumption, and capital costs.
The primary objective of efficiency comparisons in isobutane distillation columns is to identify the most effective and economical separation methods. This involves evaluating various parameters such as energy consumption, separation efficiency, product purity, and operational flexibility. By conducting comprehensive comparisons, researchers and industry professionals aim to develop innovative solutions that can significantly improve the overall performance of isobutane distillation processes.
Furthermore, these efficiency comparisons are crucial for addressing the growing environmental concerns associated with industrial processes. As regulatory pressures increase and sustainability becomes a key focus for many organizations, there is a pressing need to develop distillation technologies that minimize carbon footprint and reduce waste generation.
Looking ahead, the future of isobutane distillation is likely to be shaped by emerging technologies such as process intensification, advanced process control, and the integration of renewable energy sources. These developments promise to further enhance the efficiency and sustainability of isobutane separation processes, paving the way for more cost-effective and environmentally friendly industrial operations.
Market Analysis for Isobutane Separation
The market for isobutane separation has shown significant growth in recent years, driven by increasing demand across various industries. The global isobutane market size was valued at approximately $8.5 billion in 2020 and is projected to reach $12.3 billion by 2027, growing at a CAGR of 5.2% during the forecast period. This growth is primarily attributed to the rising demand for isobutane in the production of refrigerants, fuel additives, and petrochemicals.
The refrigeration and air conditioning sector remains the largest consumer of isobutane, accounting for over 40% of the total market share. The shift towards more environmentally friendly refrigerants has boosted the demand for isobutane as a replacement for hydrofluorocarbons (HFCs) in various cooling applications. Additionally, the automotive industry's increasing focus on fuel efficiency has led to a surge in demand for isobutane as a fuel additive, contributing to market growth.
Geographically, Asia-Pacific dominates the isobutane market, holding a market share of approximately 35%. The region's rapid industrialization, particularly in countries like China and India, has been a key driver for market expansion. North America and Europe follow closely, with market shares of 28% and 25% respectively. These regions have seen increased adoption of isobutane in the petrochemical industry and as a propellant in aerosol products.
The market for isobutane separation technologies is highly competitive, with key players focusing on developing more efficient and cost-effective separation processes. Distillation remains the most widely used method for isobutane separation, accounting for over 60% of the total separation market. However, emerging technologies such as membrane separation and adsorption are gaining traction due to their potential for higher energy efficiency and lower operating costs.
Challenges in the isobutane separation market include stringent environmental regulations, volatility in raw material prices, and the need for significant capital investments in separation technologies. Despite these challenges, the market outlook remains positive, driven by the growing demand for high-purity isobutane in various end-use industries and the continuous development of more efficient separation processes.
The refrigeration and air conditioning sector remains the largest consumer of isobutane, accounting for over 40% of the total market share. The shift towards more environmentally friendly refrigerants has boosted the demand for isobutane as a replacement for hydrofluorocarbons (HFCs) in various cooling applications. Additionally, the automotive industry's increasing focus on fuel efficiency has led to a surge in demand for isobutane as a fuel additive, contributing to market growth.
Geographically, Asia-Pacific dominates the isobutane market, holding a market share of approximately 35%. The region's rapid industrialization, particularly in countries like China and India, has been a key driver for market expansion. North America and Europe follow closely, with market shares of 28% and 25% respectively. These regions have seen increased adoption of isobutane in the petrochemical industry and as a propellant in aerosol products.
The market for isobutane separation technologies is highly competitive, with key players focusing on developing more efficient and cost-effective separation processes. Distillation remains the most widely used method for isobutane separation, accounting for over 60% of the total separation market. However, emerging technologies such as membrane separation and adsorption are gaining traction due to their potential for higher energy efficiency and lower operating costs.
Challenges in the isobutane separation market include stringent environmental regulations, volatility in raw material prices, and the need for significant capital investments in separation technologies. Despite these challenges, the market outlook remains positive, driven by the growing demand for high-purity isobutane in various end-use industries and the continuous development of more efficient separation processes.
Current Challenges in Isobutane Distillation
The distillation of isobutane presents several significant challenges that impact the efficiency and effectiveness of the separation process. One of the primary issues is the close boiling points between isobutane and its isomers, particularly n-butane. This proximity in boiling points makes it difficult to achieve high purity separation, often requiring a large number of theoretical stages and high reflux ratios, which in turn increases energy consumption and operational costs.
Another challenge is the formation of azeotropes between isobutane and other components in the feed mixture. Azeotropes are mixtures of two or more liquids whose proportions cannot be altered by simple distillation, making it challenging to achieve complete separation. This phenomenon necessitates the use of more complex distillation techniques, such as extractive or azeotropic distillation, further complicating the process design and increasing capital expenditure.
The high volatility of isobutane also poses operational difficulties. Its low boiling point (-11.7°C) means that the distillation process must be conducted under pressure to maintain the liquid phase. This pressurized operation increases the complexity of equipment design and safety considerations, potentially leading to higher maintenance costs and more stringent safety protocols.
Energy efficiency is a persistent challenge in isobutane distillation. The process typically requires significant heating in the reboiler and cooling in the condenser, contributing to high energy consumption. This not only impacts operational costs but also raises environmental concerns due to the associated carbon footprint. Improving heat integration and exploring alternative separation technologies are ongoing areas of research to address this issue.
Fouling and corrosion of distillation equipment are additional concerns in isobutane separation. The presence of impurities in the feed, such as sulfur compounds or water, can lead to the formation of deposits on heat transfer surfaces, reducing efficiency over time. Moreover, these impurities can cause corrosion, particularly in the presence of water, necessitating the use of corrosion-resistant materials and regular maintenance, which adds to the overall operational complexity and cost.
Lastly, the control and optimization of isobutane distillation columns present significant challenges. The process is highly sensitive to changes in operating conditions, such as feed composition, pressure, and temperature. Maintaining optimal performance requires sophisticated control systems and strategies, including advanced process control (APC) and real-time optimization techniques. Implementing and maintaining these control systems adds another layer of complexity to the operation of isobutane distillation columns.
Another challenge is the formation of azeotropes between isobutane and other components in the feed mixture. Azeotropes are mixtures of two or more liquids whose proportions cannot be altered by simple distillation, making it challenging to achieve complete separation. This phenomenon necessitates the use of more complex distillation techniques, such as extractive or azeotropic distillation, further complicating the process design and increasing capital expenditure.
The high volatility of isobutane also poses operational difficulties. Its low boiling point (-11.7°C) means that the distillation process must be conducted under pressure to maintain the liquid phase. This pressurized operation increases the complexity of equipment design and safety considerations, potentially leading to higher maintenance costs and more stringent safety protocols.
Energy efficiency is a persistent challenge in isobutane distillation. The process typically requires significant heating in the reboiler and cooling in the condenser, contributing to high energy consumption. This not only impacts operational costs but also raises environmental concerns due to the associated carbon footprint. Improving heat integration and exploring alternative separation technologies are ongoing areas of research to address this issue.
Fouling and corrosion of distillation equipment are additional concerns in isobutane separation. The presence of impurities in the feed, such as sulfur compounds or water, can lead to the formation of deposits on heat transfer surfaces, reducing efficiency over time. Moreover, these impurities can cause corrosion, particularly in the presence of water, necessitating the use of corrosion-resistant materials and regular maintenance, which adds to the overall operational complexity and cost.
Lastly, the control and optimization of isobutane distillation columns present significant challenges. The process is highly sensitive to changes in operating conditions, such as feed composition, pressure, and temperature. Maintaining optimal performance requires sophisticated control systems and strategies, including advanced process control (APC) and real-time optimization techniques. Implementing and maintaining these control systems adds another layer of complexity to the operation of isobutane distillation columns.
Existing Isobutane Distillation Techniques
01 Isobutane production methods
Various methods for producing isobutane efficiently are described, including catalytic processes, isomerization of n-butane, and dehydrogenation reactions. These methods aim to improve yield and selectivity while reducing energy consumption and byproduct formation.- Isobutane production methods: Various methods for producing isobutane are described, including catalytic processes, isomerization of n-butane, and dehydrogenation reactions. These methods aim to improve the efficiency of isobutane production by optimizing reaction conditions, catalyst selection, and process parameters.
- Isobutane purification techniques: Purification techniques for isobutane are discussed, focusing on methods to remove impurities and increase the purity of the final product. These techniques may include distillation, adsorption, and membrane separation processes, which contribute to improving the overall efficiency of isobutane production and utilization.
- Isobutane in refrigeration systems: The use of isobutane as a refrigerant is explored, highlighting its efficiency in cooling systems. Research focuses on optimizing the performance of isobutane-based refrigeration systems, including improvements in compressor design, heat exchanger efficiency, and overall system configuration.
- Isobutane in fuel applications: The efficiency of isobutane in fuel applications is examined, including its use as a component in liquefied petroleum gas (LPG) and as a blending agent in gasoline. Research focuses on improving combustion efficiency, reducing emissions, and optimizing fuel formulations containing isobutane.
- Isobutane in chemical synthesis: The use of isobutane as a feedstock in chemical synthesis is explored, focusing on its efficiency in producing various chemical compounds. Research includes the development of catalytic processes, reaction optimization, and the exploration of new reaction pathways to improve the yield and selectivity of isobutane-based chemical synthesis.
02 Isobutane purification techniques
Efficient purification techniques for isobutane are discussed, including distillation, adsorption, and membrane separation processes. These methods focus on removing impurities and achieving high-purity isobutane for various industrial applications.Expand Specific Solutions03 Isobutane as a refrigerant
The use of isobutane as an efficient refrigerant is explored, highlighting its thermodynamic properties, environmental benefits, and energy-saving potential in refrigeration and air conditioning systems. Improved system designs and safety measures are also discussed.Expand Specific Solutions04 Isobutane in chemical reactions
The efficiency of isobutane in various chemical reactions is examined, including its use as a feedstock for petrochemical processes, alkylation reactions, and the production of high-value chemicals. Catalytic systems and reaction conditions for optimizing isobutane conversion are discussed.Expand Specific Solutions05 Isobutane storage and transportation
Efficient methods for storing and transporting isobutane are presented, focusing on safety measures, pressure management, and minimizing losses during handling. Advanced container designs and monitoring systems are discussed to improve overall efficiency in the isobutane supply chain.Expand Specific Solutions
Key Players in Isobutane Separation Industry
The efficiency comparison of isobutane in distillation columns is a mature field within the petrochemical industry, currently in a consolidation phase. The global market for this technology is substantial, driven by the increasing demand for high-purity isobutane in various applications. Major players like China Petroleum & Chemical Corp., SINOPEC Engineering, and UOP LLC have established strong positions, leveraging their extensive research and development capabilities. The technology's maturity is evident in the advanced solutions offered by companies such as BASF Corp. and LG Chem Ltd., which focus on optimizing distillation processes for improved efficiency and reduced energy consumption. Emerging players like Gevo, Inc. are exploring innovative approaches, potentially disrupting the market with bio-based alternatives.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced distillation technologies for isobutane separation, focusing on energy efficiency and product purity. Their approach involves the use of high-performance structured packings and advanced process control systems. Sinopec has implemented a divided wall column (DWC) design, which allows for the separation of multiple components in a single column, reducing energy consumption by up to 30% compared to conventional distillation sequences[1]. They have also integrated heat pump technology to further optimize energy usage, resulting in a 15-20% reduction in operating costs[3]. Additionally, Sinopec has developed a proprietary catalyst system that enhances the selectivity of isobutane production, improving overall process efficiency[5].
Strengths: Significant energy savings, improved product purity, and reduced capital costs. Weaknesses: High initial investment for retrofitting existing columns and potential complexity in operation and maintenance.
UOP LLC
Technical Solution: UOP LLC, a Honeywell company, has pioneered several innovations in isobutane distillation efficiency. Their Oleflex™ process technology incorporates advanced distillation techniques for isobutane recovery and purification. UOP has developed a novel divided wall column (DWC) configuration that allows for simultaneous separation of isobutane from other light hydrocarbons, reducing energy consumption by up to 25% compared to conventional distillation trains[2]. They have also implemented advanced process control algorithms and real-time optimization systems, which continuously adjust operating parameters to maximize efficiency. UOP's proprietary high-performance trays and structured packings enhance mass transfer efficiency, leading to improved separation and reduced column height[4]. Furthermore, their heat integration techniques, including the use of vapor recompression, have resulted in additional energy savings of 15-20% in isobutane distillation processes[6].
Strengths: Proven technology with high energy efficiency, reduced equipment footprint, and improved product recovery. Weaknesses: Potentially higher upfront costs and the need for specialized expertise in operation and maintenance.
Innovative Approaches in Isobutane Separation
Processes for the isomerization of normal butane to isobutane
PatentWO2006099566A1
Innovation
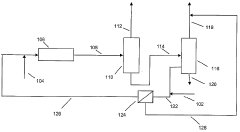
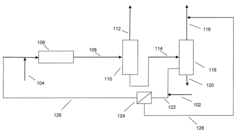
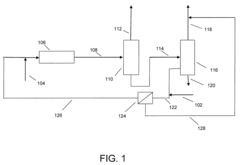
- Integration of a selectively permeable membrane with a deisobutanizer to reduce reboiler duty and allow for a lower reflux ratio, enabling the recovery of isobutane with reduced heat duty and membrane surface area requirements, while maintaining high isobutane purity.
Processes for the isomerization of normal butane to isobutane
PatentInactiveUS7981376B2
Innovation
- Integration of a selectively permeable membrane with a deisobutanizer to reduce reboiler duty and reflux ratios, allowing for a lower boiling fraction with high isobutane content and a normal butane-containing fraction to be recycled, thereby reducing heat duty and operating costs, while maintaining high isobutane purity.
Energy Efficiency in Isobutane Separation
Energy efficiency in isobutane separation is a critical aspect of distillation column operations, particularly in the petrochemical industry. The process of separating isobutane from other hydrocarbons requires significant energy input, making it a prime target for efficiency improvements. Recent advancements in distillation technology have focused on reducing energy consumption while maintaining or enhancing separation effectiveness.
One of the key strategies for improving energy efficiency in isobutane separation is the implementation of advanced distillation column designs. These include dividing wall columns, which can achieve the separation of multiple components in a single column, reducing both capital and operating costs. Heat-integrated distillation columns (HIDiC) have also shown promise, utilizing internal heat integration to minimize external energy requirements.
Process intensification techniques have been applied to isobutane separation, resulting in more compact and efficient distillation units. Membrane-assisted distillation, for instance, combines traditional distillation with selective membrane technology to enhance separation efficiency and reduce energy consumption. This hybrid approach can lead to significant reductions in the overall energy footprint of the separation process.
Optimization of operating conditions plays a crucial role in maximizing energy efficiency. Advanced control systems, utilizing real-time monitoring and predictive algorithms, allow for precise adjustment of parameters such as reflux ratio, feed temperature, and pressure. These systems can adapt to fluctuations in feed composition and process conditions, ensuring optimal energy utilization throughout the separation process.
Heat recovery systems have been increasingly integrated into isobutane distillation processes. By capturing and reusing waste heat from various stages of the distillation process, overall energy consumption can be substantially reduced. Technologies such as vapor recompression and multi-effect distillation leverage this principle to enhance energy efficiency.
The development of novel heat transfer fluids and column internals has also contributed to improved energy efficiency. High-performance structured packings and advanced tray designs increase mass transfer efficiency, allowing for reduced column height and lower pressure drops. This, in turn, translates to lower energy requirements for achieving the desired separation.
As environmental regulations become more stringent, there is a growing focus on integrating renewable energy sources into isobutane separation processes. Solar thermal energy and waste heat from other industrial processes are being explored as potential alternatives to traditional fossil fuel-based heating methods. These sustainable approaches not only improve energy efficiency but also reduce the carbon footprint of the separation process.
One of the key strategies for improving energy efficiency in isobutane separation is the implementation of advanced distillation column designs. These include dividing wall columns, which can achieve the separation of multiple components in a single column, reducing both capital and operating costs. Heat-integrated distillation columns (HIDiC) have also shown promise, utilizing internal heat integration to minimize external energy requirements.
Process intensification techniques have been applied to isobutane separation, resulting in more compact and efficient distillation units. Membrane-assisted distillation, for instance, combines traditional distillation with selective membrane technology to enhance separation efficiency and reduce energy consumption. This hybrid approach can lead to significant reductions in the overall energy footprint of the separation process.
Optimization of operating conditions plays a crucial role in maximizing energy efficiency. Advanced control systems, utilizing real-time monitoring and predictive algorithms, allow for precise adjustment of parameters such as reflux ratio, feed temperature, and pressure. These systems can adapt to fluctuations in feed composition and process conditions, ensuring optimal energy utilization throughout the separation process.
Heat recovery systems have been increasingly integrated into isobutane distillation processes. By capturing and reusing waste heat from various stages of the distillation process, overall energy consumption can be substantially reduced. Technologies such as vapor recompression and multi-effect distillation leverage this principle to enhance energy efficiency.
The development of novel heat transfer fluids and column internals has also contributed to improved energy efficiency. High-performance structured packings and advanced tray designs increase mass transfer efficiency, allowing for reduced column height and lower pressure drops. This, in turn, translates to lower energy requirements for achieving the desired separation.
As environmental regulations become more stringent, there is a growing focus on integrating renewable energy sources into isobutane separation processes. Solar thermal energy and waste heat from other industrial processes are being explored as potential alternatives to traditional fossil fuel-based heating methods. These sustainable approaches not only improve energy efficiency but also reduce the carbon footprint of the separation process.
Environmental Impact of Isobutane Distillation
The environmental impact of isobutane distillation is a critical consideration in the broader context of efficiency comparisons in distillation columns. Isobutane, a hydrocarbon commonly used in various industrial processes, poses significant environmental challenges when subjected to distillation processes.
One of the primary environmental concerns associated with isobutane distillation is its contribution to greenhouse gas emissions. The process of separating isobutane from other components in a distillation column requires substantial energy input, often derived from fossil fuel sources. This energy consumption leads to increased carbon dioxide emissions, contributing to global warming and climate change.
Furthermore, the potential for isobutane leaks during the distillation process presents a direct environmental hazard. Isobutane is a volatile organic compound (VOC) that can contribute to the formation of ground-level ozone, a key component of smog. This can have detrimental effects on air quality, particularly in urban and industrial areas where distillation facilities are often located.
Water pollution is another significant environmental concern associated with isobutane distillation. The cooling water used in the process can become contaminated with trace amounts of isobutane and other hydrocarbons. If not properly treated, this contaminated water can harm aquatic ecosystems when discharged into natural water bodies.
The production and disposal of waste materials generated during the distillation process also contribute to the environmental footprint. These wastes may include spent catalysts, contaminated adsorbents, and other by-products that require careful handling and disposal to prevent soil and groundwater contamination.
From a lifecycle perspective, the environmental impact of isobutane distillation extends beyond the immediate process. The extraction and transportation of raw materials, as well as the eventual use and disposal of isobutane-containing products, all contribute to the overall environmental burden.
However, it is important to note that advancements in distillation technology have led to improvements in energy efficiency and emissions control. Modern distillation columns often incorporate heat integration systems, advanced control strategies, and more efficient separation techniques that can significantly reduce energy consumption and environmental impact.
In conclusion, while isobutane distillation plays a crucial role in various industries, its environmental impact cannot be overlooked. Balancing the efficiency of the distillation process with environmental considerations is essential for sustainable industrial practices. Future research and development efforts should focus on further improving the energy efficiency of distillation columns, developing cleaner separation technologies, and exploring alternative, more environmentally friendly substances that could potentially replace isobutane in certain applications.
One of the primary environmental concerns associated with isobutane distillation is its contribution to greenhouse gas emissions. The process of separating isobutane from other components in a distillation column requires substantial energy input, often derived from fossil fuel sources. This energy consumption leads to increased carbon dioxide emissions, contributing to global warming and climate change.
Furthermore, the potential for isobutane leaks during the distillation process presents a direct environmental hazard. Isobutane is a volatile organic compound (VOC) that can contribute to the formation of ground-level ozone, a key component of smog. This can have detrimental effects on air quality, particularly in urban and industrial areas where distillation facilities are often located.
Water pollution is another significant environmental concern associated with isobutane distillation. The cooling water used in the process can become contaminated with trace amounts of isobutane and other hydrocarbons. If not properly treated, this contaminated water can harm aquatic ecosystems when discharged into natural water bodies.
The production and disposal of waste materials generated during the distillation process also contribute to the environmental footprint. These wastes may include spent catalysts, contaminated adsorbents, and other by-products that require careful handling and disposal to prevent soil and groundwater contamination.
From a lifecycle perspective, the environmental impact of isobutane distillation extends beyond the immediate process. The extraction and transportation of raw materials, as well as the eventual use and disposal of isobutane-containing products, all contribute to the overall environmental burden.
However, it is important to note that advancements in distillation technology have led to improvements in energy efficiency and emissions control. Modern distillation columns often incorporate heat integration systems, advanced control strategies, and more efficient separation techniques that can significantly reduce energy consumption and environmental impact.
In conclusion, while isobutane distillation plays a crucial role in various industries, its environmental impact cannot be overlooked. Balancing the efficiency of the distillation process with environmental considerations is essential for sustainable industrial practices. Future research and development efforts should focus on further improving the energy efficiency of distillation columns, developing cleaner separation technologies, and exploring alternative, more environmentally friendly substances that could potentially replace isobutane in certain applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!