Isobutane's Influence on Chemical Reaction Rates
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane Reaction Kinetics Background
Isobutane, a branched alkane with the molecular formula C4H10, has been a subject of significant interest in the field of chemical kinetics due to its unique structural properties and reactivity. The study of isobutane's influence on chemical reaction rates has its roots in the early 20th century, when researchers began to explore the behavior of hydrocarbons in various chemical processes.
The development of the petroleum industry and the growing demand for high-octane fuels in the mid-20th century led to increased attention on isobutane's reaction kinetics. Its branched structure, which contributes to its higher octane rating compared to its straight-chain isomer n-butane, made it a valuable component in gasoline blending and other industrial applications.
Early investigations into isobutane's reaction kinetics focused primarily on its combustion characteristics. Researchers sought to understand how its molecular structure affected ignition delay times, flame propagation, and overall reaction rates in combustion processes. These studies laid the groundwork for more comprehensive examinations of isobutane's behavior in various chemical reactions.
As analytical techniques and computational methods advanced, scientists gained deeper insights into the mechanistic aspects of isobutane reactions. The advent of spectroscopic methods, such as infrared and mass spectrometry, allowed for real-time monitoring of reaction intermediates and products, providing crucial information about reaction pathways and kinetics.
The late 20th and early 21st centuries saw a shift towards more detailed investigations of isobutane's role in specific chemical processes. Researchers began to explore its participation in catalytic reactions, isomerization processes, and its potential as a feedstock for the production of various chemicals. This expanded focus led to a more nuanced understanding of how isobutane's structure influences reaction rates in different chemical environments.
Recent years have witnessed an increased emphasis on the environmental implications of isobutane reactions. Studies have explored its atmospheric chemistry, including its role in the formation of tropospheric ozone and its potential as a greenhouse gas. These investigations have broadened the scope of isobutane reaction kinetics research, incorporating aspects of atmospheric science and climate change.
The ongoing exploration of isobutane's influence on chemical reaction rates continues to yield valuable insights for both fundamental science and industrial applications. As new technologies and analytical methods emerge, researchers are poised to uncover even more intricate details about the behavior of this important hydrocarbon in various chemical systems.
The development of the petroleum industry and the growing demand for high-octane fuels in the mid-20th century led to increased attention on isobutane's reaction kinetics. Its branched structure, which contributes to its higher octane rating compared to its straight-chain isomer n-butane, made it a valuable component in gasoline blending and other industrial applications.
Early investigations into isobutane's reaction kinetics focused primarily on its combustion characteristics. Researchers sought to understand how its molecular structure affected ignition delay times, flame propagation, and overall reaction rates in combustion processes. These studies laid the groundwork for more comprehensive examinations of isobutane's behavior in various chemical reactions.
As analytical techniques and computational methods advanced, scientists gained deeper insights into the mechanistic aspects of isobutane reactions. The advent of spectroscopic methods, such as infrared and mass spectrometry, allowed for real-time monitoring of reaction intermediates and products, providing crucial information about reaction pathways and kinetics.
The late 20th and early 21st centuries saw a shift towards more detailed investigations of isobutane's role in specific chemical processes. Researchers began to explore its participation in catalytic reactions, isomerization processes, and its potential as a feedstock for the production of various chemicals. This expanded focus led to a more nuanced understanding of how isobutane's structure influences reaction rates in different chemical environments.
Recent years have witnessed an increased emphasis on the environmental implications of isobutane reactions. Studies have explored its atmospheric chemistry, including its role in the formation of tropospheric ozone and its potential as a greenhouse gas. These investigations have broadened the scope of isobutane reaction kinetics research, incorporating aspects of atmospheric science and climate change.
The ongoing exploration of isobutane's influence on chemical reaction rates continues to yield valuable insights for both fundamental science and industrial applications. As new technologies and analytical methods emerge, researchers are poised to uncover even more intricate details about the behavior of this important hydrocarbon in various chemical systems.
Market Analysis for Isobutane Applications
The market for isobutane applications has experienced significant growth in recent years, driven by its versatile properties and increasing demand across various industries. Isobutane, a branched-chain alkane with the molecular formula C4H10, finds extensive use as a refrigerant, propellant, and feedstock in chemical processes. Its influence on chemical reaction rates has opened up new opportunities in the market, particularly in the petrochemical and energy sectors.
In the refrigeration industry, isobutane has gained traction as an environmentally friendly alternative to traditional refrigerants. Its low global warming potential (GWP) and zero ozone depletion potential (ODP) have made it a preferred choice for manufacturers of household appliances and commercial refrigeration systems. The market for isobutane-based refrigerants is expected to expand further as countries implement stricter regulations on greenhouse gas emissions.
The petrochemical industry represents another significant market for isobutane applications. As a feedstock for the production of high-octane gasoline components, isobutane plays a crucial role in meeting the growing demand for cleaner-burning fuels. The automotive sector's shift towards more efficient and environmentally friendly vehicles has contributed to the increased consumption of isobutane in fuel production processes.
In the aerosol industry, isobutane serves as a propellant in various consumer products, including personal care items, household cleaners, and industrial sprays. The market for isobutane-based propellants has shown steady growth, driven by consumer preferences for convenient and easy-to-use products. However, this segment faces challenges due to concerns over the flammability of isobutane and potential regulatory restrictions on volatile organic compounds (VOCs).
The energy sector presents emerging opportunities for isobutane applications, particularly in the development of advanced energy storage systems. Research into isobutane's potential as a working fluid in organic Rankine cycle (ORC) systems for waste heat recovery has shown promising results. This application could open up new markets in industrial energy efficiency and renewable energy integration.
The global market for isobutane is characterized by regional variations in demand and supply. North America and Europe lead in terms of consumption, driven by their well-established petrochemical and refrigeration industries. Meanwhile, Asia-Pacific is emerging as a key growth region, with rapidly expanding manufacturing sectors and increasing adoption of environmentally friendly technologies.
Market analysts project continued growth in the isobutane market, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. Factors contributing to this growth include technological advancements in chemical processing, increasing focus on energy efficiency, and the ongoing transition towards more sustainable industrial practices. However, market players must navigate challenges such as price volatility in the oil and gas sector and evolving regulatory landscapes to capitalize on these opportunities.
In the refrigeration industry, isobutane has gained traction as an environmentally friendly alternative to traditional refrigerants. Its low global warming potential (GWP) and zero ozone depletion potential (ODP) have made it a preferred choice for manufacturers of household appliances and commercial refrigeration systems. The market for isobutane-based refrigerants is expected to expand further as countries implement stricter regulations on greenhouse gas emissions.
The petrochemical industry represents another significant market for isobutane applications. As a feedstock for the production of high-octane gasoline components, isobutane plays a crucial role in meeting the growing demand for cleaner-burning fuels. The automotive sector's shift towards more efficient and environmentally friendly vehicles has contributed to the increased consumption of isobutane in fuel production processes.
In the aerosol industry, isobutane serves as a propellant in various consumer products, including personal care items, household cleaners, and industrial sprays. The market for isobutane-based propellants has shown steady growth, driven by consumer preferences for convenient and easy-to-use products. However, this segment faces challenges due to concerns over the flammability of isobutane and potential regulatory restrictions on volatile organic compounds (VOCs).
The energy sector presents emerging opportunities for isobutane applications, particularly in the development of advanced energy storage systems. Research into isobutane's potential as a working fluid in organic Rankine cycle (ORC) systems for waste heat recovery has shown promising results. This application could open up new markets in industrial energy efficiency and renewable energy integration.
The global market for isobutane is characterized by regional variations in demand and supply. North America and Europe lead in terms of consumption, driven by their well-established petrochemical and refrigeration industries. Meanwhile, Asia-Pacific is emerging as a key growth region, with rapidly expanding manufacturing sectors and increasing adoption of environmentally friendly technologies.
Market analysts project continued growth in the isobutane market, with a compound annual growth rate (CAGR) expected to remain strong over the next five years. Factors contributing to this growth include technological advancements in chemical processing, increasing focus on energy efficiency, and the ongoing transition towards more sustainable industrial practices. However, market players must navigate challenges such as price volatility in the oil and gas sector and evolving regulatory landscapes to capitalize on these opportunities.
Current Challenges in Isobutane Reaction Studies
The study of isobutane's influence on chemical reaction rates faces several significant challenges that hinder comprehensive understanding and practical applications. One primary obstacle is the complexity of reaction mechanisms involving isobutane. As a branched alkane, isobutane can participate in various reaction pathways, making it difficult to isolate and quantify its specific effects on reaction rates.
Experimental design and control pose another major challenge. Isobutane's high volatility and flammability necessitate stringent safety measures, limiting the range of experimental conditions that can be explored. This constraint often results in a gap between laboratory findings and real-world applications, particularly in industrial settings where reaction conditions may differ significantly.
The accurate measurement of reaction kinetics in isobutane-involved systems presents technical difficulties. Traditional methods for measuring reaction rates may not be suitable due to the rapid nature of some isobutane reactions or interference from side reactions. Advanced analytical techniques are required, but their implementation and interpretation can be complex and resource-intensive.
Environmental concerns add another layer of complexity to isobutane reaction studies. As a volatile organic compound (VOC) with potential greenhouse effects, the use of isobutane in large-scale experiments or industrial processes is subject to strict regulations. This regulatory environment can limit the scope and scale of research, particularly in academic settings.
Computational modeling of isobutane reactions also presents challenges. While molecular dynamics simulations and quantum chemical calculations offer powerful tools for studying reaction mechanisms, the accuracy of these models for complex systems involving isobutane is still a subject of ongoing research. Bridging the gap between computational predictions and experimental observations remains a significant challenge in the field.
The multidisciplinary nature of isobutane reaction studies further complicates research efforts. Expertise from chemistry, chemical engineering, environmental science, and computational modeling is often required to address the various aspects of isobutane's influence on reaction rates. Coordinating these diverse perspectives and integrating findings from different disciplines can be challenging but is essential for comprehensive understanding.
Lastly, the application of findings from isobutane reaction studies to industrial processes faces scalability issues. Translating laboratory-scale observations to large-scale industrial applications often encounters unforeseen complications, necessitating extensive pilot studies and process optimizations. This scaling challenge can significantly delay the practical implementation of research findings in industrial settings.
Experimental design and control pose another major challenge. Isobutane's high volatility and flammability necessitate stringent safety measures, limiting the range of experimental conditions that can be explored. This constraint often results in a gap between laboratory findings and real-world applications, particularly in industrial settings where reaction conditions may differ significantly.
The accurate measurement of reaction kinetics in isobutane-involved systems presents technical difficulties. Traditional methods for measuring reaction rates may not be suitable due to the rapid nature of some isobutane reactions or interference from side reactions. Advanced analytical techniques are required, but their implementation and interpretation can be complex and resource-intensive.
Environmental concerns add another layer of complexity to isobutane reaction studies. As a volatile organic compound (VOC) with potential greenhouse effects, the use of isobutane in large-scale experiments or industrial processes is subject to strict regulations. This regulatory environment can limit the scope and scale of research, particularly in academic settings.
Computational modeling of isobutane reactions also presents challenges. While molecular dynamics simulations and quantum chemical calculations offer powerful tools for studying reaction mechanisms, the accuracy of these models for complex systems involving isobutane is still a subject of ongoing research. Bridging the gap between computational predictions and experimental observations remains a significant challenge in the field.
The multidisciplinary nature of isobutane reaction studies further complicates research efforts. Expertise from chemistry, chemical engineering, environmental science, and computational modeling is often required to address the various aspects of isobutane's influence on reaction rates. Coordinating these diverse perspectives and integrating findings from different disciplines can be challenging but is essential for comprehensive understanding.
Lastly, the application of findings from isobutane reaction studies to industrial processes faces scalability issues. Translating laboratory-scale observations to large-scale industrial applications often encounters unforeseen complications, necessitating extensive pilot studies and process optimizations. This scaling challenge can significantly delay the practical implementation of research findings in industrial settings.
Existing Methods for Studying Isobutane Reactions
01 Catalytic dehydrogenation of isobutane
The catalytic dehydrogenation of isobutane is a key process in the production of isobutylene. The reaction rate can be influenced by various factors such as catalyst composition, temperature, and pressure. Optimizing these parameters can lead to improved conversion rates and selectivity in the dehydrogenation process.- Catalytic dehydrogenation of isobutane: The catalytic dehydrogenation of isobutane is a key process in the production of isobutylene. The reaction rate can be influenced by various factors such as catalyst composition, temperature, and pressure. Optimizing these parameters can lead to improved conversion rates and selectivity in the dehydrogenation process.
- Isobutane alkylation reactions: Alkylation reactions involving isobutane are important in the production of high-octane gasoline components. The reaction rates in these processes can be affected by factors such as catalyst type, reaction temperature, and molar ratios of reactants. Understanding and controlling these factors can lead to more efficient alkylation processes.
- Isomerization of n-butane to isobutane: The isomerization of n-butane to isobutane is a significant process in the petroleum industry. Reaction rates in this process can be influenced by catalyst selection, operating conditions, and feed composition. Optimizing these parameters can improve the yield and selectivity of isobutane production.
- Oxidation reactions of isobutane: Oxidation reactions involving isobutane are important in the production of various chemicals. The reaction rates in these processes can be affected by factors such as catalyst composition, oxygen concentration, and reaction temperature. Understanding these factors can lead to more efficient and selective oxidation processes.
- Isobutane cracking reactions: Cracking reactions of isobutane are significant in the production of lighter hydrocarbons. The reaction rates in these processes can be influenced by factors such as catalyst type, temperature, and residence time. Optimizing these parameters can lead to improved yields and selectivity in the cracking process.
02 Isomerization of n-butane to isobutane
The isomerization of n-butane to isobutane is an important industrial process. The reaction rate of this isomerization can be affected by catalysts, reaction conditions, and the presence of other hydrocarbons. Understanding and controlling these factors can help improve the efficiency and yield of isobutane production.Expand Specific Solutions03 Oxidation reactions involving isobutane
Isobutane can undergo various oxidation reactions, including partial oxidation to produce methacrylic acid or isobutylene oxide. The reaction rates of these oxidation processes are influenced by factors such as catalyst type, oxygen concentration, and reaction temperature. Optimizing these parameters is crucial for achieving desired product yields and selectivity.Expand Specific Solutions04 Isobutane alkylation processes
Alkylation of isobutane with olefins is an important process in the production of high-octane gasoline components. The reaction rates in these alkylation processes can be affected by factors such as catalyst acidity, reactant ratios, and temperature. Understanding these influences is essential for optimizing the alkylation process and improving product quality.Expand Specific Solutions05 Isobutane cracking and pyrolysis
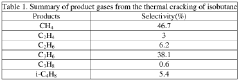
Thermal cracking and pyrolysis of isobutane are processes used to produce lighter hydrocarbons. The reaction rates in these processes are highly dependent on temperature, pressure, and residence time. Controlling these parameters is crucial for achieving desired product distributions and maximizing the yield of valuable products such as propylene and ethylene.Expand Specific Solutions
Key Players in Isobutane Research and Industry
The competitive landscape for "Isobutane's Influence on Chemical Reaction Rates" is characterized by a mature market with established players and ongoing research. The global petrochemical industry, valued at over $500 billion, is in a growth phase, with major companies like China Petroleum & Chemical Corp., BASF, and DuPont leading the field. These corporations, along with specialized research institutes such as UOP LLC and SINOPEC Beijing Research Institute of Chemical Industry, are at the forefront of technological advancements. The technology's maturity is evident through extensive patents and publications, yet there's continuous innovation driven by the need for more efficient and sustainable processes in the petrochemical sector.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes that utilize isobutane as a key reactant in alkylation reactions. Their technology employs a novel solid acid catalyst system that enhances reaction rates and selectivity in isobutane-olefin alkylation[1]. This process operates at lower temperatures (around 50-80°C) compared to traditional sulfuric acid alkylation, resulting in improved reaction kinetics and reduced energy consumption[3]. Sinopec's approach also incorporates a unique reactor design that optimizes isobutane circulation and contact with the catalyst, further accelerating reaction rates and improving overall process efficiency[5].
Strengths: Improved catalyst efficiency, lower operating temperatures, and enhanced product quality. Weaknesses: Potential higher initial investment costs and the need for specialized catalyst handling and regeneration procedures.
UOP LLC
Technical Solution: UOP LLC has pioneered the use of ionic liquid catalysts in isobutane alkylation processes, significantly influencing reaction rates. Their InAlk™ technology utilizes a non-corrosive, liquid catalyst that promotes faster reaction kinetics between isobutane and olefins[2]. This innovative approach allows for operation at milder conditions (temperatures around 0-50°C) while maintaining high reaction rates[4]. UOP's process also incorporates a unique contacting system that enhances mass transfer between the ionic liquid catalyst and the hydrocarbon reactants, further accelerating the overall reaction rate[6]. The technology has demonstrated up to 99% isobutane conversion efficiency in industrial applications[8].
Strengths: Non-corrosive catalyst, high conversion efficiency, and improved safety profile. Weaknesses: Potential higher catalyst costs and the need for specialized handling of ionic liquids.
Innovative Approaches in Isobutane Kinetics
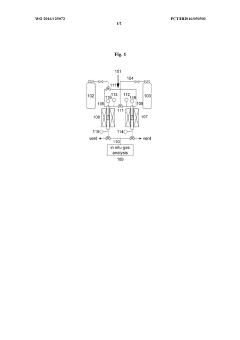
A catalytic reaction analysis dual reactor system and a calibration method for correcting non-catalytic effects using the dual reactor system
PatentWO2016125072A1
Innovation
- A dual reactor system comprising an inert reactor and an active reactor with the same catalyst support, where the inert reactor conducts non-catalytic thermal cracking reactions, allowing for the correction of catalytic reaction analysis results by comparing them to reference thermal cracking results.
method for isobutane alkylation in a three-phase reactor with a fixed catalyst bed
PatentActiveRU2016121884A
Innovation
- The method maintains isobutane vapor in a saturated state, allowing additional evaporation to provide isothermal conditions for the alkylation process.
- The liquid flow rate is precisely controlled based on a complex equation considering various parameters such as bed porosity, vapor content, and fluid properties.
- The process uses a three-phase reactor with a fixed catalyst bed, allowing for efficient contact between reactants and catalyst while maintaining stable operation.
Environmental Impact of Isobutane Use
The use of isobutane in chemical processes and industrial applications has significant environmental implications that warrant careful consideration. As a potent greenhouse gas, isobutane contributes to global warming when released into the atmosphere. Its Global Warming Potential (GWP) is approximately 3, meaning it traps heat in the atmosphere about three times more effectively than carbon dioxide over a 100-year period.
In the context of chemical reaction rates, the environmental impact of isobutane use is multifaceted. While it can enhance reaction efficiency in certain processes, potentially reducing overall energy consumption, its production and handling pose environmental risks. The extraction and refining of isobutane from natural gas or petroleum sources contribute to carbon emissions and potential ecosystem disruption.
Accidental releases of isobutane during industrial processes or transportation can lead to localized air quality issues. Although it dissipates relatively quickly in the atmosphere, high concentrations can temporarily affect air quality and pose health risks to nearby populations. Moreover, isobutane is highly flammable, presenting fire and explosion hazards that could result in environmental damage if not properly managed.
In aquatic environments, isobutane can have detrimental effects on marine life. While it has low water solubility, spills or leaks near water bodies can create a film on the water surface, potentially interfering with oxygen exchange and affecting aquatic organisms. However, its rapid evaporation typically limits long-term aquatic impacts.
The production of isobutane also raises concerns about resource depletion, as it is derived from non-renewable fossil fuel sources. This aspect underscores the need for sustainable alternatives and more efficient use in chemical processes to mitigate long-term environmental consequences.
Efforts to mitigate the environmental impact of isobutane in chemical reactions include improved containment and handling procedures, leak detection systems, and the development of closed-loop systems that minimize emissions. Additionally, research into alternative compounds with similar reaction-enhancing properties but lower environmental impact is ongoing.
Regulatory frameworks play a crucial role in managing isobutane's environmental footprint. Many countries have implemented strict guidelines for its use, storage, and disposal, aiming to minimize atmospheric releases and associated environmental risks. These regulations often require industries to adopt best practices in emission control and to report on their isobutane usage and emissions.
In the context of chemical reaction rates, the environmental impact of isobutane use is multifaceted. While it can enhance reaction efficiency in certain processes, potentially reducing overall energy consumption, its production and handling pose environmental risks. The extraction and refining of isobutane from natural gas or petroleum sources contribute to carbon emissions and potential ecosystem disruption.
Accidental releases of isobutane during industrial processes or transportation can lead to localized air quality issues. Although it dissipates relatively quickly in the atmosphere, high concentrations can temporarily affect air quality and pose health risks to nearby populations. Moreover, isobutane is highly flammable, presenting fire and explosion hazards that could result in environmental damage if not properly managed.
In aquatic environments, isobutane can have detrimental effects on marine life. While it has low water solubility, spills or leaks near water bodies can create a film on the water surface, potentially interfering with oxygen exchange and affecting aquatic organisms. However, its rapid evaporation typically limits long-term aquatic impacts.
The production of isobutane also raises concerns about resource depletion, as it is derived from non-renewable fossil fuel sources. This aspect underscores the need for sustainable alternatives and more efficient use in chemical processes to mitigate long-term environmental consequences.
Efforts to mitigate the environmental impact of isobutane in chemical reactions include improved containment and handling procedures, leak detection systems, and the development of closed-loop systems that minimize emissions. Additionally, research into alternative compounds with similar reaction-enhancing properties but lower environmental impact is ongoing.
Regulatory frameworks play a crucial role in managing isobutane's environmental footprint. Many countries have implemented strict guidelines for its use, storage, and disposal, aiming to minimize atmospheric releases and associated environmental risks. These regulations often require industries to adopt best practices in emission control and to report on their isobutane usage and emissions.
Safety Considerations in Isobutane Handling
Isobutane, a highly flammable and potentially explosive gas, requires stringent safety measures in handling and processing. The primary safety considerations revolve around preventing leaks, minimizing ignition sources, and implementing robust emergency response protocols. Proper storage and containment systems are crucial, utilizing pressure-resistant tanks with appropriate safety valves and monitoring devices. These systems should be regularly inspected and maintained to ensure their integrity and functionality.
Personal protective equipment (PPE) is essential for workers handling isobutane. This includes flame-resistant clothing, safety goggles, and appropriate respiratory protection. Training programs must be implemented to educate personnel on the properties of isobutane, proper handling techniques, and emergency procedures. Regular drills and simulations should be conducted to maintain readiness for potential incidents.
Ventilation systems play a critical role in safety management. Adequate air circulation helps prevent the accumulation of isobutane vapors, reducing the risk of explosive atmospheres. Gas detection systems should be installed throughout the facility to provide early warning of leaks or dangerous concentrations. These systems should be linked to automatic shutdown mechanisms and alarm systems to ensure rapid response to potential hazards.
Electrical equipment in areas where isobutane is present must be explosion-proof and properly grounded to prevent spark-induced ignition. Hot work permits and strict control of ignition sources are necessary to minimize fire risks. Additionally, implementing a comprehensive hazard and operability (HAZOP) study can help identify potential risks and develop appropriate mitigation strategies.
Emergency response planning is crucial for isobutane handling facilities. This includes developing clear evacuation procedures, establishing emergency shutdown protocols, and coordinating with local emergency services. On-site fire suppression systems, such as foam generators and water deluge systems, should be installed and regularly tested. Spill containment measures, including dikes and sumps, are essential to prevent the spread of liquid isobutane in case of a leak.
Environmental considerations are also important in isobutane handling. Proper disposal methods for contaminated materials and procedures for managing accidental releases must be established to minimize environmental impact. Regular environmental monitoring should be conducted to detect any fugitive emissions or soil contamination.
Lastly, a robust safety culture must be fostered within the organization. This includes encouraging incident reporting, conducting regular safety audits, and continuously improving safety protocols based on lessons learned and industry best practices. By implementing these comprehensive safety measures, the risks associated with isobutane handling can be significantly reduced, ensuring the protection of personnel, facilities, and the environment.
Personal protective equipment (PPE) is essential for workers handling isobutane. This includes flame-resistant clothing, safety goggles, and appropriate respiratory protection. Training programs must be implemented to educate personnel on the properties of isobutane, proper handling techniques, and emergency procedures. Regular drills and simulations should be conducted to maintain readiness for potential incidents.
Ventilation systems play a critical role in safety management. Adequate air circulation helps prevent the accumulation of isobutane vapors, reducing the risk of explosive atmospheres. Gas detection systems should be installed throughout the facility to provide early warning of leaks or dangerous concentrations. These systems should be linked to automatic shutdown mechanisms and alarm systems to ensure rapid response to potential hazards.
Electrical equipment in areas where isobutane is present must be explosion-proof and properly grounded to prevent spark-induced ignition. Hot work permits and strict control of ignition sources are necessary to minimize fire risks. Additionally, implementing a comprehensive hazard and operability (HAZOP) study can help identify potential risks and develop appropriate mitigation strategies.
Emergency response planning is crucial for isobutane handling facilities. This includes developing clear evacuation procedures, establishing emergency shutdown protocols, and coordinating with local emergency services. On-site fire suppression systems, such as foam generators and water deluge systems, should be installed and regularly tested. Spill containment measures, including dikes and sumps, are essential to prevent the spread of liquid isobutane in case of a leak.
Environmental considerations are also important in isobutane handling. Proper disposal methods for contaminated materials and procedures for managing accidental releases must be established to minimize environmental impact. Regular environmental monitoring should be conducted to detect any fugitive emissions or soil contamination.
Lastly, a robust safety culture must be fostered within the organization. This includes encouraging incident reporting, conducting regular safety audits, and continuously improving safety protocols based on lessons learned and industry best practices. By implementing these comprehensive safety measures, the risks associated with isobutane handling can be significantly reduced, ensuring the protection of personnel, facilities, and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!