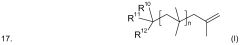How Isobutane Acts as a Catalytic Agent in Polymer Synthesis
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane Catalysis Background and Objectives
Isobutane catalysis in polymer synthesis has emerged as a significant area of research and development in the field of materials science and chemical engineering. The journey of isobutane as a catalytic agent can be traced back to the mid-20th century when scientists began exploring alternative methods for polymer production. This technology has since evolved, driven by the need for more efficient and environmentally friendly synthesis processes.
The primary objective of utilizing isobutane as a catalytic agent is to enhance the efficiency and selectivity of polymer synthesis reactions. By acting as a catalyst, isobutane facilitates the formation of specific polymer structures while minimizing unwanted side reactions. This catalytic approach aims to improve the overall yield of desired polymers, reduce energy consumption, and decrease the production of waste byproducts.
Over the years, the role of isobutane in polymer synthesis has expanded beyond its initial applications. Researchers have discovered its potential in various polymerization processes, including the production of polyolefins, polyurethanes, and other specialty polymers. The versatility of isobutane as a catalytic agent has led to its integration into both academic research and industrial production settings.
One of the key drivers behind the continued interest in isobutane catalysis is the growing demand for sustainable and eco-friendly manufacturing processes. As environmental concerns become increasingly prominent, the chemical industry is under pressure to develop greener alternatives to traditional synthesis methods. Isobutane catalysis offers a promising solution by potentially reducing the use of harmful solvents and minimizing the carbon footprint of polymer production.
The technological evolution of isobutane catalysis has been marked by several significant milestones. These include the development of novel catalyst systems, the optimization of reaction conditions, and the integration of advanced analytical techniques for monitoring and controlling the polymerization process. Each advancement has contributed to a deeper understanding of the catalytic mechanisms involved and has paved the way for more sophisticated applications.
Looking ahead, the future of isobutane catalysis in polymer synthesis is poised for further innovation. Researchers are exploring new catalyst designs, investigating the potential of hybrid catalytic systems, and leveraging computational modeling to predict and optimize reaction outcomes. The ultimate goal is to develop highly efficient, selective, and sustainable polymerization processes that can meet the growing global demand for advanced polymer materials.
The primary objective of utilizing isobutane as a catalytic agent is to enhance the efficiency and selectivity of polymer synthesis reactions. By acting as a catalyst, isobutane facilitates the formation of specific polymer structures while minimizing unwanted side reactions. This catalytic approach aims to improve the overall yield of desired polymers, reduce energy consumption, and decrease the production of waste byproducts.
Over the years, the role of isobutane in polymer synthesis has expanded beyond its initial applications. Researchers have discovered its potential in various polymerization processes, including the production of polyolefins, polyurethanes, and other specialty polymers. The versatility of isobutane as a catalytic agent has led to its integration into both academic research and industrial production settings.
One of the key drivers behind the continued interest in isobutane catalysis is the growing demand for sustainable and eco-friendly manufacturing processes. As environmental concerns become increasingly prominent, the chemical industry is under pressure to develop greener alternatives to traditional synthesis methods. Isobutane catalysis offers a promising solution by potentially reducing the use of harmful solvents and minimizing the carbon footprint of polymer production.
The technological evolution of isobutane catalysis has been marked by several significant milestones. These include the development of novel catalyst systems, the optimization of reaction conditions, and the integration of advanced analytical techniques for monitoring and controlling the polymerization process. Each advancement has contributed to a deeper understanding of the catalytic mechanisms involved and has paved the way for more sophisticated applications.
Looking ahead, the future of isobutane catalysis in polymer synthesis is poised for further innovation. Researchers are exploring new catalyst designs, investigating the potential of hybrid catalytic systems, and leveraging computational modeling to predict and optimize reaction outcomes. The ultimate goal is to develop highly efficient, selective, and sustainable polymerization processes that can meet the growing global demand for advanced polymer materials.
Market Analysis for Isobutane-Catalyzed Polymers
The market for isobutane-catalyzed polymers has shown significant growth in recent years, driven by increasing demand for high-performance plastics across various industries. These polymers, synthesized using isobutane as a catalytic agent, offer unique properties that make them attractive for a wide range of applications.
In the automotive sector, isobutane-catalyzed polymers are gaining traction due to their lightweight nature and excellent mechanical properties. As automakers strive to improve fuel efficiency and reduce emissions, these materials are increasingly being used in vehicle components, contributing to overall weight reduction without compromising structural integrity.
The packaging industry represents another key market for isobutane-catalyzed polymers. Their superior barrier properties and chemical resistance make them ideal for food packaging, extending shelf life and ensuring product safety. The growing emphasis on sustainable packaging solutions has further boosted the demand for these polymers, as they can be engineered to be recyclable or biodegradable.
In the construction sector, isobutane-catalyzed polymers are finding applications in insulation materials, sealants, and structural components. Their durability, weather resistance, and thermal insulation properties make them valuable in improving energy efficiency in buildings.
The electronics industry is also a significant consumer of these polymers. Their excellent electrical insulation properties and heat resistance make them suitable for use in electronic components, circuit boards, and device housings. As the demand for smaller, more powerful electronic devices continues to grow, so does the market for these specialized polymers.
Geographically, North America and Europe currently lead the market for isobutane-catalyzed polymers, owing to their advanced manufacturing capabilities and stringent regulations promoting the use of high-performance materials. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing disposable incomes, and growing awareness of sustainable materials.
Market analysts project a compound annual growth rate (CAGR) for the isobutane-catalyzed polymer market in the mid-single digits over the next five years. This growth is attributed to ongoing research and development efforts to expand the application scope of these polymers and improve their performance characteristics.
However, the market faces challenges such as volatility in raw material prices and increasing environmental concerns regarding the use of petroleum-based products. These factors are driving research into bio-based alternatives and more sustainable production methods, which could reshape the market landscape in the long term.
In the automotive sector, isobutane-catalyzed polymers are gaining traction due to their lightweight nature and excellent mechanical properties. As automakers strive to improve fuel efficiency and reduce emissions, these materials are increasingly being used in vehicle components, contributing to overall weight reduction without compromising structural integrity.
The packaging industry represents another key market for isobutane-catalyzed polymers. Their superior barrier properties and chemical resistance make them ideal for food packaging, extending shelf life and ensuring product safety. The growing emphasis on sustainable packaging solutions has further boosted the demand for these polymers, as they can be engineered to be recyclable or biodegradable.
In the construction sector, isobutane-catalyzed polymers are finding applications in insulation materials, sealants, and structural components. Their durability, weather resistance, and thermal insulation properties make them valuable in improving energy efficiency in buildings.
The electronics industry is also a significant consumer of these polymers. Their excellent electrical insulation properties and heat resistance make them suitable for use in electronic components, circuit boards, and device housings. As the demand for smaller, more powerful electronic devices continues to grow, so does the market for these specialized polymers.
Geographically, North America and Europe currently lead the market for isobutane-catalyzed polymers, owing to their advanced manufacturing capabilities and stringent regulations promoting the use of high-performance materials. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing disposable incomes, and growing awareness of sustainable materials.
Market analysts project a compound annual growth rate (CAGR) for the isobutane-catalyzed polymer market in the mid-single digits over the next five years. This growth is attributed to ongoing research and development efforts to expand the application scope of these polymers and improve their performance characteristics.
However, the market faces challenges such as volatility in raw material prices and increasing environmental concerns regarding the use of petroleum-based products. These factors are driving research into bio-based alternatives and more sustainable production methods, which could reshape the market landscape in the long term.
Current Challenges in Isobutane Catalytic Processes
Despite the widespread use of isobutane as a catalytic agent in polymer synthesis, several challenges persist in its application, hindering optimal performance and efficiency. One of the primary issues is the control of reaction selectivity. Isobutane-catalyzed processes often yield a mixture of products, including unwanted side reactions, which can compromise the purity and quality of the desired polymer. Achieving high selectivity towards the target product remains a significant challenge, particularly in complex polymerization reactions.
Another major hurdle is the stability of isobutane under various reaction conditions. High temperatures and pressures, often required for efficient polymerization, can lead to the degradation of isobutane, reducing its catalytic effectiveness over time. This instability not only affects the reaction yield but also introduces impurities into the final product, necessitating additional purification steps and increasing production costs.
The activation of isobutane for catalytic processes presents another challenge. While isobutane can act as an effective catalyst, its activation often requires harsh conditions or the use of co-catalysts. Finding more efficient and environmentally friendly methods for activating isobutane without compromising its catalytic properties is an ongoing area of research.
Catalyst recovery and recycling pose significant challenges in isobutane-catalyzed polymer synthesis. The separation of the catalyst from the polymer product can be complex and energy-intensive, particularly in homogeneous catalytic systems. Developing effective methods for catalyst recovery and reuse is crucial for improving the economic viability and sustainability of these processes.
The environmental impact of isobutane-based catalytic processes is also a growing concern. As a volatile organic compound, isobutane can contribute to air pollution and greenhouse gas emissions if not properly contained and managed. Addressing these environmental concerns while maintaining process efficiency is a delicate balance that researchers and industry professionals continue to grapple with.
Scalability and process control present additional challenges, especially when transitioning from laboratory-scale experiments to industrial production. Maintaining consistent catalytic performance and product quality at larger scales requires sophisticated engineering solutions and precise control mechanisms, which can be technically challenging and economically demanding.
Lastly, the optimization of reaction kinetics and thermodynamics in isobutane-catalyzed polymerizations remains an ongoing challenge. Understanding and controlling the complex interplay between reaction parameters, such as temperature, pressure, and catalyst concentration, is crucial for maximizing yield and product quality. This requires advanced modeling and experimental techniques to elucidate reaction mechanisms and optimize process conditions.
Another major hurdle is the stability of isobutane under various reaction conditions. High temperatures and pressures, often required for efficient polymerization, can lead to the degradation of isobutane, reducing its catalytic effectiveness over time. This instability not only affects the reaction yield but also introduces impurities into the final product, necessitating additional purification steps and increasing production costs.
The activation of isobutane for catalytic processes presents another challenge. While isobutane can act as an effective catalyst, its activation often requires harsh conditions or the use of co-catalysts. Finding more efficient and environmentally friendly methods for activating isobutane without compromising its catalytic properties is an ongoing area of research.
Catalyst recovery and recycling pose significant challenges in isobutane-catalyzed polymer synthesis. The separation of the catalyst from the polymer product can be complex and energy-intensive, particularly in homogeneous catalytic systems. Developing effective methods for catalyst recovery and reuse is crucial for improving the economic viability and sustainability of these processes.
The environmental impact of isobutane-based catalytic processes is also a growing concern. As a volatile organic compound, isobutane can contribute to air pollution and greenhouse gas emissions if not properly contained and managed. Addressing these environmental concerns while maintaining process efficiency is a delicate balance that researchers and industry professionals continue to grapple with.
Scalability and process control present additional challenges, especially when transitioning from laboratory-scale experiments to industrial production. Maintaining consistent catalytic performance and product quality at larger scales requires sophisticated engineering solutions and precise control mechanisms, which can be technically challenging and economically demanding.
Lastly, the optimization of reaction kinetics and thermodynamics in isobutane-catalyzed polymerizations remains an ongoing challenge. Understanding and controlling the complex interplay between reaction parameters, such as temperature, pressure, and catalyst concentration, is crucial for maximizing yield and product quality. This requires advanced modeling and experimental techniques to elucidate reaction mechanisms and optimize process conditions.
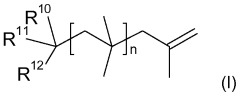
Existing Isobutane Catalytic Mechanisms in Polymer Synthesis
01 Production and purification of isobutane
Various methods for producing and purifying isobutane are described. These include processes for separating isobutane from other hydrocarbons, catalytic conversion of other compounds to isobutane, and techniques for improving the purity of isobutane streams.- Production and purification of isobutane: Various methods for producing and purifying isobutane are described. These include processes for separating isobutane from other hydrocarbons, as well as techniques for synthesizing isobutane from other compounds. The purification methods aim to obtain high-purity isobutane for industrial applications.
- Isobutane as a refrigerant: Isobutane is utilized as an environmentally friendly refrigerant in various cooling systems. Its properties make it suitable for use in refrigerators, air conditioners, and other cooling applications. The use of isobutane as a refrigerant is explored in several patents, detailing its advantages and implementation methods.
- Isobutane in fuel compositions: Isobutane is used as a component in various fuel compositions. It can be blended with other hydrocarbons to improve the performance of fuels for internal combustion engines. Some patents describe methods for incorporating isobutane into fuel mixtures to enhance combustion efficiency and reduce emissions.
- Chemical reactions involving isobutane: Several patents focus on chemical reactions involving isobutane. These include processes for converting isobutane into other valuable chemicals, such as isobutylene or tertiary butyl alcohol. The reactions often involve catalysts and specific reaction conditions to achieve desired products efficiently.
- Isobutane in aerosol propellants: Isobutane is widely used as a propellant in aerosol products. It is often combined with other gases to create propellant mixtures with desired properties. Patents in this category describe formulations and methods for using isobutane in various aerosol applications, including personal care products and industrial sprays.
02 Isobutane as a refrigerant
Isobutane is utilized as an environmentally friendly refrigerant in various cooling systems. Its properties make it suitable for use in refrigeration and air conditioning applications, often as a replacement for traditional refrigerants with higher global warming potential.Expand Specific Solutions03 Isobutane in chemical reactions
Isobutane serves as a key reactant or intermediate in various chemical processes. It is used in the production of other chemicals, including the synthesis of high-octane gasoline components, polymers, and other valuable industrial products.Expand Specific Solutions04 Isobutane in aerosol propellants
Isobutane is commonly used as a propellant in aerosol products. Its properties make it suitable for various applications, including personal care products, household cleaners, and industrial sprays. The use of isobutane as a propellant often involves considerations of safety and environmental impact.Expand Specific Solutions05 Isobutane in fuel applications
Isobutane is utilized in various fuel applications, including as a component in liquefied petroleum gas (LPG) mixtures and as a feedstock for the production of high-octane gasoline components. Its properties contribute to improved fuel performance and efficiency in certain applications.Expand Specific Solutions
Key Industry Players in Isobutane-Based Polymer Production
The competitive landscape for isobutane as a catalytic agent in polymer synthesis is characterized by a mature market with established players and ongoing research. The global market for catalysts in polymer production is substantial, estimated to be in the billions of dollars annually. Major chemical companies like BASF, ExxonMobil, and Sinopec are key players, leveraging their extensive R&D capabilities and production facilities. The technology is well-developed, with companies like LyondellBasell and SABIC offering specialized catalysts. Academic institutions such as Beijing University of Chemical Technology and research organizations like IFP Energies Nouvelles contribute to advancing the field. Emerging players from Asia, including Wanhua Chemical and LG Chem, are increasingly active, reflecting the shifting global manufacturing landscape.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to using isobutane as a catalytic agent in polymer synthesis. Their process involves a gas-phase polymerization reactor where isobutane acts as both a diluent and a chain transfer agent[1]. This method allows for precise control of polymer molecular weight and enhances the overall efficiency of the polymerization process. BASF's technology utilizes a specialized catalyst system that interacts synergistically with isobutane, promoting rapid and uniform polymer growth[3]. The company has also implemented advanced process control systems to optimize the isobutane concentration and reaction conditions, resulting in improved product quality and reduced energy consumption[5].
Strengths: Precise molecular weight control, improved energy efficiency, and enhanced product quality. Weaknesses: May require specialized equipment and careful process control, potentially increasing operational complexity.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel isobutane-assisted catalytic system for polymer synthesis. Their approach involves using isobutane as a co-catalyst in conjunction with traditional metallocenes or Ziegler-Natta catalysts[2]. This innovative method enhances catalyst activity and allows for better control of polymer properties. Sinopec's technology incorporates a proprietary reactor design that optimizes the distribution and interaction of isobutane with the catalyst and monomer[4]. The company has also implemented advanced online monitoring systems to maintain optimal isobutane concentrations throughout the polymerization process, ensuring consistent product quality[6].
Strengths: Enhanced catalyst activity, improved polymer property control, and consistent product quality. Weaknesses: May require significant modifications to existing production facilities and careful handling of isobutane.
Innovative Approaches in Isobutane Catalysis Research
Method for producing highly reactive polyisobutenes
PatentInactiveEP1362063A1
Innovation
- A process involving cationic polymerization of isobutene using boron trifluoride and a secondary alcohol, where the catalyst is deactivated by adding a first portion of water at the reaction temperature, followed by a second portion at a higher temperature to prevent isomerization, ensuring the polymerization is terminated efficiently.
Process for preparing high-reactivity isobutene homo- or copolymers
PatentWO2013021058A1
Innovation
- A process involving polymerization of isobutene using a catalyst system comprising a Lewis acid and a donor, with an organic sulfonic acid as the initiator, specifically using a complex of methanesulfonic acid or other sulfonic acids with Lewis acids like aluminum trichloride, to achieve high terminal vinylidene double bond content while maintaining a narrow molecular weight distribution.
Environmental Impact of Isobutane Catalysis in Polymer Industry
The use of isobutane as a catalytic agent in polymer synthesis has significant environmental implications for the polymer industry. While isobutane catalysis offers several advantages in terms of efficiency and product quality, its environmental impact must be carefully considered and managed.
One of the primary environmental concerns associated with isobutane catalysis is its contribution to air pollution. Isobutane is a volatile organic compound (VOC) that can easily escape into the atmosphere during the manufacturing process. When released, it can react with other pollutants in the presence of sunlight to form ground-level ozone, a major component of smog. This can lead to respiratory issues and other health problems in affected areas.
Furthermore, isobutane is a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide. Any leaks or emissions of isobutane during the polymer synthesis process can contribute to climate change. The polymer industry must implement strict emission control measures and leak detection systems to minimize these environmental impacts.
Water pollution is another concern related to isobutane catalysis in polymer production. Wastewater from the manufacturing process may contain traces of isobutane or its byproducts. If not properly treated, this can contaminate local water sources, potentially harming aquatic ecosystems and human health. Advanced wastewater treatment technologies are essential to mitigate this risk.
The production and transportation of isobutane itself also have environmental implications. The extraction and refining processes for isobutane, typically derived from natural gas or petroleum, can result in habitat disruption, energy consumption, and additional greenhouse gas emissions. The polymer industry must consider the entire life cycle of isobutane when assessing its environmental footprint.
On the positive side, isobutane catalysis can lead to more efficient polymer production processes, potentially reducing overall energy consumption and waste generation. This improved efficiency may partially offset some of the negative environmental impacts associated with its use. Additionally, the enhanced properties of polymers produced using isobutane catalysis may lead to more durable and longer-lasting products, potentially reducing the need for frequent replacements and associated waste.
To address these environmental challenges, the polymer industry is increasingly focusing on developing greener alternatives and improving existing processes. This includes research into bio-based catalysts, closed-loop systems to minimize isobutane emissions, and more efficient recycling technologies for polymers produced using isobutane catalysis. Regulatory bodies are also implementing stricter environmental standards, pushing the industry towards more sustainable practices.
In conclusion, while isobutane catalysis offers significant benefits in polymer synthesis, its environmental impact is a critical consideration for the industry. Balancing the advantages of this technology with its potential environmental risks requires ongoing research, innovation, and responsible management practices throughout the polymer production value chain.
One of the primary environmental concerns associated with isobutane catalysis is its contribution to air pollution. Isobutane is a volatile organic compound (VOC) that can easily escape into the atmosphere during the manufacturing process. When released, it can react with other pollutants in the presence of sunlight to form ground-level ozone, a major component of smog. This can lead to respiratory issues and other health problems in affected areas.
Furthermore, isobutane is a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide. Any leaks or emissions of isobutane during the polymer synthesis process can contribute to climate change. The polymer industry must implement strict emission control measures and leak detection systems to minimize these environmental impacts.
Water pollution is another concern related to isobutane catalysis in polymer production. Wastewater from the manufacturing process may contain traces of isobutane or its byproducts. If not properly treated, this can contaminate local water sources, potentially harming aquatic ecosystems and human health. Advanced wastewater treatment technologies are essential to mitigate this risk.
The production and transportation of isobutane itself also have environmental implications. The extraction and refining processes for isobutane, typically derived from natural gas or petroleum, can result in habitat disruption, energy consumption, and additional greenhouse gas emissions. The polymer industry must consider the entire life cycle of isobutane when assessing its environmental footprint.
On the positive side, isobutane catalysis can lead to more efficient polymer production processes, potentially reducing overall energy consumption and waste generation. This improved efficiency may partially offset some of the negative environmental impacts associated with its use. Additionally, the enhanced properties of polymers produced using isobutane catalysis may lead to more durable and longer-lasting products, potentially reducing the need for frequent replacements and associated waste.
To address these environmental challenges, the polymer industry is increasingly focusing on developing greener alternatives and improving existing processes. This includes research into bio-based catalysts, closed-loop systems to minimize isobutane emissions, and more efficient recycling technologies for polymers produced using isobutane catalysis. Regulatory bodies are also implementing stricter environmental standards, pushing the industry towards more sustainable practices.
In conclusion, while isobutane catalysis offers significant benefits in polymer synthesis, its environmental impact is a critical consideration for the industry. Balancing the advantages of this technology with its potential environmental risks requires ongoing research, innovation, and responsible management practices throughout the polymer production value chain.
Regulatory Framework for Isobutane Use in Chemical Processes
The regulatory framework for isobutane use in chemical processes, particularly in polymer synthesis, is a complex and evolving landscape. Governments and international bodies have established stringent guidelines to ensure the safe handling, storage, and utilization of isobutane in industrial settings. These regulations primarily focus on environmental protection, worker safety, and product quality assurance.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating isobutane use under the Clean Air Act. The EPA has classified isobutane as a volatile organic compound (VOC) and has set specific emission standards for its industrial application. Additionally, the Occupational Safety and Health Administration (OSHA) has established workplace exposure limits and safety protocols for handling isobutane in chemical processes.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which requires manufacturers and importers to register isobutane and provide detailed safety information. The European Chemicals Agency (ECHA) oversees this process and ensures compliance with REACH requirements. Furthermore, the EU's Seveso III Directive addresses the prevention and control of major accidents involving dangerous substances, including isobutane.
In Asia, countries like China and Japan have also developed comprehensive regulatory frameworks. China's Ministry of Ecology and Environment has issued guidelines for the management of volatile organic compounds, including isobutane, in industrial processes. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of chemical substances, including isobutane, to prevent environmental pollution.
International standards, such as those set by the International Organization for Standardization (ISO), provide guidelines for the safe handling and storage of isobutane in chemical processes. These standards are often adopted or referenced by national regulatory bodies to ensure consistency in safety practices across borders.
Specific to polymer synthesis, regulatory bodies have established guidelines for the use of isobutane as a catalytic agent. These regulations often address issues such as residual catalyst levels in final products, emission control during the synthesis process, and proper disposal of waste materials containing isobutane.
As environmental concerns continue to grow, regulatory frameworks are evolving to address the potential impact of isobutane on climate change. Many countries are implementing stricter controls on greenhouse gas emissions, which may affect the use of isobutane in industrial processes. This trend is likely to drive innovation in catalyst technologies and process optimization to minimize environmental impact while maintaining production efficiency.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating isobutane use under the Clean Air Act. The EPA has classified isobutane as a volatile organic compound (VOC) and has set specific emission standards for its industrial application. Additionally, the Occupational Safety and Health Administration (OSHA) has established workplace exposure limits and safety protocols for handling isobutane in chemical processes.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which requires manufacturers and importers to register isobutane and provide detailed safety information. The European Chemicals Agency (ECHA) oversees this process and ensures compliance with REACH requirements. Furthermore, the EU's Seveso III Directive addresses the prevention and control of major accidents involving dangerous substances, including isobutane.
In Asia, countries like China and Japan have also developed comprehensive regulatory frameworks. China's Ministry of Ecology and Environment has issued guidelines for the management of volatile organic compounds, including isobutane, in industrial processes. Japan's Chemical Substances Control Law (CSCL) regulates the manufacture, import, and use of chemical substances, including isobutane, to prevent environmental pollution.
International standards, such as those set by the International Organization for Standardization (ISO), provide guidelines for the safe handling and storage of isobutane in chemical processes. These standards are often adopted or referenced by national regulatory bodies to ensure consistency in safety practices across borders.
Specific to polymer synthesis, regulatory bodies have established guidelines for the use of isobutane as a catalytic agent. These regulations often address issues such as residual catalyst levels in final products, emission control during the synthesis process, and proper disposal of waste materials containing isobutane.
As environmental concerns continue to grow, regulatory frameworks are evolving to address the potential impact of isobutane on climate change. Many countries are implementing stricter controls on greenhouse gas emissions, which may affect the use of isobutane in industrial processes. This trend is likely to drive innovation in catalyst technologies and process optimization to minimize environmental impact while maintaining production efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!