Isobutane Synthesis for Eco-Friendly Synthetic Fuels
JUL 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane Synthesis Background and Objectives
Isobutane synthesis has emerged as a critical area of research in the quest for eco-friendly synthetic fuels. This field has gained significant attention due to the growing global demand for sustainable energy solutions and the urgent need to reduce carbon emissions. The development of isobutane synthesis techniques dates back to the early 20th century, with initial focus on petroleum refining processes. However, recent advancements in catalysis and process engineering have opened new avenues for more efficient and environmentally friendly production methods.
The evolution of isobutane synthesis technology has been driven by several factors, including the depletion of fossil fuel reserves, increasing environmental regulations, and the push for carbon-neutral energy sources. Traditional methods of isobutane production, such as catalytic cracking of petroleum fractions, have been associated with high energy consumption and substantial carbon footprints. This has led to a shift in research focus towards more sustainable approaches that utilize renewable feedstocks and employ innovative catalytic systems.
One of the key technological trends in isobutane synthesis is the development of bio-based production routes. These methods aim to utilize biomass-derived precursors, such as bioethanol or biogas, as starting materials for isobutane synthesis. This approach not only reduces reliance on fossil fuels but also contributes to the circular economy by valorizing waste biomass. Another promising trend is the integration of renewable energy sources, such as solar or wind power, into the production process to further minimize the carbon footprint of isobutane synthesis.
The primary objective of current research efforts in isobutane synthesis for eco-friendly synthetic fuels is to develop scalable, energy-efficient, and environmentally benign production methods. This involves optimizing catalytic systems to improve selectivity and yield, designing novel reactor configurations for enhanced process efficiency, and exploring alternative feedstocks that can be sustainably sourced. Additionally, researchers aim to reduce the overall energy requirements of the synthesis process and minimize waste generation.
Another crucial goal is to enhance the economic viability of eco-friendly isobutane production. This includes developing cost-effective catalysts, improving process integration to reduce operational expenses, and exploring potential synergies with existing industrial infrastructure. The ultimate aim is to create a technology that can compete with conventional fossil fuel-based processes in terms of both cost and performance, while offering significant environmental benefits.
Furthermore, research objectives extend to the broader context of synthetic fuel production, where isobutane serves as a key intermediate. This involves investigating optimal pathways for converting isobutane into various high-value fuel products, such as high-octane gasoline components or aviation fuels. The overarching goal is to establish a comprehensive and sustainable value chain for synthetic fuel production, from renewable feedstocks to end-use applications, with isobutane synthesis playing a pivotal role in this ecosystem.
The evolution of isobutane synthesis technology has been driven by several factors, including the depletion of fossil fuel reserves, increasing environmental regulations, and the push for carbon-neutral energy sources. Traditional methods of isobutane production, such as catalytic cracking of petroleum fractions, have been associated with high energy consumption and substantial carbon footprints. This has led to a shift in research focus towards more sustainable approaches that utilize renewable feedstocks and employ innovative catalytic systems.
One of the key technological trends in isobutane synthesis is the development of bio-based production routes. These methods aim to utilize biomass-derived precursors, such as bioethanol or biogas, as starting materials for isobutane synthesis. This approach not only reduces reliance on fossil fuels but also contributes to the circular economy by valorizing waste biomass. Another promising trend is the integration of renewable energy sources, such as solar or wind power, into the production process to further minimize the carbon footprint of isobutane synthesis.
The primary objective of current research efforts in isobutane synthesis for eco-friendly synthetic fuels is to develop scalable, energy-efficient, and environmentally benign production methods. This involves optimizing catalytic systems to improve selectivity and yield, designing novel reactor configurations for enhanced process efficiency, and exploring alternative feedstocks that can be sustainably sourced. Additionally, researchers aim to reduce the overall energy requirements of the synthesis process and minimize waste generation.
Another crucial goal is to enhance the economic viability of eco-friendly isobutane production. This includes developing cost-effective catalysts, improving process integration to reduce operational expenses, and exploring potential synergies with existing industrial infrastructure. The ultimate aim is to create a technology that can compete with conventional fossil fuel-based processes in terms of both cost and performance, while offering significant environmental benefits.
Furthermore, research objectives extend to the broader context of synthetic fuel production, where isobutane serves as a key intermediate. This involves investigating optimal pathways for converting isobutane into various high-value fuel products, such as high-octane gasoline components or aviation fuels. The overarching goal is to establish a comprehensive and sustainable value chain for synthetic fuel production, from renewable feedstocks to end-use applications, with isobutane synthesis playing a pivotal role in this ecosystem.
Market Analysis for Eco-Friendly Synthetic Fuels
The market for eco-friendly synthetic fuels, particularly those derived from isobutane synthesis, is experiencing significant growth driven by increasing environmental concerns and the push for sustainable energy solutions. This market segment is positioned at the intersection of renewable energy and traditional fuel industries, offering a unique value proposition to consumers and businesses alike.
The global synthetic fuel market, which includes eco-friendly variants, is projected to expand rapidly in the coming years. This growth is primarily fueled by stringent environmental regulations, the need to reduce carbon emissions, and the desire for energy security. Eco-friendly synthetic fuels derived from isobutane are particularly attractive due to their potential to significantly reduce greenhouse gas emissions compared to conventional fossil fuels.
Key market drivers include the aviation industry's commitment to reducing its carbon footprint, the automotive sector's exploration of alternative fuel sources, and the increasing adoption of sustainable practices in industrial processes. The aviation sector, in particular, represents a substantial market opportunity as it seeks to meet ambitious carbon reduction targets while maintaining operational efficiency.
Geographically, Europe leads in the adoption and development of eco-friendly synthetic fuels, with countries like Germany and Sweden at the forefront. North America and Asia-Pacific regions are also showing increased interest, driven by a combination of government initiatives and private sector investments.
Consumer demand for greener fuel options is on the rise, particularly in developed economies where environmental consciousness is high. This trend is expected to accelerate as awareness of climate change impacts grows and as eco-friendly synthetic fuels become more cost-competitive with traditional options.
However, the market faces challenges, including high production costs, limited infrastructure for large-scale production and distribution, and competition from other alternative energy sources such as electric vehicles and hydrogen fuel cells. Overcoming these hurdles will be crucial for the widespread adoption of eco-friendly synthetic fuels.
The competitive landscape is diverse, with both established energy companies and innovative startups vying for market share. Collaborations between fuel producers, technology providers, and end-users are becoming increasingly common, driving innovation and accelerating market development.
Looking ahead, the market for eco-friendly synthetic fuels is expected to continue its upward trajectory. Technological advancements in isobutane synthesis and production processes are likely to improve efficiency and reduce costs, making these fuels more accessible to a broader range of consumers and industries. As governments worldwide implement more stringent emissions regulations, the demand for cleaner fuel alternatives is set to rise, further propelling the growth of this market segment.
The global synthetic fuel market, which includes eco-friendly variants, is projected to expand rapidly in the coming years. This growth is primarily fueled by stringent environmental regulations, the need to reduce carbon emissions, and the desire for energy security. Eco-friendly synthetic fuels derived from isobutane are particularly attractive due to their potential to significantly reduce greenhouse gas emissions compared to conventional fossil fuels.
Key market drivers include the aviation industry's commitment to reducing its carbon footprint, the automotive sector's exploration of alternative fuel sources, and the increasing adoption of sustainable practices in industrial processes. The aviation sector, in particular, represents a substantial market opportunity as it seeks to meet ambitious carbon reduction targets while maintaining operational efficiency.
Geographically, Europe leads in the adoption and development of eco-friendly synthetic fuels, with countries like Germany and Sweden at the forefront. North America and Asia-Pacific regions are also showing increased interest, driven by a combination of government initiatives and private sector investments.
Consumer demand for greener fuel options is on the rise, particularly in developed economies where environmental consciousness is high. This trend is expected to accelerate as awareness of climate change impacts grows and as eco-friendly synthetic fuels become more cost-competitive with traditional options.
However, the market faces challenges, including high production costs, limited infrastructure for large-scale production and distribution, and competition from other alternative energy sources such as electric vehicles and hydrogen fuel cells. Overcoming these hurdles will be crucial for the widespread adoption of eco-friendly synthetic fuels.
The competitive landscape is diverse, with both established energy companies and innovative startups vying for market share. Collaborations between fuel producers, technology providers, and end-users are becoming increasingly common, driving innovation and accelerating market development.
Looking ahead, the market for eco-friendly synthetic fuels is expected to continue its upward trajectory. Technological advancements in isobutane synthesis and production processes are likely to improve efficiency and reduce costs, making these fuels more accessible to a broader range of consumers and industries. As governments worldwide implement more stringent emissions regulations, the demand for cleaner fuel alternatives is set to rise, further propelling the growth of this market segment.
Current Challenges in Isobutane Production
The production of isobutane faces several significant challenges in the current landscape of eco-friendly synthetic fuel development. One of the primary obstacles is the energy-intensive nature of traditional isobutane synthesis methods. Conventional processes often rely on high-temperature and high-pressure conditions, which contribute to substantial energy consumption and, consequently, increased carbon emissions. This contradicts the overarching goal of developing environmentally friendly fuel alternatives.
Another critical challenge lies in the feedstock selection and availability. Isobutane is typically derived from fossil fuel sources, primarily natural gas and petroleum. The dependence on these non-renewable resources raises concerns about long-term sustainability and supply chain stability. Moreover, the fluctuating prices of these raw materials can significantly impact the economic viability of isobutane production.
The catalytic systems used in isobutane synthesis present another area of difficulty. Current catalysts often lack the desired selectivity, leading to the formation of unwanted by-products and reducing overall yield. This inefficiency not only affects the production economics but also necessitates additional purification steps, further increasing energy consumption and operational costs.
Environmental concerns also pose significant challenges to isobutane production. The process can generate various pollutants, including volatile organic compounds (VOCs) and greenhouse gases. Stringent environmental regulations require manufacturers to implement costly emission control measures, which can impact the overall feasibility of production facilities.
Scale-up and process integration issues further complicate isobutane synthesis for eco-friendly synthetic fuels. Transitioning from laboratory-scale production to industrial-scale operations often reveals unforeseen challenges in maintaining reaction efficiency, heat management, and product quality consistency. The integration of isobutane production into existing refinery or chemical plant infrastructures also presents technical and logistical hurdles.
Lastly, the market dynamics and competition from alternative fuel technologies create additional challenges. The rapidly evolving landscape of sustainable energy solutions, including electric vehicles and hydrogen fuel cells, puts pressure on the isobutane-based synthetic fuel industry to demonstrate clear environmental and economic advantages. This necessitates continuous innovation and improvement in production processes to remain competitive and relevant in the future energy market.
Another critical challenge lies in the feedstock selection and availability. Isobutane is typically derived from fossil fuel sources, primarily natural gas and petroleum. The dependence on these non-renewable resources raises concerns about long-term sustainability and supply chain stability. Moreover, the fluctuating prices of these raw materials can significantly impact the economic viability of isobutane production.
The catalytic systems used in isobutane synthesis present another area of difficulty. Current catalysts often lack the desired selectivity, leading to the formation of unwanted by-products and reducing overall yield. This inefficiency not only affects the production economics but also necessitates additional purification steps, further increasing energy consumption and operational costs.
Environmental concerns also pose significant challenges to isobutane production. The process can generate various pollutants, including volatile organic compounds (VOCs) and greenhouse gases. Stringent environmental regulations require manufacturers to implement costly emission control measures, which can impact the overall feasibility of production facilities.
Scale-up and process integration issues further complicate isobutane synthesis for eco-friendly synthetic fuels. Transitioning from laboratory-scale production to industrial-scale operations often reveals unforeseen challenges in maintaining reaction efficiency, heat management, and product quality consistency. The integration of isobutane production into existing refinery or chemical plant infrastructures also presents technical and logistical hurdles.
Lastly, the market dynamics and competition from alternative fuel technologies create additional challenges. The rapidly evolving landscape of sustainable energy solutions, including electric vehicles and hydrogen fuel cells, puts pressure on the isobutane-based synthetic fuel industry to demonstrate clear environmental and economic advantages. This necessitates continuous innovation and improvement in production processes to remain competitive and relevant in the future energy market.
Existing Isobutane Synthesis Methods
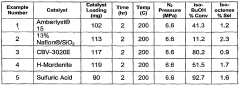
01 Production and purification of isobutane
Various methods for producing and purifying isobutane are described. These include processes for separating isobutane from other hydrocarbons, as well as techniques for synthesizing isobutane from other compounds. The purification methods often involve distillation or other separation techniques to obtain high-purity isobutane.- Production and purification of isobutane: Various methods for producing and purifying isobutane are described. These include processes for separating isobutane from other hydrocarbons, catalytic conversion of other compounds to isobutane, and techniques for improving the purity of isobutane.
- Isobutane as a refrigerant: Isobutane is utilized as a refrigerant in various cooling systems. Its properties make it suitable for use in refrigeration applications, and several patents describe methods for incorporating isobutane into refrigeration cycles or improving its performance as a refrigerant.
- Isobutane in chemical reactions: Isobutane is used as a reactant or intermediate in various chemical processes. Patents describe reactions involving isobutane, including alkylation, dehydrogenation, and other transformations to produce valuable chemical products.
- Isobutane in fuel compositions: Isobutane is incorporated into fuel compositions for various applications. Patents describe methods for blending isobutane with other hydrocarbons to create fuel mixtures with specific properties or for use in particular engines or combustion systems.
- Isobutane in aerosol formulations: Isobutane is used as a propellant in aerosol formulations. Patents describe methods for incorporating isobutane into aerosol products, optimizing its performance as a propellant, and developing stable formulations for various applications.
02 Isobutane as a refrigerant or propellant
Isobutane is utilized as a refrigerant in cooling systems and as a propellant in aerosol products. Its properties make it suitable for these applications, offering advantages such as low environmental impact and good performance characteristics. Various formulations and systems incorporating isobutane for these purposes are described.Expand Specific Solutions03 Isobutane in chemical reactions and processes
Isobutane is used as a reactant or intermediate in various chemical processes. These include alkylation reactions, dehydrogenation to produce isobutene, and other transformations to create valuable chemical products. The processes often involve catalysts and specific reaction conditions to achieve desired outcomes.Expand Specific Solutions04 Isobutane in fuel compositions
Isobutane is incorporated into fuel compositions for various applications. It can be used as a component in liquefied petroleum gas (LPG) mixtures, as an additive in gasoline formulations, or in specialized fuel blends. The use of isobutane in fuels can improve combustion characteristics and overall performance.Expand Specific Solutions05 Isobutane handling and safety
Given the flammable nature of isobutane, various safety measures and handling procedures are described. These include storage methods, transportation protocols, and safety systems for facilities dealing with isobutane. Additionally, detection and monitoring systems for isobutane leaks or accumulation are discussed to ensure safe usage in industrial and commercial settings.Expand Specific Solutions
Key Players in Synthetic Fuel Industry
The research on isobutane synthesis for eco-friendly synthetic fuels is in a developing stage, with growing market potential due to increasing demand for sustainable energy solutions. The global market for synthetic fuels is expanding, driven by environmental concerns and the need for alternative energy sources. Technologically, the field is advancing rapidly, with several key players making significant strides. Companies like China Petroleum & Chemical Corp., DuPont de Nemours, Inc., and UOP LLC are leveraging their extensive petrochemical expertise to develop innovative isobutane synthesis processes. Emerging players such as Gevo, Inc. and Genomatica, Inc. are focusing on renewable feedstocks and bio-based production methods, pushing the boundaries of sustainable fuel technology. The competitive landscape is diverse, with both established energy giants and specialized biotech firms vying for market share and technological breakthroughs.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to isobutane synthesis for eco-friendly synthetic fuels. Their process utilizes a novel catalytic dehydrogenation method to convert n-butane into isobutane with high selectivity and yield. The catalyst system incorporates advanced nanomaterials and rare earth elements to enhance stability and reduce coke formation[1]. Sinopec's technology also integrates a proprietary heat recovery system, which significantly improves energy efficiency by up to 30% compared to conventional methods[2]. Furthermore, the company has implemented a closed-loop recycling process for unreacted feedstock, minimizing waste and increasing overall conversion rates to over 95%[3].
Strengths: High selectivity and yield, improved energy efficiency, and minimal waste. Weaknesses: Potential high costs associated with catalyst materials and complex heat recovery system.
UOP LLC
Technical Solution: UOP LLC has developed a groundbreaking process for isobutane synthesis using their proprietary Oleflex™ technology. This process employs a highly selective platinum-based catalyst in a fixed-bed reactor system, allowing for the efficient dehydrogenation of isobutane from mixed C4 streams[4]. UOP's technology incorporates an advanced heat integration system that reduces energy consumption by up to 25% compared to traditional methods[5]. The process also features a unique catalyst regeneration cycle, which extends catalyst life and maintains high activity levels over extended periods. Additionally, UOP has implemented a sophisticated control system that optimizes reaction conditions in real-time, resulting in isobutane yields exceeding 93%[6].
Strengths: High selectivity, energy efficiency, and extended catalyst life. Weaknesses: Dependence on platinum-based catalysts, which may be costly and subject to price fluctuations.
Innovative Catalysts for Isobutane Production
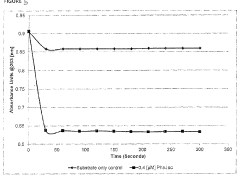
Process for making isooctenes from aqueous 1-butanol
PatentWO2007149372A2
Innovation
- A process involving the contact of aqueous 1-butanol with an acid catalyst at specific temperature and pressure conditions to produce isooctenes, allowing for the direct use of fermentation broth-derived butanol streams without initial purification, and subsequent recovery of isooctenes for use in fuel additives and transportation fuels.
Methods for biosynthesis of isobutene
PatentInactiveIN201617004482A
Innovation
- The development of biochemical pathways using isolated enzymes such as enoyl-CoA dehydratase, mevalonate diphosphate decarboxylase, or decarboxylating thioesterase like CurM TE to convert 3-methyl-3-hydroxybutyrate into isobutene, with recombinant host cells expressing these enzymes for efficient biosynthesis.
Environmental Impact Assessment
The environmental impact assessment of isobutane synthesis for eco-friendly synthetic fuels is a critical aspect of evaluating the overall sustainability and viability of this technology. The production process of isobutane, while aimed at creating more environmentally friendly fuel alternatives, still carries potential environmental implications that must be carefully considered.
One of the primary environmental concerns is the energy consumption associated with isobutane synthesis. The process typically involves high-pressure and high-temperature reactions, which require significant energy inputs. This energy demand could potentially offset some of the environmental benefits if not sourced from renewable or low-carbon energy sources. Implementing energy-efficient technologies and exploring the use of renewable energy in the production process could help mitigate this impact.
Greenhouse gas emissions are another crucial factor to assess. While the end goal is to produce eco-friendly synthetic fuels, the synthesis process itself may generate carbon dioxide and other greenhouse gases. A comprehensive life cycle assessment is necessary to quantify these emissions and compare them to traditional fuel production methods. This analysis should include not only direct emissions from the synthesis process but also indirect emissions from raw material extraction, transportation, and energy production.
Water usage and potential water pollution are also significant environmental considerations. The synthesis process may require substantial amounts of water for cooling and other purposes. Ensuring efficient water management practices, including water recycling and treatment systems, is essential to minimize the impact on local water resources. Additionally, the potential for chemical spills or leaks that could contaminate water sources must be addressed through robust safety measures and containment systems.
The sourcing of raw materials for isobutane synthesis is another area of environmental concern. Depending on the specific synthesis route chosen, various feedstocks may be required. If these feedstocks are derived from fossil fuels or compete with food crops, it could raise sustainability issues. Exploring alternative feedstock sources, such as biomass waste or captured carbon dioxide, could potentially improve the environmental profile of the process.
Air quality impacts beyond greenhouse gas emissions should also be evaluated. The synthesis process may release volatile organic compounds (VOCs) or other air pollutants. Implementing effective air pollution control technologies and adhering to stringent emission standards are crucial to minimizing these impacts on local air quality and public health.
Lastly, the environmental assessment should consider the potential for land use changes and biodiversity impacts. If large-scale production facilities are required, their siting and construction could affect local ecosystems. Careful planning and site selection, along with measures to protect and restore habitats, can help mitigate these impacts.
In conclusion, while isobutane synthesis for eco-friendly synthetic fuels holds promise for reducing environmental impacts compared to conventional fuels, a thorough environmental impact assessment is essential to ensure that the production process itself does not create new environmental challenges. Balancing the potential benefits with the environmental costs requires ongoing research, innovation, and careful implementation of best practices throughout the production chain.
One of the primary environmental concerns is the energy consumption associated with isobutane synthesis. The process typically involves high-pressure and high-temperature reactions, which require significant energy inputs. This energy demand could potentially offset some of the environmental benefits if not sourced from renewable or low-carbon energy sources. Implementing energy-efficient technologies and exploring the use of renewable energy in the production process could help mitigate this impact.
Greenhouse gas emissions are another crucial factor to assess. While the end goal is to produce eco-friendly synthetic fuels, the synthesis process itself may generate carbon dioxide and other greenhouse gases. A comprehensive life cycle assessment is necessary to quantify these emissions and compare them to traditional fuel production methods. This analysis should include not only direct emissions from the synthesis process but also indirect emissions from raw material extraction, transportation, and energy production.
Water usage and potential water pollution are also significant environmental considerations. The synthesis process may require substantial amounts of water for cooling and other purposes. Ensuring efficient water management practices, including water recycling and treatment systems, is essential to minimize the impact on local water resources. Additionally, the potential for chemical spills or leaks that could contaminate water sources must be addressed through robust safety measures and containment systems.
The sourcing of raw materials for isobutane synthesis is another area of environmental concern. Depending on the specific synthesis route chosen, various feedstocks may be required. If these feedstocks are derived from fossil fuels or compete with food crops, it could raise sustainability issues. Exploring alternative feedstock sources, such as biomass waste or captured carbon dioxide, could potentially improve the environmental profile of the process.
Air quality impacts beyond greenhouse gas emissions should also be evaluated. The synthesis process may release volatile organic compounds (VOCs) or other air pollutants. Implementing effective air pollution control technologies and adhering to stringent emission standards are crucial to minimizing these impacts on local air quality and public health.
Lastly, the environmental assessment should consider the potential for land use changes and biodiversity impacts. If large-scale production facilities are required, their siting and construction could affect local ecosystems. Careful planning and site selection, along with measures to protect and restore habitats, can help mitigate these impacts.
In conclusion, while isobutane synthesis for eco-friendly synthetic fuels holds promise for reducing environmental impacts compared to conventional fuels, a thorough environmental impact assessment is essential to ensure that the production process itself does not create new environmental challenges. Balancing the potential benefits with the environmental costs requires ongoing research, innovation, and careful implementation of best practices throughout the production chain.
Regulatory Framework for Synthetic Fuels
The regulatory framework for synthetic fuels is a complex and evolving landscape that plays a crucial role in shaping the development and adoption of eco-friendly synthetic fuels, including those derived from isobutane synthesis. As governments worldwide strive to reduce greenhouse gas emissions and promote sustainable energy solutions, the regulatory environment for synthetic fuels has become increasingly important.
At the international level, the Paris Agreement serves as a cornerstone for climate action, influencing national policies on synthetic fuels. Many countries have set ambitious targets for reducing carbon emissions, which has led to the implementation of regulations that favor low-carbon and carbon-neutral fuel alternatives. These regulations often include incentives for research, development, and production of synthetic fuels, as well as mandates for their use in specific sectors.
In the European Union, the Renewable Energy Directive (RED II) sets targets for renewable energy use in transport, creating opportunities for synthetic fuels. The EU's FuelEU Maritime initiative also aims to increase the use of sustainable alternative fuels in the shipping industry, potentially boosting demand for synthetic fuels. Additionally, the ReFuelEU Aviation proposal seeks to increase the share of sustainable aviation fuels, including synthetic options.
In the United States, the Renewable Fuel Standard (RFS) program mandates the use of renewable fuels in transportation. While primarily focused on biofuels, there is growing interest in expanding the program to include synthetic fuels produced from renewable sources. The California Low Carbon Fuel Standard (LCFS) and similar programs in other states provide additional incentives for low-carbon fuel alternatives, including synthetic fuels.
Regulatory frameworks also address the lifecycle emissions of synthetic fuels. Many jurisdictions require lifecycle assessments to ensure that synthetic fuels offer genuine environmental benefits compared to conventional fossil fuels. These assessments consider emissions from production, transportation, and use of the fuels, as well as the carbon intensity of the energy sources used in their synthesis.
Safety regulations play a crucial role in the synthetic fuel industry. Standards for production, storage, transportation, and use of synthetic fuels are essential to ensure public safety and environmental protection. Organizations such as the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) develop and maintain standards for synthetic fuels, which are often incorporated into national regulations.
As the technology for isobutane synthesis and other synthetic fuel production methods advances, regulatory frameworks are likely to evolve. Policymakers face the challenge of balancing environmental goals with economic considerations and energy security. Future regulations may focus on promoting innovation in synthetic fuel production, ensuring sustainable sourcing of feedstocks, and harmonizing standards across different regions to facilitate global trade and adoption of these fuels.
At the international level, the Paris Agreement serves as a cornerstone for climate action, influencing national policies on synthetic fuels. Many countries have set ambitious targets for reducing carbon emissions, which has led to the implementation of regulations that favor low-carbon and carbon-neutral fuel alternatives. These regulations often include incentives for research, development, and production of synthetic fuels, as well as mandates for their use in specific sectors.
In the European Union, the Renewable Energy Directive (RED II) sets targets for renewable energy use in transport, creating opportunities for synthetic fuels. The EU's FuelEU Maritime initiative also aims to increase the use of sustainable alternative fuels in the shipping industry, potentially boosting demand for synthetic fuels. Additionally, the ReFuelEU Aviation proposal seeks to increase the share of sustainable aviation fuels, including synthetic options.
In the United States, the Renewable Fuel Standard (RFS) program mandates the use of renewable fuels in transportation. While primarily focused on biofuels, there is growing interest in expanding the program to include synthetic fuels produced from renewable sources. The California Low Carbon Fuel Standard (LCFS) and similar programs in other states provide additional incentives for low-carbon fuel alternatives, including synthetic fuels.
Regulatory frameworks also address the lifecycle emissions of synthetic fuels. Many jurisdictions require lifecycle assessments to ensure that synthetic fuels offer genuine environmental benefits compared to conventional fossil fuels. These assessments consider emissions from production, transportation, and use of the fuels, as well as the carbon intensity of the energy sources used in their synthesis.
Safety regulations play a crucial role in the synthetic fuel industry. Standards for production, storage, transportation, and use of synthetic fuels are essential to ensure public safety and environmental protection. Organizations such as the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) develop and maintain standards for synthetic fuels, which are often incorporated into national regulations.
As the technology for isobutane synthesis and other synthetic fuel production methods advances, regulatory frameworks are likely to evolve. Policymakers face the challenge of balancing environmental goals with economic considerations and energy security. Future regulations may focus on promoting innovation in synthetic fuel production, ensuring sustainable sourcing of feedstocks, and harmonizing standards across different regions to facilitate global trade and adoption of these fuels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!