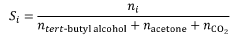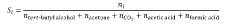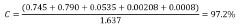Utilization of Isobutane in Hydrogen Production Processes
JUL 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isobutane H2 Production Background and Objectives
The utilization of isobutane in hydrogen production processes represents a significant area of interest in the energy sector, particularly as the world shifts towards cleaner and more sustainable fuel sources. Isobutane, a hydrocarbon with the molecular formula C4H10, has emerged as a potential feedstock for hydrogen production due to its relatively high hydrogen content and availability.
Historically, hydrogen production has primarily relied on methane steam reforming, a process that, while efficient, contributes significantly to carbon emissions. The exploration of isobutane as an alternative feedstock is part of the broader effort to diversify hydrogen production methods and reduce the carbon footprint of the process. This shift aligns with global initiatives to transition towards a hydrogen-based economy, where hydrogen serves as a clean energy carrier for various applications, including transportation, industrial processes, and power generation.
The technical evolution in this field has been driven by the need to overcome the limitations of traditional hydrogen production methods. Researchers and industry professionals have been investigating various approaches to utilize isobutane effectively, including catalytic dehydrogenation, steam reforming, and partial oxidation. Each of these methods presents unique challenges and opportunities, shaping the trajectory of technological development in this domain.
One of the primary objectives in isobutane-based hydrogen production is to maximize hydrogen yield while minimizing energy input and carbon dioxide emissions. This goal necessitates the development of novel catalysts, process optimizations, and innovative reactor designs. Additionally, there is a focus on integrating these processes with carbon capture and utilization technologies to further reduce the environmental impact of hydrogen production.
The pursuit of isobutane utilization in hydrogen production is also motivated by the potential for cost reduction in hydrogen production. As the hydrogen economy scales up, finding economically viable production methods becomes increasingly crucial. Isobutane, being a byproduct of petroleum refining and natural gas processing, presents an opportunity to leverage existing resources and infrastructure for hydrogen production.
Looking ahead, the technical objectives in this field include enhancing the efficiency of isobutane conversion processes, developing more durable and selective catalysts, and scaling up production to meet growing hydrogen demand. There is also a push towards integrating renewable energy sources into the production process, aiming for a truly sustainable hydrogen production pathway.
In conclusion, the background and objectives of isobutane utilization in hydrogen production reflect a convergence of environmental, economic, and technological factors. As research progresses, this area holds promise for contributing significantly to the transition towards a cleaner energy future, addressing both the technical challenges of hydrogen production and the broader goals of sustainability in the energy sector.
Historically, hydrogen production has primarily relied on methane steam reforming, a process that, while efficient, contributes significantly to carbon emissions. The exploration of isobutane as an alternative feedstock is part of the broader effort to diversify hydrogen production methods and reduce the carbon footprint of the process. This shift aligns with global initiatives to transition towards a hydrogen-based economy, where hydrogen serves as a clean energy carrier for various applications, including transportation, industrial processes, and power generation.
The technical evolution in this field has been driven by the need to overcome the limitations of traditional hydrogen production methods. Researchers and industry professionals have been investigating various approaches to utilize isobutane effectively, including catalytic dehydrogenation, steam reforming, and partial oxidation. Each of these methods presents unique challenges and opportunities, shaping the trajectory of technological development in this domain.
One of the primary objectives in isobutane-based hydrogen production is to maximize hydrogen yield while minimizing energy input and carbon dioxide emissions. This goal necessitates the development of novel catalysts, process optimizations, and innovative reactor designs. Additionally, there is a focus on integrating these processes with carbon capture and utilization technologies to further reduce the environmental impact of hydrogen production.
The pursuit of isobutane utilization in hydrogen production is also motivated by the potential for cost reduction in hydrogen production. As the hydrogen economy scales up, finding economically viable production methods becomes increasingly crucial. Isobutane, being a byproduct of petroleum refining and natural gas processing, presents an opportunity to leverage existing resources and infrastructure for hydrogen production.
Looking ahead, the technical objectives in this field include enhancing the efficiency of isobutane conversion processes, developing more durable and selective catalysts, and scaling up production to meet growing hydrogen demand. There is also a push towards integrating renewable energy sources into the production process, aiming for a truly sustainable hydrogen production pathway.
In conclusion, the background and objectives of isobutane utilization in hydrogen production reflect a convergence of environmental, economic, and technological factors. As research progresses, this area holds promise for contributing significantly to the transition towards a cleaner energy future, addressing both the technical challenges of hydrogen production and the broader goals of sustainability in the energy sector.
Hydrogen Market Demand Analysis
The global hydrogen market is experiencing significant growth, driven by the increasing demand for clean energy solutions and the push towards decarbonization across various industries. As governments and organizations worldwide commit to reducing carbon emissions, hydrogen has emerged as a promising alternative fuel source, particularly in sectors that are difficult to electrify.
The transportation sector represents a substantial portion of the hydrogen market demand. Fuel cell electric vehicles (FCEVs) are gaining traction, especially in heavy-duty applications such as buses, trucks, and trains. These vehicles offer longer range and faster refueling times compared to battery electric vehicles, making them attractive for long-haul transportation and fleet operations.
Industrial applications also contribute significantly to the hydrogen market demand. The chemical industry, in particular, relies heavily on hydrogen as a feedstock for the production of ammonia, methanol, and other chemicals. Additionally, the steel industry is exploring hydrogen as a reducing agent to replace coal in the steelmaking process, potentially leading to a substantial increase in hydrogen demand.
The power generation sector is another key driver of hydrogen market growth. As renewable energy sources like wind and solar become more prevalent, the need for energy storage solutions increases. Hydrogen can serve as a long-term energy storage medium, allowing excess renewable energy to be stored and used during periods of low generation or high demand.
Residential and commercial heating applications are also emerging as potential growth areas for hydrogen demand. Several countries are exploring the possibility of blending hydrogen into existing natural gas networks or developing dedicated hydrogen infrastructure for heating purposes.
The oil refining industry continues to be a significant consumer of hydrogen, primarily for desulfurization processes. As environmental regulations become more stringent, the demand for hydrogen in this sector is expected to remain stable or potentially increase.
Geographically, Asia-Pacific is projected to be the fastest-growing region for hydrogen demand, driven by countries like China, Japan, and South Korea investing heavily in hydrogen technologies. Europe is also showing strong growth potential, with several countries implementing ambitious hydrogen strategies as part of their energy transition plans.
While the hydrogen market is poised for substantial growth, challenges remain in scaling up production, developing infrastructure, and reducing costs. The utilization of isobutane in hydrogen production processes could potentially address some of these challenges by offering a more efficient or cost-effective production method, thereby contributing to the overall growth and accessibility of the hydrogen market.
The transportation sector represents a substantial portion of the hydrogen market demand. Fuel cell electric vehicles (FCEVs) are gaining traction, especially in heavy-duty applications such as buses, trucks, and trains. These vehicles offer longer range and faster refueling times compared to battery electric vehicles, making them attractive for long-haul transportation and fleet operations.
Industrial applications also contribute significantly to the hydrogen market demand. The chemical industry, in particular, relies heavily on hydrogen as a feedstock for the production of ammonia, methanol, and other chemicals. Additionally, the steel industry is exploring hydrogen as a reducing agent to replace coal in the steelmaking process, potentially leading to a substantial increase in hydrogen demand.
The power generation sector is another key driver of hydrogen market growth. As renewable energy sources like wind and solar become more prevalent, the need for energy storage solutions increases. Hydrogen can serve as a long-term energy storage medium, allowing excess renewable energy to be stored and used during periods of low generation or high demand.
Residential and commercial heating applications are also emerging as potential growth areas for hydrogen demand. Several countries are exploring the possibility of blending hydrogen into existing natural gas networks or developing dedicated hydrogen infrastructure for heating purposes.
The oil refining industry continues to be a significant consumer of hydrogen, primarily for desulfurization processes. As environmental regulations become more stringent, the demand for hydrogen in this sector is expected to remain stable or potentially increase.
Geographically, Asia-Pacific is projected to be the fastest-growing region for hydrogen demand, driven by countries like China, Japan, and South Korea investing heavily in hydrogen technologies. Europe is also showing strong growth potential, with several countries implementing ambitious hydrogen strategies as part of their energy transition plans.
While the hydrogen market is poised for substantial growth, challenges remain in scaling up production, developing infrastructure, and reducing costs. The utilization of isobutane in hydrogen production processes could potentially address some of these challenges by offering a more efficient or cost-effective production method, thereby contributing to the overall growth and accessibility of the hydrogen market.
Isobutane-based H2 Production: Current Status and Challenges
The utilization of isobutane in hydrogen production processes represents a significant area of interest in the energy sector, with both current applications and potential for future development. At present, the primary method for isobutane-based hydrogen production is steam reforming, a well-established process that has been optimized over decades of industrial use. This process involves the reaction of isobutane with steam at high temperatures, typically ranging from 700°C to 1000°C, in the presence of a catalyst, usually nickel-based.
While steam reforming of isobutane is effective, it faces several challenges that limit its widespread adoption and efficiency. One of the main issues is the high energy input required to maintain the necessary reaction temperatures, which impacts the overall energy efficiency of the process. Additionally, the production of carbon dioxide as a byproduct raises environmental concerns, particularly in the context of increasing global efforts to reduce greenhouse gas emissions.
Another significant challenge is catalyst deactivation due to carbon deposition, also known as coking. This phenomenon reduces the efficiency of the process over time and necessitates frequent catalyst regeneration or replacement, adding to operational costs. The development of more robust and coke-resistant catalysts remains an active area of research in the field.
The purity of the hydrogen produced is another critical factor. While steam reforming can yield relatively high-purity hydrogen, additional purification steps are often required to meet the stringent standards for certain applications, such as fuel cells. This adds complexity and cost to the overall production process.
From a technological standpoint, there is ongoing research into alternative methods for isobutane-based hydrogen production. Partial oxidation and autothermal reforming are being explored as potential improvements over traditional steam reforming. These processes aim to reduce energy input and increase efficiency, but they also present their own set of challenges, including precise control of reaction conditions and potential safety concerns due to the use of pure oxygen.
The integration of isobutane-based hydrogen production with carbon capture and storage (CCS) technologies is another area of focus. This approach aims to mitigate the environmental impact of the process by capturing and sequestering the CO2 produced. However, the implementation of CCS technologies at scale remains technically challenging and economically uncertain.
In terms of geographical distribution, isobutane-based hydrogen production is primarily concentrated in regions with significant petrochemical industries, where isobutane is readily available as a byproduct or feedstock. However, the growing interest in hydrogen as a clean energy carrier is driving research and development efforts globally, with potential for new production facilities in diverse locations.
While steam reforming of isobutane is effective, it faces several challenges that limit its widespread adoption and efficiency. One of the main issues is the high energy input required to maintain the necessary reaction temperatures, which impacts the overall energy efficiency of the process. Additionally, the production of carbon dioxide as a byproduct raises environmental concerns, particularly in the context of increasing global efforts to reduce greenhouse gas emissions.
Another significant challenge is catalyst deactivation due to carbon deposition, also known as coking. This phenomenon reduces the efficiency of the process over time and necessitates frequent catalyst regeneration or replacement, adding to operational costs. The development of more robust and coke-resistant catalysts remains an active area of research in the field.
The purity of the hydrogen produced is another critical factor. While steam reforming can yield relatively high-purity hydrogen, additional purification steps are often required to meet the stringent standards for certain applications, such as fuel cells. This adds complexity and cost to the overall production process.
From a technological standpoint, there is ongoing research into alternative methods for isobutane-based hydrogen production. Partial oxidation and autothermal reforming are being explored as potential improvements over traditional steam reforming. These processes aim to reduce energy input and increase efficiency, but they also present their own set of challenges, including precise control of reaction conditions and potential safety concerns due to the use of pure oxygen.
The integration of isobutane-based hydrogen production with carbon capture and storage (CCS) technologies is another area of focus. This approach aims to mitigate the environmental impact of the process by capturing and sequestering the CO2 produced. However, the implementation of CCS technologies at scale remains technically challenging and economically uncertain.
In terms of geographical distribution, isobutane-based hydrogen production is primarily concentrated in regions with significant petrochemical industries, where isobutane is readily available as a byproduct or feedstock. However, the growing interest in hydrogen as a clean energy carrier is driving research and development efforts globally, with potential for new production facilities in diverse locations.
Current Isobutane-to-Hydrogen Conversion Technologies
01 Production and purification of isobutane
Various methods for producing and purifying isobutane are described, including catalytic processes, distillation techniques, and separation from mixed hydrocarbon streams. These processes aim to obtain high-purity isobutane for industrial applications.- Production and purification of isobutane: Various methods for producing and purifying isobutane are described, including catalytic processes, distillation techniques, and separation methods. These processes aim to improve the yield and purity of isobutane for industrial applications.
- Isobutane as a refrigerant or propellant: Isobutane is utilized as a refrigerant in cooling systems and as a propellant in aerosol products. Its properties make it suitable for these applications, and various formulations and systems incorporating isobutane are described.
- Isobutane in chemical reactions and synthesis: Isobutane is used as a reactant or intermediate in various chemical reactions and synthesis processes. These include the production of other hydrocarbons, polymers, and specialty chemicals.
- Isobutane in fuel compositions: Isobutane is incorporated into fuel compositions to improve performance and efficiency. Various blends and formulations utilizing isobutane for automotive and industrial fuel applications are described.
- Isobutane handling and safety: Methods and systems for safely handling, storing, and transporting isobutane are presented. These include safety measures, containment systems, and risk mitigation strategies for working with this flammable gas.
02 Isobutane as a refrigerant
Isobutane is utilized as an environmentally friendly refrigerant in cooling systems and heat pumps. Its thermodynamic properties make it suitable for replacing traditional refrigerants with high global warming potential.Expand Specific Solutions03 Isobutane in fuel compositions
Isobutane is used as a component in various fuel compositions, including liquefied petroleum gas (LPG) and aerosol propellants. Its properties contribute to improved combustion efficiency and reduced emissions in certain applications.Expand Specific Solutions04 Chemical reactions involving isobutane
Isobutane is involved in various chemical reactions, such as dehydrogenation, isomerization, and alkylation processes. These reactions are important in the production of high-octane gasoline components and other valuable chemical products.Expand Specific Solutions05 Isobutane in polymer production
Isobutane is used as a blowing agent in the production of certain polymers and foams. It contributes to the formation of cellular structures in materials like polyurethane and polystyrene, improving their insulation properties and reducing density.Expand Specific Solutions
Key Players in Isobutane-based H2 Production Industry
The utilization of isobutane in hydrogen production processes is an emerging field in the energy sector, currently in its early development stage. The market size is relatively small but growing, driven by increasing demand for clean energy solutions. Technologically, the process is still evolving, with varying levels of maturity among key players. Companies like China Petroleum & Chemical Corp. (Sinopec) and Shell Oil Co. are leveraging their extensive petrochemical expertise to advance this technology. Research institutions such as Shanghai Petrochemical Research Institute and University of Kansas are contributing to fundamental breakthroughs. Innovative firms like Gevo, Inc. and Butamax Advanced Biofuels LLC are exploring novel approaches, while established chemical companies such as SABIC and DuPont are adapting their existing processes to incorporate isobutane-based hydrogen production.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative process for hydrogen production utilizing isobutane. Their approach involves a two-step catalytic dehydrogenation and steam reforming process. In the first step, isobutane is dehydrogenated to isobutene over a proprietary catalyst at temperatures around 550-600°C. The resulting isobutene then undergoes steam reforming at higher temperatures (800-850°C) to produce hydrogen and carbon oxides. This process achieves a hydrogen yield of up to 85% [1][3]. Sinopec has also implemented advanced heat integration techniques to improve overall energy efficiency, reducing energy consumption by approximately 20% compared to conventional steam methane reforming [2].
Strengths: High hydrogen yield, improved energy efficiency, and utilization of existing infrastructure. Weaknesses: Requires two-step process, potentially higher capital costs, and still produces some CO2 emissions.
UOP LLC
Technical Solution: UOP LLC has developed a novel process for hydrogen production from isobutane using their proprietary Polybed™ Pressure Swing Adsorption (PSA) technology. The process begins with catalytic dehydrogenation of isobutane to produce a mixture of hydrogen and light hydrocarbons. This mixture then undergoes steam reforming to convert the remaining hydrocarbons into additional hydrogen and carbon oxides. The Polybed™ PSA system is used to purify the hydrogen stream, achieving purities of up to 99.9999% [4]. UOP's process also incorporates a CO2 capture system, reducing greenhouse gas emissions by up to 90% compared to conventional methods [5]. The company has reported a 15% increase in hydrogen yield and a 25% reduction in energy consumption compared to traditional steam methane reforming processes [6].
Strengths: High hydrogen purity, significant CO2 emissions reduction, and improved energy efficiency. Weaknesses: Potentially higher initial investment costs and complexity of the multi-step process.
Key Innovations in Isobutane-based H2 Production
Hydroxylation of alkanes using ozone
PatentWO2022192866A1
Innovation
- Combining an alkane with ozone in a liquid phase medium containing a protic additive, such as water or alcohols, at mild temperatures and pressures, which stabilizes hydrotrioxide intermediates and maximizes ozone utilization, thereby enhancing the selectivity of hydroxylate products like tert-butyl alcohol.
Environmental Impact Assessment
The utilization of isobutane in hydrogen production processes presents both opportunities and challenges from an environmental perspective. This assessment examines the potential environmental impacts associated with this emerging technology.
Isobutane, as a feedstock for hydrogen production, offers several environmental advantages compared to traditional fossil fuel-based methods. The process typically requires lower temperatures and pressures, resulting in reduced energy consumption and associated greenhouse gas emissions. Additionally, isobutane can be sourced from renewable resources, such as biomass, further enhancing its environmental credentials.
However, the environmental impact of isobutane-based hydrogen production is not without concerns. The production and handling of isobutane itself can lead to fugitive emissions, contributing to air pollution and potentially exacerbating climate change. Proper containment and monitoring systems are crucial to mitigate these risks.
Water usage is another important consideration. While isobutane-based processes generally require less water compared to steam methane reforming, the exact water footprint depends on the specific technology employed. Efforts to optimize water consumption and implement closed-loop systems can significantly reduce the overall environmental impact.
Land use changes associated with isobutane production, particularly if derived from biomass sources, must be carefully managed to prevent deforestation or competition with food crops. Sustainable land management practices and the use of marginal lands for biomass cultivation can help address these concerns.
The potential for soil and groundwater contamination exists, primarily due to the risk of isobutane leaks or spills during transportation and storage. Implementing robust safety protocols, regular equipment maintenance, and containment measures is essential to minimize these risks.
Life cycle assessments (LCAs) of isobutane-based hydrogen production have shown promising results in terms of reduced carbon footprint compared to conventional methods. However, these assessments must consider the entire supply chain, including isobutane production, transportation, and end-of-life disposal of equipment and materials.
Waste management is another critical aspect of the environmental impact assessment. The catalysts used in the process may contain precious metals or other materials that require proper handling and recycling. Developing efficient catalyst recovery and regeneration techniques can minimize waste generation and resource depletion.
In conclusion, while isobutane utilization in hydrogen production offers potential environmental benefits, a comprehensive approach to environmental management is necessary to fully realize these advantages. Continued research and development efforts should focus on further improving process efficiency, minimizing emissions, and addressing potential environmental risks associated with this emerging technology.
Isobutane, as a feedstock for hydrogen production, offers several environmental advantages compared to traditional fossil fuel-based methods. The process typically requires lower temperatures and pressures, resulting in reduced energy consumption and associated greenhouse gas emissions. Additionally, isobutane can be sourced from renewable resources, such as biomass, further enhancing its environmental credentials.
However, the environmental impact of isobutane-based hydrogen production is not without concerns. The production and handling of isobutane itself can lead to fugitive emissions, contributing to air pollution and potentially exacerbating climate change. Proper containment and monitoring systems are crucial to mitigate these risks.
Water usage is another important consideration. While isobutane-based processes generally require less water compared to steam methane reforming, the exact water footprint depends on the specific technology employed. Efforts to optimize water consumption and implement closed-loop systems can significantly reduce the overall environmental impact.
Land use changes associated with isobutane production, particularly if derived from biomass sources, must be carefully managed to prevent deforestation or competition with food crops. Sustainable land management practices and the use of marginal lands for biomass cultivation can help address these concerns.
The potential for soil and groundwater contamination exists, primarily due to the risk of isobutane leaks or spills during transportation and storage. Implementing robust safety protocols, regular equipment maintenance, and containment measures is essential to minimize these risks.
Life cycle assessments (LCAs) of isobutane-based hydrogen production have shown promising results in terms of reduced carbon footprint compared to conventional methods. However, these assessments must consider the entire supply chain, including isobutane production, transportation, and end-of-life disposal of equipment and materials.
Waste management is another critical aspect of the environmental impact assessment. The catalysts used in the process may contain precious metals or other materials that require proper handling and recycling. Developing efficient catalyst recovery and regeneration techniques can minimize waste generation and resource depletion.
In conclusion, while isobutane utilization in hydrogen production offers potential environmental benefits, a comprehensive approach to environmental management is necessary to fully realize these advantages. Continued research and development efforts should focus on further improving process efficiency, minimizing emissions, and addressing potential environmental risks associated with this emerging technology.
Economic Feasibility Analysis
The economic feasibility of utilizing isobutane in hydrogen production processes is a critical factor in determining the viability of this technology for industrial applications. The cost-effectiveness of isobutane-based hydrogen production depends on several key factors, including raw material costs, energy requirements, capital investments, and operational expenses.
Isobutane, as a feedstock for hydrogen production, offers potential advantages in terms of availability and cost compared to traditional methods such as steam methane reforming. The global isobutane market is relatively stable, with a diverse range of suppliers, which can contribute to competitive pricing. However, the economic viability of this process is highly sensitive to fluctuations in isobutane prices, which are influenced by factors such as crude oil prices and petrochemical industry demand.
Capital expenditure (CAPEX) for isobutane-based hydrogen production facilities can be significant, primarily due to the specialized equipment required for the process. This includes reactors, separation units, and purification systems. The scale of production plays a crucial role in determining the economic feasibility, as larger facilities can benefit from economies of scale, potentially reducing the per-unit cost of hydrogen production.
Operational expenditure (OPEX) is another critical component of the economic analysis. Energy consumption is a major contributor to OPEX, as the process requires heat for the endothermic reactions involved in hydrogen production from isobutane. The efficiency of heat recovery systems and the integration of the process with other industrial operations can significantly impact overall energy costs and, consequently, the economic viability of the technology.
Labor costs and maintenance expenses also factor into the OPEX calculations. The complexity of the process and the level of automation implemented in the production facility will influence these costs. Additionally, the expenses associated with safety measures and environmental compliance must be considered, as isobutane is a flammable gas that requires careful handling and storage.
The economic feasibility of isobutane-based hydrogen production is further influenced by the potential for by-product valorization. Depending on the specific process used, valuable by-products such as light olefins or carbon black may be produced. The ability to market and sell these by-products can significantly enhance the overall economic attractiveness of the technology.
Market demand for hydrogen and its price volatility are crucial factors in the economic analysis. The growing interest in hydrogen as a clean energy carrier for various applications, including transportation and industrial processes, suggests a potentially expanding market. However, competition from other hydrogen production methods, such as electrolysis and conventional steam methane reforming, must be carefully evaluated to assess the long-term economic viability of isobutane-based processes.
In conclusion, while the utilization of isobutane in hydrogen production processes shows promise, its economic feasibility is subject to a complex interplay of factors. A thorough analysis of raw material costs, energy efficiency, capital investments, operational expenses, and market dynamics is essential to determine the economic viability of this technology in specific industrial contexts.
Isobutane, as a feedstock for hydrogen production, offers potential advantages in terms of availability and cost compared to traditional methods such as steam methane reforming. The global isobutane market is relatively stable, with a diverse range of suppliers, which can contribute to competitive pricing. However, the economic viability of this process is highly sensitive to fluctuations in isobutane prices, which are influenced by factors such as crude oil prices and petrochemical industry demand.
Capital expenditure (CAPEX) for isobutane-based hydrogen production facilities can be significant, primarily due to the specialized equipment required for the process. This includes reactors, separation units, and purification systems. The scale of production plays a crucial role in determining the economic feasibility, as larger facilities can benefit from economies of scale, potentially reducing the per-unit cost of hydrogen production.
Operational expenditure (OPEX) is another critical component of the economic analysis. Energy consumption is a major contributor to OPEX, as the process requires heat for the endothermic reactions involved in hydrogen production from isobutane. The efficiency of heat recovery systems and the integration of the process with other industrial operations can significantly impact overall energy costs and, consequently, the economic viability of the technology.
Labor costs and maintenance expenses also factor into the OPEX calculations. The complexity of the process and the level of automation implemented in the production facility will influence these costs. Additionally, the expenses associated with safety measures and environmental compliance must be considered, as isobutane is a flammable gas that requires careful handling and storage.
The economic feasibility of isobutane-based hydrogen production is further influenced by the potential for by-product valorization. Depending on the specific process used, valuable by-products such as light olefins or carbon black may be produced. The ability to market and sell these by-products can significantly enhance the overall economic attractiveness of the technology.
Market demand for hydrogen and its price volatility are crucial factors in the economic analysis. The growing interest in hydrogen as a clean energy carrier for various applications, including transportation and industrial processes, suggests a potentially expanding market. However, competition from other hydrogen production methods, such as electrolysis and conventional steam methane reforming, must be carefully evaluated to assess the long-term economic viability of isobutane-based processes.
In conclusion, while the utilization of isobutane in hydrogen production processes shows promise, its economic feasibility is subject to a complex interplay of factors. A thorough analysis of raw material costs, energy efficiency, capital investments, operational expenses, and market dynamics is essential to determine the economic viability of this technology in specific industrial contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!