Silicon anode SEI engineering with fluorinated solvents and additives
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicon Anode Development Background and Objectives
Silicon anodes have emerged as a promising alternative to traditional graphite anodes in lithium-ion batteries due to their significantly higher theoretical capacity (approximately 4200 mAh/g compared to graphite's 372 mAh/g). The development of silicon anodes can be traced back to the early 2000s when researchers began exploring silicon as an anode material to meet the growing demand for higher energy density batteries in portable electronics, electric vehicles, and grid storage applications.
Despite silicon's theoretical advantages, its commercial adoption has been hindered by several critical challenges. The most significant issue is the substantial volume expansion (up to 300-400%) during lithiation, leading to mechanical degradation, pulverization, and rapid capacity fading. Additionally, the continuous formation and breakdown of the solid-electrolyte interphase (SEI) on silicon surfaces consumes electrolyte and lithium ions, further contributing to capacity loss and battery failure.
The SEI layer, a passivation film formed on electrode surfaces during the initial charging cycles, plays a crucial role in battery performance and longevity. For silicon anodes, engineering a stable and flexible SEI layer has become a central focus of research efforts worldwide. Traditional electrolyte systems designed for graphite anodes have proven inadequate for silicon, necessitating innovative approaches to SEI engineering.
Fluorinated solvents and additives have emerged as promising candidates for silicon anode SEI engineering due to their unique properties. Fluorine-containing compounds typically exhibit higher oxidative stability, lower flammability, and can form more robust SEI layers that better accommodate silicon's volume changes. Early research has demonstrated that fluorinated electrolyte components can significantly improve the cycling stability of silicon anodes by forming more flexible and durable SEI layers.
The primary objective of silicon anode SEI engineering with fluorinated solvents and additives is to develop electrolyte systems that enable stable, long-cycle-life silicon-based anodes for next-generation lithium-ion batteries. Specific goals include designing fluorinated electrolyte formulations that form mechanically robust yet flexible SEI layers, understanding the fundamental mechanisms of SEI formation and evolution with these novel electrolytes, and ultimately enabling silicon-dominant anodes with practical loading levels for commercial applications.
This research aims to bridge the gap between silicon's theoretical promise and commercial viability, potentially revolutionizing energy storage capabilities across multiple industries. Success in this field could enable electric vehicles with significantly extended range, longer-lasting consumer electronics, and more efficient renewable energy storage systems, addressing critical global challenges in energy sustainability and climate change mitigation.
Despite silicon's theoretical advantages, its commercial adoption has been hindered by several critical challenges. The most significant issue is the substantial volume expansion (up to 300-400%) during lithiation, leading to mechanical degradation, pulverization, and rapid capacity fading. Additionally, the continuous formation and breakdown of the solid-electrolyte interphase (SEI) on silicon surfaces consumes electrolyte and lithium ions, further contributing to capacity loss and battery failure.
The SEI layer, a passivation film formed on electrode surfaces during the initial charging cycles, plays a crucial role in battery performance and longevity. For silicon anodes, engineering a stable and flexible SEI layer has become a central focus of research efforts worldwide. Traditional electrolyte systems designed for graphite anodes have proven inadequate for silicon, necessitating innovative approaches to SEI engineering.
Fluorinated solvents and additives have emerged as promising candidates for silicon anode SEI engineering due to their unique properties. Fluorine-containing compounds typically exhibit higher oxidative stability, lower flammability, and can form more robust SEI layers that better accommodate silicon's volume changes. Early research has demonstrated that fluorinated electrolyte components can significantly improve the cycling stability of silicon anodes by forming more flexible and durable SEI layers.
The primary objective of silicon anode SEI engineering with fluorinated solvents and additives is to develop electrolyte systems that enable stable, long-cycle-life silicon-based anodes for next-generation lithium-ion batteries. Specific goals include designing fluorinated electrolyte formulations that form mechanically robust yet flexible SEI layers, understanding the fundamental mechanisms of SEI formation and evolution with these novel electrolytes, and ultimately enabling silicon-dominant anodes with practical loading levels for commercial applications.
This research aims to bridge the gap between silicon's theoretical promise and commercial viability, potentially revolutionizing energy storage capabilities across multiple industries. Success in this field could enable electric vehicles with significantly extended range, longer-lasting consumer electronics, and more efficient renewable energy storage systems, addressing critical global challenges in energy sustainability and climate change mitigation.
Market Analysis for Advanced Battery Technologies
The global advanced battery market is experiencing unprecedented growth, driven primarily by the electric vehicle (EV) revolution and renewable energy storage demands. Current market valuations exceed $90 billion, with projections indicating a compound annual growth rate of 18-20% through 2030. Silicon anode technology represents one of the most promising segments within this market, with specific growth rates exceeding 25% annually as manufacturers seek higher energy density solutions.
Consumer electronics continues to be a significant market driver, but the automotive sector has emerged as the dominant force shaping battery technology development. Major automakers have committed over $500 billion collectively toward electrification strategies, creating substantial demand for next-generation battery technologies. Silicon anode batteries with engineered SEI layers using fluorinated components are positioned to capture significant market share due to their superior performance characteristics.
The energy storage system (ESS) market presents another substantial opportunity, valued at approximately $12 billion currently and expected to reach $31 billion by 2030. Grid-scale applications particularly benefit from the improved cycle life that advanced SEI engineering can provide, creating a secondary market driver for fluorinated solvent and additive technologies.
Regional analysis reveals Asia-Pacific dominates manufacturing capacity, with China controlling nearly 75% of global battery production. However, recent geopolitical tensions have accelerated localization efforts in North America and Europe, with new battery gigafactories representing over $40 billion in announced investments across these regions. This regionalization trend creates market entry opportunities for innovative silicon anode technologies with engineered SEI solutions.
Customer segmentation shows three primary markets for advanced silicon anode batteries: premium EVs seeking maximum range, consumer electronics requiring high energy density, and aerospace applications where weight considerations are paramount. Each segment values different aspects of SEI engineering, with automotive customers prioritizing cycle life, electronics manufacturers focusing on fast charging capabilities, and aerospace applications emphasizing safety and thermal stability.
Price sensitivity analysis indicates that while silicon anode batteries currently command a 15-20% premium over conventional lithium-ion batteries, this gap is narrowing as manufacturing scales. The additional cost of fluorinated solvents and additives for SEI engineering adds approximately 5-8% to production costs, but delivers performance improvements that justify the premium for high-end applications.
Market barriers include supply chain constraints for fluorinated compounds, intellectual property landscapes dominated by several key players, and regulatory considerations regarding some fluorinated materials. Despite these challenges, market forecasts suggest silicon anode batteries with engineered SEI layers will capture 30-35% of the premium battery market by 2028.
Consumer electronics continues to be a significant market driver, but the automotive sector has emerged as the dominant force shaping battery technology development. Major automakers have committed over $500 billion collectively toward electrification strategies, creating substantial demand for next-generation battery technologies. Silicon anode batteries with engineered SEI layers using fluorinated components are positioned to capture significant market share due to their superior performance characteristics.
The energy storage system (ESS) market presents another substantial opportunity, valued at approximately $12 billion currently and expected to reach $31 billion by 2030. Grid-scale applications particularly benefit from the improved cycle life that advanced SEI engineering can provide, creating a secondary market driver for fluorinated solvent and additive technologies.
Regional analysis reveals Asia-Pacific dominates manufacturing capacity, with China controlling nearly 75% of global battery production. However, recent geopolitical tensions have accelerated localization efforts in North America and Europe, with new battery gigafactories representing over $40 billion in announced investments across these regions. This regionalization trend creates market entry opportunities for innovative silicon anode technologies with engineered SEI solutions.
Customer segmentation shows three primary markets for advanced silicon anode batteries: premium EVs seeking maximum range, consumer electronics requiring high energy density, and aerospace applications where weight considerations are paramount. Each segment values different aspects of SEI engineering, with automotive customers prioritizing cycle life, electronics manufacturers focusing on fast charging capabilities, and aerospace applications emphasizing safety and thermal stability.
Price sensitivity analysis indicates that while silicon anode batteries currently command a 15-20% premium over conventional lithium-ion batteries, this gap is narrowing as manufacturing scales. The additional cost of fluorinated solvents and additives for SEI engineering adds approximately 5-8% to production costs, but delivers performance improvements that justify the premium for high-end applications.
Market barriers include supply chain constraints for fluorinated compounds, intellectual property landscapes dominated by several key players, and regulatory considerations regarding some fluorinated materials. Despite these challenges, market forecasts suggest silicon anode batteries with engineered SEI layers will capture 30-35% of the premium battery market by 2028.
Current Challenges in Silicon Anode SEI Formation
Silicon anodes in lithium-ion batteries face significant challenges in forming stable and effective Solid Electrolyte Interphase (SEI) layers. The primary issue stems from the massive volume expansion (up to 300%) that silicon undergoes during lithiation, which repeatedly breaks the SEI layer, exposing fresh silicon surfaces to the electrolyte. This continuous SEI reformation consumes lithium ions and electrolyte components, leading to capacity fade and shortened battery life.
Conventional electrolyte systems designed for graphite anodes prove inadequate for silicon anodes due to their inability to form flexible and robust SEI layers that can accommodate the extreme volume changes. The SEI formed with standard carbonate-based electrolytes tends to be brittle and prone to cracking when subjected to the mechanical stress of silicon expansion and contraction.
Another critical challenge is the composition and morphology of the SEI layer on silicon surfaces. Unlike graphite anodes, silicon forms complex SEI structures with varying chemical compositions depending on the electrolyte formulation. The SEI often contains lithium silicates, lithium fluorides, and various organic compounds, but achieving the optimal ratio and distribution of these components remains elusive.
Electrolyte decomposition at silicon surfaces occurs at different potentials compared to graphite, requiring specialized additives to control the SEI formation process. Without proper engineering, parasitic reactions can dominate, leading to excessive gassing, electrolyte depletion, and impedance growth.
The interface between silicon and the current collector presents additional challenges, as delamination can occur during cycling due to the mechanical stresses. This further complicates SEI engineering as it creates discontinuities in electron transport pathways and exposes new surfaces to electrolyte attack.
Fluorinated solvents and additives have shown promise in addressing these challenges by forming more stable and flexible SEI layers. However, their implementation faces obstacles including high cost, potential toxicity, and compatibility issues with other battery components. The fluorinated compounds can also lead to increased impedance if not properly balanced in the electrolyte formulation.
Temperature sensitivity represents another significant hurdle, as silicon anodes with fluorinated electrolyte systems often exhibit poor performance at low temperatures due to increased SEI resistance. Conversely, at elevated temperatures, accelerated side reactions can compromise the protective nature of the SEI layer.
Achieving long-term cycling stability remains the ultimate challenge, requiring an SEI that can maintain its protective properties over thousands of cycles while accommodating the silicon's volumetric changes and preventing continuous electrolyte decomposition.
Conventional electrolyte systems designed for graphite anodes prove inadequate for silicon anodes due to their inability to form flexible and robust SEI layers that can accommodate the extreme volume changes. The SEI formed with standard carbonate-based electrolytes tends to be brittle and prone to cracking when subjected to the mechanical stress of silicon expansion and contraction.
Another critical challenge is the composition and morphology of the SEI layer on silicon surfaces. Unlike graphite anodes, silicon forms complex SEI structures with varying chemical compositions depending on the electrolyte formulation. The SEI often contains lithium silicates, lithium fluorides, and various organic compounds, but achieving the optimal ratio and distribution of these components remains elusive.
Electrolyte decomposition at silicon surfaces occurs at different potentials compared to graphite, requiring specialized additives to control the SEI formation process. Without proper engineering, parasitic reactions can dominate, leading to excessive gassing, electrolyte depletion, and impedance growth.
The interface between silicon and the current collector presents additional challenges, as delamination can occur during cycling due to the mechanical stresses. This further complicates SEI engineering as it creates discontinuities in electron transport pathways and exposes new surfaces to electrolyte attack.
Fluorinated solvents and additives have shown promise in addressing these challenges by forming more stable and flexible SEI layers. However, their implementation faces obstacles including high cost, potential toxicity, and compatibility issues with other battery components. The fluorinated compounds can also lead to increased impedance if not properly balanced in the electrolyte formulation.
Temperature sensitivity represents another significant hurdle, as silicon anodes with fluorinated electrolyte systems often exhibit poor performance at low temperatures due to increased SEI resistance. Conversely, at elevated temperatures, accelerated side reactions can compromise the protective nature of the SEI layer.
Achieving long-term cycling stability remains the ultimate challenge, requiring an SEI that can maintain its protective properties over thousands of cycles while accommodating the silicon's volumetric changes and preventing continuous electrolyte decomposition.
Fluorinated Electrolyte Solutions Overview
01 Electrolyte additives for SEI formation and stability
Various electrolyte additives can be incorporated to enhance the formation and stability of the solid electrolyte interphase (SEI) layer on silicon anodes. These additives help create a more uniform and robust SEI layer that can accommodate the volume changes of silicon during cycling. Specific compounds such as fluoroethylene carbonate (FEC), vinylene carbonate (VC), and lithium difluoro(oxalato)borate (LiDFOB) have been shown to significantly improve the SEI stability and consequently enhance the cycling performance of silicon anodes.- Electrolyte additives for SEI formation and stability: Various electrolyte additives can be incorporated to enhance the formation and stability of the solid electrolyte interphase (SEI) layer on silicon anodes. These additives can include fluorinated compounds, carbonates, and other organic molecules that decompose preferentially on the silicon surface to form a stable protective layer. The resulting SEI helps to prevent continuous electrolyte decomposition, reduces irreversible capacity loss, and improves cycling stability of silicon anodes.
- Artificial SEI coatings and surface modifications: Pre-formed artificial SEI layers or surface modifications can be applied to silicon anodes before battery assembly to improve stability and performance. These coatings can include inorganic materials, polymers, or composite structures that mimic the protective properties of naturally formed SEI but with enhanced mechanical stability. Such artificial SEI layers can accommodate the large volume changes of silicon during cycling while maintaining electrode integrity and ionic conductivity.
- Nanostructured silicon designs for SEI stability: Nanostructured silicon designs, such as nanoparticles, nanowires, and porous structures, can be engineered to improve SEI stability. These nanostructures provide better accommodation of volume changes during lithiation/delithiation cycles, reducing mechanical stress on the SEI layer. The controlled void spaces and surface features in these nanostructures help maintain SEI integrity over extended cycling, leading to improved capacity retention and battery lifespan.
- Composite silicon anodes with carbon materials: Silicon-carbon composite anodes can be designed to enhance SEI stability and performance. By incorporating carbon materials such as graphene, carbon nanotubes, or amorphous carbon, these composites provide structural support for silicon and create a more stable interface for SEI formation. The carbon component helps buffer volume changes, improves electrical conductivity, and can serve as a protective scaffold for the SEI layer, resulting in better cycling performance.
- Advanced binders and conductive additives for SEI engineering: Specialized binders and conductive additives can be incorporated into silicon anode formulations to enhance SEI stability. These materials help maintain electrode integrity during volume changes and can participate in forming a more robust SEI layer. Functional binders with reactive groups can chemically bond with silicon surfaces or SEI components, while conductive additives ensure uniform current distribution and SEI formation across the electrode surface, leading to improved electrochemical performance.
02 Protective coatings and surface modifications
Surface modifications and protective coatings on silicon particles can significantly improve SEI stability. These include carbon coatings, metal oxide layers, and polymer films that serve as artificial SEI layers or templates for stable SEI formation. Such modifications help mitigate the direct contact between silicon and the electrolyte, reducing continuous SEI formation and electrolyte consumption. They also provide mechanical support to accommodate volume changes during lithiation/delithiation cycles, thereby enhancing the overall electrochemical performance and cycle life of silicon anodes.Expand Specific Solutions03 Nanostructured silicon designs for SEI stability
Nanostructured silicon designs, including silicon nanowires, nanoparticles, and porous structures, offer improved SEI stability by providing better accommodation for volume expansion during cycling. These structures create more stable interfaces with the electrolyte and allow for more uniform SEI formation. The increased surface area and shortened lithium diffusion paths in nanostructured silicon also contribute to enhanced electrochemical performance, while the engineered void spaces help maintain structural integrity during repeated cycling.Expand Specific Solutions04 Silicon-carbon composite structures
Silicon-carbon composite structures represent an effective approach to enhance SEI stability and performance of silicon anodes. By integrating silicon with various carbon materials such as graphene, carbon nanotubes, or amorphous carbon, these composites provide mechanical support and electrical conductivity while buffering the volume changes of silicon. The carbon component helps form a more stable SEI layer and prevents direct exposure of silicon to the electrolyte, resulting in improved cycling stability and rate capability of the anode material.Expand Specific Solutions05 Advanced binder systems for SEI engineering
Advanced binder systems play a crucial role in SEI engineering for silicon anodes. Functional polymeric binders with specific chemical groups (such as carboxyl, hydroxyl, or amide) can interact with both silicon particles and the SEI components, leading to enhanced mechanical integrity and electrochemical stability. These binders help maintain electrode structure during volume changes, prevent silicon particle isolation, and contribute to the formation of a more flexible and durable SEI layer. Self-healing binders and those with conductive properties further improve the overall performance and longevity of silicon anodes.Expand Specific Solutions
Leading Companies in Silicon Anode Technology
Silicon anode SEI engineering with fluorinated solvents and additives is currently in the growth phase of industry development, with an expanding market driven by demand for higher energy density batteries. The global market for silicon anode materials is projected to reach significant scale as electric vehicle adoption accelerates. Technologically, this field remains in mid-maturity, with companies at varying stages of development. Leading players like LG Energy Solution, CATL, and Enevate are advancing commercial applications, while specialized innovators such as NanoGraf, Nexeon, and StoreDot focus on breakthrough silicon anode technologies. Research institutions including Carnegie Mellon University and Argonne National Laboratory contribute fundamental knowledge, particularly in SEI formation mechanisms with fluorinated compounds. The competitive landscape features both established battery manufacturers and emerging technology companies working to overcome silicon expansion challenges through engineered SEI layers.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an innovative silicon anode SEI engineering approach utilizing fluorinated electrolyte systems. Their technology employs a multi-component fluorinated solvent mixture (primarily FEC and FEMC) combined with LiDFOB and LiFSI additives to create a robust and stable SEI layer on silicon anodes. This formulation creates a fluorine-rich protective film that effectively mitigates silicon's volumetric expansion issues during cycling. CATL's research demonstrates that their engineered electrolyte forms a LiF-rich SEI layer that significantly improves the cycling stability of high-capacity silicon anodes. Their proprietary electrolyte composition achieves over 80% capacity retention after 500 cycles in high-silicon-content anodes (>50% Si), representing a substantial improvement over conventional electrolytes[1]. The company has also developed a pre-lithiation technique that works synergistically with their fluorinated electrolyte system to compensate for initial capacity loss during SEI formation.
Strengths: Superior cycling stability with high silicon content anodes; reduced irreversible capacity loss; excellent compatibility with existing manufacturing processes. Weaknesses: Higher cost of fluorinated components compared to conventional electrolytes; potential environmental concerns with some fluorinated compounds; limited performance data at extreme temperature conditions.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has pioneered a silicon anode SEI engineering approach using a dual-functional fluorinated electrolyte system. Their technology combines fluoroethylene carbonate (FEC) as the primary solvent with strategically selected fluorinated additives including LiPF6 and novel organo-fluorine compounds. This formulation creates a mechanically robust and ionically conductive SEI layer that accommodates silicon's volume expansion. LG's research shows their engineered fluorinated electrolyte forms a uniform, elastic SEI layer rich in LiF and Si-F compounds that significantly enhances cycling performance[2]. Their silicon anode cells utilizing this technology demonstrate approximately 91% capacity retention after 300 cycles at 1C rate, with initial specific capacities exceeding 3500 mAh/g for the silicon component. LG has also developed a gradient concentration fluorinated electrolyte system that creates an optimized SEI structure with distinct inner and outer layers, providing both mechanical stability and efficient ion transport properties.
Strengths: Exceptional cycling stability; high initial capacity retention; compatible with high-energy density cell designs; scalable manufacturing process. Weaknesses: Higher production costs due to specialized fluorinated components; potential safety concerns with some fluorinated additives at elevated temperatures; limited long-term stability data beyond 500 cycles.
Key Innovations in Fluorinated Additives
Silicon-based energy storage devices with fluorinated polymer containing electrolyte additives
PatentActiveUS11888114B2
Innovation
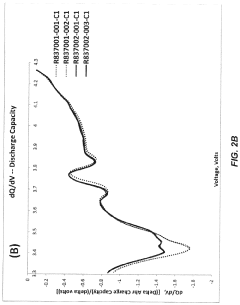
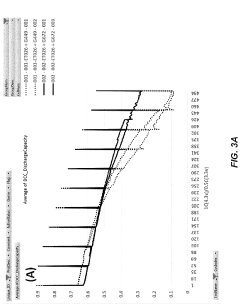
- The development of fluorinated polymer electrolyte additives that form stable, electronically insulating but ionically conducting solid electrolyte interphase (SEI) layers on silicon anodes and cathode surfaces, enhancing mechanical strength and thermal stability, and reducing flammability, thereby improving the electrochemical performance and safety of lithium-ion batteries.
Surface-fluorinated silicon-containing electrodes
PatentActiveUS11870055B2
Innovation
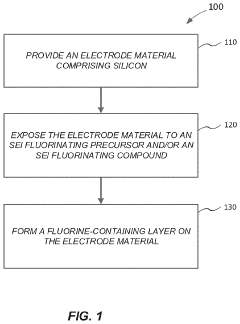
- A method involving surface modification of silicon particles with a fluorine-containing layer, formed through exposure to SEI fluorinating precursors or compounds, such as metal fluoride compounds or fluorine-doped metal oxides, to create a stable and robust SEI layer that enhances mechanical strength and electrochemical stability.
Environmental Impact of Fluorinated Battery Components
The increasing adoption of fluorinated solvents and additives in silicon anode SEI engineering raises significant environmental concerns that warrant careful examination. These compounds, while beneficial for battery performance, contain fluorine atoms that form persistent environmental pollutants when released. Perfluorinated compounds (PFCs) and other fluorinated materials can persist in the environment for decades or even centuries, earning them the designation as "forever chemicals."
The production process of fluorinated battery components generates substantial greenhouse gas emissions, particularly hydrofluorocarbons (HFCs) and perfluorocarbons (PFCs), which have global warming potentials hundreds to thousands of times greater than carbon dioxide. Manufacturing facilities producing these compounds often release these gases during synthesis, purification, and waste treatment processes, contributing to climate change disproportionately to their production volume.
Water contamination represents another critical environmental challenge. Fluorinated compounds can leach into groundwater and surface water systems through improper disposal of batteries or manufacturing waste. These chemicals are extremely difficult to remove using conventional water treatment methods, requiring specialized advanced oxidation processes or activated carbon filtration systems that are energy-intensive and expensive to implement at scale.
Bioaccumulation of fluorinated compounds in wildlife and humans presents long-term ecological and health risks. Studies have documented the presence of fluorinated organic compounds in marine mammals, birds, and fish, even in remote Arctic regions, demonstrating their global transport capabilities. These compounds can disrupt endocrine systems, affect reproductive capabilities, and potentially cause developmental abnormalities in exposed organisms.
End-of-life management of batteries containing fluorinated components presents additional environmental challenges. Current recycling infrastructure is inadequately equipped to safely process and contain these compounds, potentially releasing them during shredding, smelting, or other recycling operations. The thermal decomposition of fluorinated compounds can generate highly toxic hydrogen fluoride gas and other hazardous byproducts.
Regulatory frameworks worldwide are increasingly focusing on restricting perfluorinated compounds, with the European Union's REACH regulations and the Stockholm Convention on Persistent Organic Pollutants targeting many fluorinated substances. Battery manufacturers employing these materials may face growing regulatory pressure, potential bans, or mandatory substitution requirements in the coming years, creating market uncertainty and potential liability issues.
The production process of fluorinated battery components generates substantial greenhouse gas emissions, particularly hydrofluorocarbons (HFCs) and perfluorocarbons (PFCs), which have global warming potentials hundreds to thousands of times greater than carbon dioxide. Manufacturing facilities producing these compounds often release these gases during synthesis, purification, and waste treatment processes, contributing to climate change disproportionately to their production volume.
Water contamination represents another critical environmental challenge. Fluorinated compounds can leach into groundwater and surface water systems through improper disposal of batteries or manufacturing waste. These chemicals are extremely difficult to remove using conventional water treatment methods, requiring specialized advanced oxidation processes or activated carbon filtration systems that are energy-intensive and expensive to implement at scale.
Bioaccumulation of fluorinated compounds in wildlife and humans presents long-term ecological and health risks. Studies have documented the presence of fluorinated organic compounds in marine mammals, birds, and fish, even in remote Arctic regions, demonstrating their global transport capabilities. These compounds can disrupt endocrine systems, affect reproductive capabilities, and potentially cause developmental abnormalities in exposed organisms.
End-of-life management of batteries containing fluorinated components presents additional environmental challenges. Current recycling infrastructure is inadequately equipped to safely process and contain these compounds, potentially releasing them during shredding, smelting, or other recycling operations. The thermal decomposition of fluorinated compounds can generate highly toxic hydrogen fluoride gas and other hazardous byproducts.
Regulatory frameworks worldwide are increasingly focusing on restricting perfluorinated compounds, with the European Union's REACH regulations and the Stockholm Convention on Persistent Organic Pollutants targeting many fluorinated substances. Battery manufacturers employing these materials may face growing regulatory pressure, potential bans, or mandatory substitution requirements in the coming years, creating market uncertainty and potential liability issues.
Scalability and Manufacturing Considerations
The scalability of silicon anode technology with fluorinated electrolyte systems presents significant manufacturing challenges that must be addressed for commercial viability. Current laboratory-scale synthesis methods for fluorinated solvents and additives typically involve complex chemical processes with expensive precursors and specialized equipment. These methods often yield small quantities suitable for research but face substantial hurdles when considered for industrial production volumes required by the battery industry.
Cost considerations represent a primary barrier to widespread adoption. Fluorinated compounds generally command premium prices due to the complexity of fluorination processes and the high cost of fluorine-containing raw materials. For instance, fluoroethylene carbonate (FEC) and fluorinated ethers can cost 5-10 times more than their non-fluorinated counterparts, significantly impacting the overall cell cost structure. Economic viability requires either cost reduction through process optimization or demonstration of performance benefits that justify the premium.
Safety and environmental concerns also present significant manufacturing challenges. Many fluorination processes involve hazardous reagents such as hydrogen fluoride or sulfur tetrafluoride, requiring specialized containment systems and safety protocols. The potential for hydrogen fluoride generation during processing necessitates robust engineering controls and worker protection measures. Additionally, the environmental impact of fluorinated compound production and disposal must be carefully managed to comply with increasingly stringent regulations worldwide.
Process integration represents another critical consideration. Introducing fluorinated electrolyte components into existing battery manufacturing lines requires validation of compatibility with current mixing, filling, and sealing equipment. Potential issues include material compatibility with pumps and seals, as well as modified viscosity profiles affecting dispensing accuracy. Manufacturers must also consider the stability of these compounds during the entire battery assembly process, including potential degradation during high-temperature electrode drying steps.
Quality control methodologies need significant development for industrial implementation. Analytical techniques must be established to verify the purity and concentration of fluorinated components in electrolyte mixtures at production scale. This includes rapid in-line testing capabilities to ensure batch-to-batch consistency. Furthermore, long-term stability testing protocols must be developed to guarantee that the beneficial SEI-forming properties of these compounds remain effective throughout the battery's intended service life under various operating conditions.
Cost considerations represent a primary barrier to widespread adoption. Fluorinated compounds generally command premium prices due to the complexity of fluorination processes and the high cost of fluorine-containing raw materials. For instance, fluoroethylene carbonate (FEC) and fluorinated ethers can cost 5-10 times more than their non-fluorinated counterparts, significantly impacting the overall cell cost structure. Economic viability requires either cost reduction through process optimization or demonstration of performance benefits that justify the premium.
Safety and environmental concerns also present significant manufacturing challenges. Many fluorination processes involve hazardous reagents such as hydrogen fluoride or sulfur tetrafluoride, requiring specialized containment systems and safety protocols. The potential for hydrogen fluoride generation during processing necessitates robust engineering controls and worker protection measures. Additionally, the environmental impact of fluorinated compound production and disposal must be carefully managed to comply with increasingly stringent regulations worldwide.
Process integration represents another critical consideration. Introducing fluorinated electrolyte components into existing battery manufacturing lines requires validation of compatibility with current mixing, filling, and sealing equipment. Potential issues include material compatibility with pumps and seals, as well as modified viscosity profiles affecting dispensing accuracy. Manufacturers must also consider the stability of these compounds during the entire battery assembly process, including potential degradation during high-temperature electrode drying steps.
Quality control methodologies need significant development for industrial implementation. Analytical techniques must be established to verify the purity and concentration of fluorinated components in electrolyte mixtures at production scale. This includes rapid in-line testing capabilities to ensure batch-to-batch consistency. Furthermore, long-term stability testing protocols must be developed to guarantee that the beneficial SEI-forming properties of these compounds remain effective throughout the battery's intended service life under various operating conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!