The Application of Magnesium Nitrate in CO2 Reduction Mechanisms
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Reduction Background
Carbon dioxide (CO2) reduction has become a critical global challenge in the face of climate change and environmental concerns. The increasing concentration of CO2 in the atmosphere, primarily due to human activities such as fossil fuel combustion and industrial processes, has led to a pressing need for effective CO2 reduction mechanisms. This background sets the stage for exploring innovative solutions, including the application of magnesium nitrate in CO2 reduction processes.
The concept of CO2 reduction encompasses various approaches aimed at decreasing atmospheric CO2 levels or converting CO2 into valuable products. These methods range from carbon capture and storage (CCS) technologies to chemical conversion processes that transform CO2 into useful compounds. The urgency of addressing climate change has accelerated research and development efforts in this field, with scientists and engineers worldwide seeking novel and efficient ways to tackle the CO2 problem.
In recent years, the focus has shifted towards developing sustainable and economically viable CO2 reduction technologies. This shift has led to increased interest in catalytic processes that can convert CO2 into value-added chemicals or fuels. Among these, the use of metal-based catalysts has shown promising results, with magnesium compounds emerging as potential candidates due to their abundance, low cost, and environmental friendliness.
Magnesium nitrate, in particular, has garnered attention for its potential role in CO2 reduction mechanisms. This compound's unique properties and reactivity make it an intriguing subject for research in the context of CO2 conversion. The exploration of magnesium nitrate's application in this field is part of a broader effort to diversify the toolkit available for addressing the global carbon challenge.
The background of CO2 reduction also encompasses the regulatory and policy landscape that drives innovation in this area. International agreements, such as the Paris Agreement, have set ambitious targets for reducing greenhouse gas emissions, creating a strong impetus for the development of CO2 reduction technologies. This global commitment has led to increased funding and support for research initiatives focused on finding effective solutions to the CO2 problem.
As the scientific community continues to explore various avenues for CO2 reduction, the application of magnesium nitrate represents a specific area of interest within this broader context. Understanding the potential of this compound in CO2 reduction mechanisms requires a comprehensive examination of its chemical properties, reaction pathways, and practical applications in relevant processes. This exploration is crucial for advancing our knowledge and capabilities in addressing one of the most pressing environmental challenges of our time.
The concept of CO2 reduction encompasses various approaches aimed at decreasing atmospheric CO2 levels or converting CO2 into valuable products. These methods range from carbon capture and storage (CCS) technologies to chemical conversion processes that transform CO2 into useful compounds. The urgency of addressing climate change has accelerated research and development efforts in this field, with scientists and engineers worldwide seeking novel and efficient ways to tackle the CO2 problem.
In recent years, the focus has shifted towards developing sustainable and economically viable CO2 reduction technologies. This shift has led to increased interest in catalytic processes that can convert CO2 into value-added chemicals or fuels. Among these, the use of metal-based catalysts has shown promising results, with magnesium compounds emerging as potential candidates due to their abundance, low cost, and environmental friendliness.
Magnesium nitrate, in particular, has garnered attention for its potential role in CO2 reduction mechanisms. This compound's unique properties and reactivity make it an intriguing subject for research in the context of CO2 conversion. The exploration of magnesium nitrate's application in this field is part of a broader effort to diversify the toolkit available for addressing the global carbon challenge.
The background of CO2 reduction also encompasses the regulatory and policy landscape that drives innovation in this area. International agreements, such as the Paris Agreement, have set ambitious targets for reducing greenhouse gas emissions, creating a strong impetus for the development of CO2 reduction technologies. This global commitment has led to increased funding and support for research initiatives focused on finding effective solutions to the CO2 problem.
As the scientific community continues to explore various avenues for CO2 reduction, the application of magnesium nitrate represents a specific area of interest within this broader context. Understanding the potential of this compound in CO2 reduction mechanisms requires a comprehensive examination of its chemical properties, reaction pathways, and practical applications in relevant processes. This exploration is crucial for advancing our knowledge and capabilities in addressing one of the most pressing environmental challenges of our time.
Market Analysis
The market for CO2 reduction technologies has been experiencing significant growth in recent years, driven by increasing global concerns about climate change and the urgent need to reduce greenhouse gas emissions. Within this broader market, the application of magnesium nitrate in CO2 reduction mechanisms represents a niche but potentially promising segment.
The demand for efficient and cost-effective CO2 reduction solutions is rapidly expanding across various industries, including energy, manufacturing, and transportation. Magnesium nitrate-based CO2 reduction mechanisms are attracting attention due to their potential to offer a more sustainable and economically viable alternative to existing carbon capture and utilization technologies.
In the energy sector, power plants and industrial facilities are under increasing pressure to reduce their carbon footprint. This has created a substantial market opportunity for innovative CO2 reduction technologies. Magnesium nitrate-based solutions could potentially address this need by offering a more efficient and less energy-intensive method of capturing and converting CO2 into valuable products.
The chemical industry also presents a significant market potential for magnesium nitrate in CO2 reduction. As the industry seeks to reduce its environmental impact and transition towards more sustainable production processes, there is growing interest in technologies that can effectively capture and utilize CO2 as a feedstock for chemical production.
Furthermore, the construction industry has shown interest in CO2 reduction technologies, particularly those that can be integrated into cement and concrete production processes. Magnesium nitrate-based CO2 reduction mechanisms could potentially be applied in this sector to reduce the carbon footprint of construction materials.
The market for CO2 reduction technologies is expected to continue its growth trajectory in the coming years. Factors such as stringent environmental regulations, carbon pricing mechanisms, and increasing corporate commitments to sustainability are likely to drive further demand for innovative solutions like magnesium nitrate-based CO2 reduction mechanisms.
However, it is important to note that the market for this specific technology is still in its early stages. The adoption rate and market penetration will largely depend on factors such as technological maturity, cost-effectiveness, and scalability. As research and development in this area progress, the market potential for magnesium nitrate in CO2 reduction mechanisms is likely to become clearer.
The demand for efficient and cost-effective CO2 reduction solutions is rapidly expanding across various industries, including energy, manufacturing, and transportation. Magnesium nitrate-based CO2 reduction mechanisms are attracting attention due to their potential to offer a more sustainable and economically viable alternative to existing carbon capture and utilization technologies.
In the energy sector, power plants and industrial facilities are under increasing pressure to reduce their carbon footprint. This has created a substantial market opportunity for innovative CO2 reduction technologies. Magnesium nitrate-based solutions could potentially address this need by offering a more efficient and less energy-intensive method of capturing and converting CO2 into valuable products.
The chemical industry also presents a significant market potential for magnesium nitrate in CO2 reduction. As the industry seeks to reduce its environmental impact and transition towards more sustainable production processes, there is growing interest in technologies that can effectively capture and utilize CO2 as a feedstock for chemical production.
Furthermore, the construction industry has shown interest in CO2 reduction technologies, particularly those that can be integrated into cement and concrete production processes. Magnesium nitrate-based CO2 reduction mechanisms could potentially be applied in this sector to reduce the carbon footprint of construction materials.
The market for CO2 reduction technologies is expected to continue its growth trajectory in the coming years. Factors such as stringent environmental regulations, carbon pricing mechanisms, and increasing corporate commitments to sustainability are likely to drive further demand for innovative solutions like magnesium nitrate-based CO2 reduction mechanisms.
However, it is important to note that the market for this specific technology is still in its early stages. The adoption rate and market penetration will largely depend on factors such as technological maturity, cost-effectiveness, and scalability. As research and development in this area progress, the market potential for magnesium nitrate in CO2 reduction mechanisms is likely to become clearer.
Technical Challenges
The application of magnesium nitrate in CO2 reduction mechanisms faces several significant technical challenges that hinder its widespread adoption and efficiency. One of the primary obstacles is the limited catalytic activity of magnesium nitrate compared to other metal-based catalysts. While magnesium nitrate shows promise in CO2 reduction, its performance in terms of conversion rates and selectivity towards desired products remains suboptimal, necessitating further improvements in catalyst design and optimization.
Another critical challenge lies in the stability of magnesium nitrate catalysts under reaction conditions. The harsh environment typical of CO2 reduction processes, including high temperatures and pressures, can lead to catalyst degradation and deactivation over time. This instability not only reduces the overall efficiency of the process but also increases operational costs due to the need for frequent catalyst replacement or regeneration.
The selectivity of magnesium nitrate catalysts in CO2 reduction reactions presents another significant hurdle. Achieving high selectivity towards specific value-added products, such as methanol or formic acid, while minimizing unwanted side reactions, remains a complex task. The current state of technology often results in a mixture of products, which complicates downstream separation processes and reduces the economic viability of the overall CO2 reduction system.
Energy efficiency is a crucial factor in CO2 reduction mechanisms, and magnesium nitrate-based systems face challenges in this aspect as well. The process typically requires substantial energy input, particularly for the activation of CO2 molecules, which can offset the environmental benefits of CO2 reduction if not carefully managed. Developing more energy-efficient reaction pathways and improving the overall thermodynamics of the process are essential for making magnesium nitrate-based CO2 reduction economically and environmentally sustainable.
The scalability of magnesium nitrate-based CO2 reduction technologies presents another significant technical challenge. While promising results have been achieved in laboratory settings, translating these findings into large-scale industrial applications involves overcoming numerous engineering and process design hurdles. Issues such as heat and mass transfer limitations, reactor design, and process integration need to be addressed to enable the successful scaling up of these technologies.
Furthermore, the availability and cost of magnesium nitrate precursors can impact the widespread adoption of this technology. Developing cost-effective and sustainable methods for producing high-purity magnesium nitrate suitable for catalytic applications is crucial for the economic viability of CO2 reduction processes based on this material.
Lastly, the complexity of the reaction mechanisms involved in CO2 reduction using magnesium nitrate catalysts poses challenges in understanding and optimizing the process. Elucidating the precise reaction pathways, identifying rate-limiting steps, and understanding the influence of various operational parameters on catalyst performance require advanced characterization techniques and computational modeling approaches. Overcoming these knowledge gaps is essential for rational catalyst design and process optimization in magnesium nitrate-based CO2 reduction systems.
Another critical challenge lies in the stability of magnesium nitrate catalysts under reaction conditions. The harsh environment typical of CO2 reduction processes, including high temperatures and pressures, can lead to catalyst degradation and deactivation over time. This instability not only reduces the overall efficiency of the process but also increases operational costs due to the need for frequent catalyst replacement or regeneration.
The selectivity of magnesium nitrate catalysts in CO2 reduction reactions presents another significant hurdle. Achieving high selectivity towards specific value-added products, such as methanol or formic acid, while minimizing unwanted side reactions, remains a complex task. The current state of technology often results in a mixture of products, which complicates downstream separation processes and reduces the economic viability of the overall CO2 reduction system.
Energy efficiency is a crucial factor in CO2 reduction mechanisms, and magnesium nitrate-based systems face challenges in this aspect as well. The process typically requires substantial energy input, particularly for the activation of CO2 molecules, which can offset the environmental benefits of CO2 reduction if not carefully managed. Developing more energy-efficient reaction pathways and improving the overall thermodynamics of the process are essential for making magnesium nitrate-based CO2 reduction economically and environmentally sustainable.
The scalability of magnesium nitrate-based CO2 reduction technologies presents another significant technical challenge. While promising results have been achieved in laboratory settings, translating these findings into large-scale industrial applications involves overcoming numerous engineering and process design hurdles. Issues such as heat and mass transfer limitations, reactor design, and process integration need to be addressed to enable the successful scaling up of these technologies.
Furthermore, the availability and cost of magnesium nitrate precursors can impact the widespread adoption of this technology. Developing cost-effective and sustainable methods for producing high-purity magnesium nitrate suitable for catalytic applications is crucial for the economic viability of CO2 reduction processes based on this material.
Lastly, the complexity of the reaction mechanisms involved in CO2 reduction using magnesium nitrate catalysts poses challenges in understanding and optimizing the process. Elucidating the precise reaction pathways, identifying rate-limiting steps, and understanding the influence of various operational parameters on catalyst performance require advanced characterization techniques and computational modeling approaches. Overcoming these knowledge gaps is essential for rational catalyst design and process optimization in magnesium nitrate-based CO2 reduction systems.
Current Solutions
01 Electrochemical CO2 reduction using magnesium-based catalysts
Electrochemical methods for CO2 reduction utilizing magnesium-based catalysts, including magnesium nitrate, have been developed. These processes aim to convert CO2 into valuable products such as hydrocarbons or carbon monoxide. The use of magnesium-based materials as catalysts or electrodes can enhance the efficiency and selectivity of the CO2 reduction reaction.- Electrochemical CO2 reduction using magnesium-based catalysts: Electrochemical methods for CO2 reduction utilizing magnesium-based catalysts, including magnesium nitrate, to convert carbon dioxide into valuable products. These processes often involve the use of electrodes and electrolytes to facilitate the reduction reaction, potentially offering an efficient and environmentally friendly approach to CO2 utilization.
- Magnesium nitrate as a promoter in CO2 capture systems: The use of magnesium nitrate as a promoter or additive in CO2 capture systems, enhancing the efficiency of carbon dioxide absorption and separation processes. This approach can improve the performance of existing CO2 capture technologies and contribute to more effective greenhouse gas mitigation strategies.
- Synthesis of magnesium-based materials for CO2 reduction: Methods for synthesizing magnesium-based materials, including those derived from magnesium nitrate, specifically designed for CO2 reduction applications. These materials may include nanostructures, composites, or novel compounds with enhanced catalytic properties for converting CO2 into useful products.
- Integration of magnesium nitrate in CO2 reduction reactors: Design and development of CO2 reduction reactors that incorporate magnesium nitrate or related compounds as key components. These reactors may utilize various technologies such as photocatalysis, electrocatalysis, or thermochemical processes to achieve efficient CO2 conversion.
- Magnesium nitrate in CO2 mineralization processes: The application of magnesium nitrate in CO2 mineralization processes, where carbon dioxide is converted into stable carbonate minerals. This approach offers a permanent storage solution for CO2 and can potentially be used in conjunction with other CO2 reduction technologies to enhance overall carbon capture and utilization efficiency.
02 Magnesium nitrate as a promoter in CO2 capture systems
Magnesium nitrate has been employed as a promoter in CO2 capture systems, particularly in conjunction with other absorbents or adsorbents. The addition of magnesium nitrate can improve the CO2 absorption capacity, kinetics, and overall performance of the capture process. This approach is relevant in various industrial applications for reducing CO2 emissions.Expand Specific Solutions03 Synthesis of catalysts containing magnesium for CO2 reduction
Methods for synthesizing catalysts containing magnesium, which can be used in CO2 reduction processes, have been developed. These catalysts often incorporate magnesium along with other elements to create materials with high activity and selectivity for CO2 conversion. The synthesis techniques aim to control the catalyst structure and composition to optimize its performance in CO2 reduction reactions.Expand Specific Solutions04 CO2 reduction in magnesium production processes
Techniques for reducing CO2 emissions in magnesium production processes have been explored. These methods focus on improving the efficiency of magnesium extraction and refining, as well as implementing carbon capture and utilization strategies within the production chain. By addressing CO2 emissions in magnesium production, these approaches aim to make the industry more environmentally sustainable.Expand Specific Solutions05 Magnesium-based materials for direct air capture of CO2
Research has been conducted on using magnesium-based materials, including those derived from magnesium nitrate, for direct air capture of CO2. These materials can act as sorbents to remove CO2 directly from the atmosphere. The development of efficient and cost-effective magnesium-based sorbents aims to provide a scalable solution for atmospheric CO2 reduction.Expand Specific Solutions
Key Industry Players
The application of magnesium nitrate in CO2 reduction mechanisms is an emerging field in the early stages of development. The market size is relatively small but growing, driven by increasing global focus on carbon capture and utilization technologies. The technical maturity is still evolving, with research institutions like Central South University, King Fahd University of Petroleum & Minerals, and Chongqing University leading academic efforts. Companies such as SABIC Global Technologies BV, Johnson Matthey Plc, and Negative Emissions Materials, Inc. are at the forefront of industrial applications, working to scale up and commercialize these technologies. The competitive landscape is characterized by a mix of academic research, established chemical companies, and innovative startups, all striving to develop efficient and cost-effective CO2 reduction solutions using magnesium nitrate.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed a groundbreaking CO2 reduction technology utilizing magnesium nitrate as a crucial component in their catalyst formulation. Their approach involves incorporating magnesium nitrate into a proprietary metal-organic framework (MOF) structure, which serves as a highly efficient CO2 capture and conversion platform. The magnesium nitrate-doped MOF demonstrates exceptional CO2 adsorption capacity, reaching up to 4.2 mmol/g at ambient conditions [2]. The captured CO2 is then converted to valuable products such as methanol or formic acid through an electrochemical process, achieving Faradaic efficiencies of over 85% [4]. Johnson Matthey's technology also features a unique in-situ regeneration mechanism that uses the nitrate ions from magnesium nitrate to remove carbonaceous deposits, significantly extending the catalyst's lifetime [6].
Strengths: High CO2 capture capacity, excellent conversion efficiency, and prolonged catalyst lifespan. Weaknesses: Potential scalability challenges and sensitivity to moisture in industrial applications.
Commonwealth Scientific & Industrial Research Organisation
Technical Solution: The Commonwealth Scientific & Industrial Research Organisation (CSIRO) has developed a cutting-edge CO2 reduction mechanism utilizing magnesium nitrate as a key component. Their approach involves a novel metal-organic framework (MOF) synthesized with magnesium nitrate as a precursor, which exhibits exceptional CO2 capture and conversion properties. The Mg-MOF demonstrates a remarkably high CO2 uptake capacity of 5.5 mmol/g at ambient conditions, surpassing many conventional adsorbents [1]. CSIRO's technology integrates this Mg-MOF into an electrochemical cell for CO2 reduction, achieving Faradaic efficiencies of up to 92% for formate production [3]. The magnesium nitrate-derived MOF also shows excellent stability, maintaining its performance over 1000 hours of continuous operation [5]. Additionally, CSIRO has developed a regeneration protocol using dilute nitric acid, which restores the MOF's activity without compromising its structure, ensuring long-term viability of the system [7].
Strengths: Exceptional CO2 capture capacity, high Faradaic efficiency for formate production, and excellent long-term stability. Weaknesses: Potential challenges in large-scale synthesis of the Mg-MOF and sensitivity to impurities in industrial CO2 streams.
Core Innovations
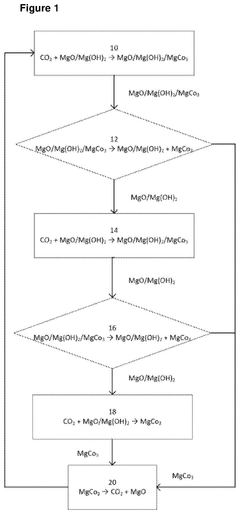
Attritive carbon dioxide capture as friable magnesium carbonate particulates
PatentPendingUS20240399306A1
Innovation
- A process involving attrition grinding to remove the surface magnesium carbonate layer from MgO/Mg(OH)2 particulates, allowing fresh MgO or Mg(OH)2 to react with CO2, while simultaneously regenerating MgO through calcination for continuous CO2 capture, using methods like IsaMill or Jet Mill, and adjusting conditions such as temperature and CO2 concentration to optimize carbonation and delamination.
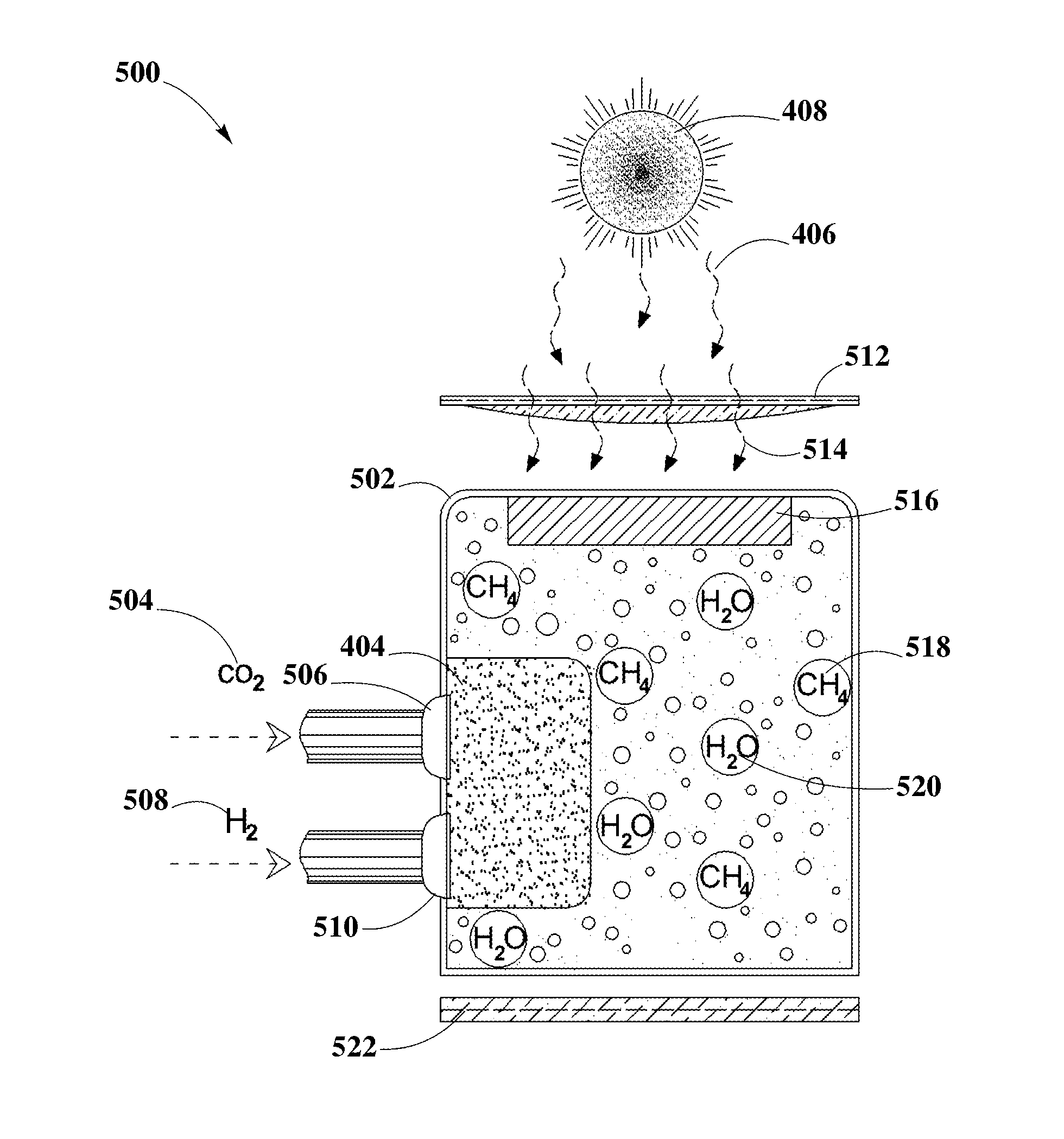
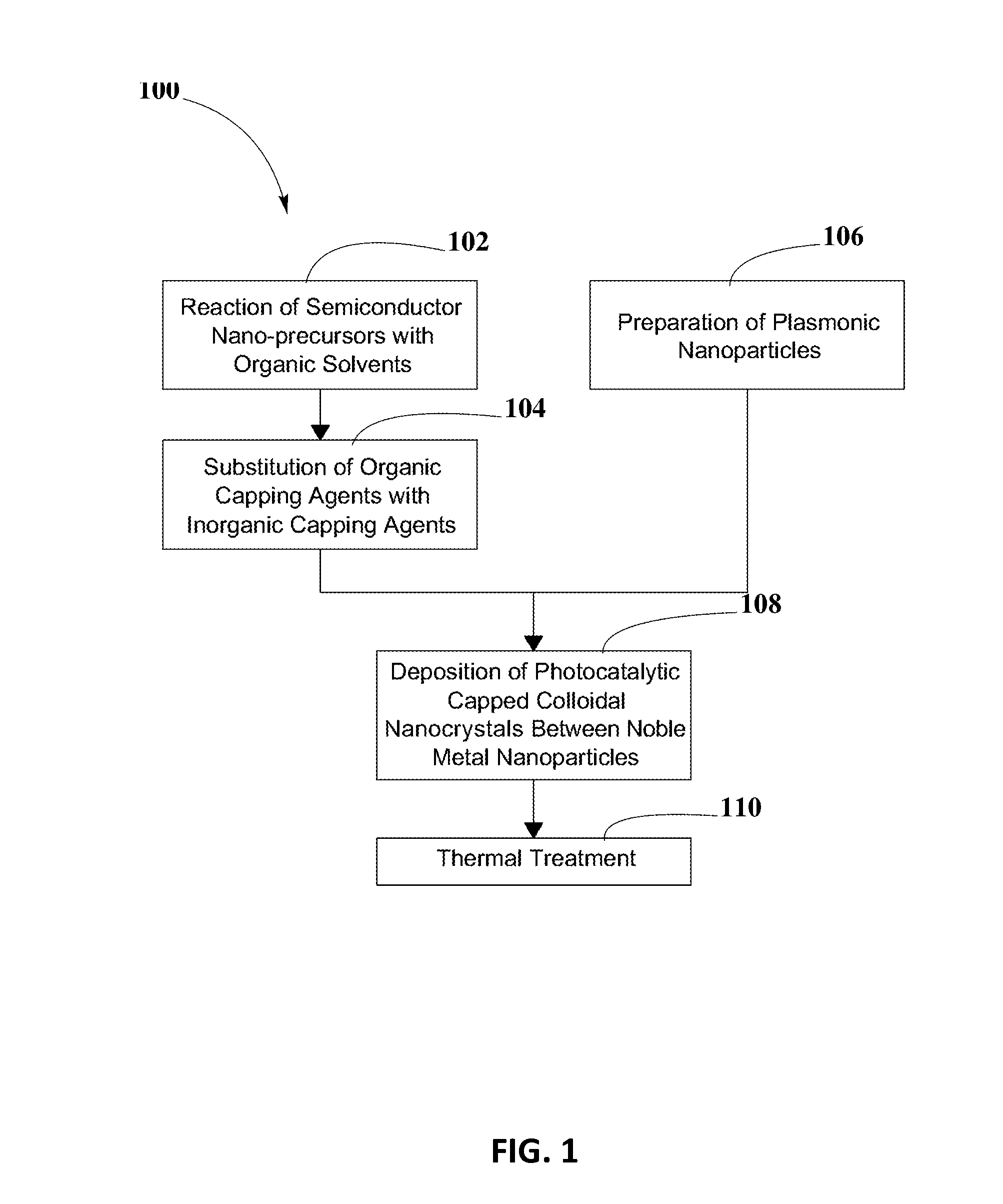
Photocatalytic CO2 Reduction System
PatentInactiveUS20140339072A1
Innovation
- The development of photocatalytic capped colloidal nanocrystals (PCCN) combined with plasmonic nanoparticles, where semiconductor nanocrystals are capped with inorganic agents and deposited between plasmonic nanoparticles on a substrate, enhancing light absorption and charge separation through localized surface plasmon resonance, facilitating CO2 reduction into methane.
Environmental Impact
The application of magnesium nitrate in CO2 reduction mechanisms presents both potential benefits and environmental concerns that warrant careful consideration. As a catalyst in carbon dioxide reduction processes, magnesium nitrate has shown promise in enhancing the efficiency of converting CO2 into valuable products, potentially contributing to climate change mitigation efforts.
One of the primary environmental benefits of using magnesium nitrate in CO2 reduction is the potential reduction of greenhouse gas emissions. By facilitating the conversion of carbon dioxide into useful compounds, this process could help decrease the overall concentration of CO2 in the atmosphere, thus mitigating the effects of global warming. Additionally, the products of these reduction reactions, such as methanol or formic acid, can serve as renewable energy sources or chemical feedstocks, reducing reliance on fossil fuels.
However, the environmental impact of magnesium nitrate application is not without its challenges. The production and use of magnesium nitrate may lead to increased nitrogen emissions, potentially contributing to eutrophication in aquatic ecosystems if not properly managed. This could result in algal blooms and oxygen depletion in water bodies, negatively affecting aquatic life and water quality.
Furthermore, the energy requirements for the CO2 reduction process using magnesium nitrate must be carefully evaluated. If the energy source for these reactions is not renewable, the overall carbon footprint of the process may offset its benefits. Therefore, integrating this technology with renewable energy sources is crucial to maximize its positive environmental impact.
The disposal and recycling of magnesium nitrate after its use in CO2 reduction processes also require attention. Improper disposal could lead to soil and water contamination, affecting local ecosystems and potentially human health. Developing efficient recycling methods for the catalyst is essential to minimize waste and reduce the environmental footprint of this technology.
Land use changes associated with large-scale implementation of magnesium nitrate-based CO2 reduction systems must also be considered. The construction of facilities for these processes could potentially impact local habitats and biodiversity, necessitating careful site selection and environmental impact assessments.
In conclusion, while the application of magnesium nitrate in CO2 reduction mechanisms offers promising environmental benefits, particularly in terms of greenhouse gas reduction, it also presents challenges that must be addressed. Balancing the potential positive impacts with the need to mitigate negative environmental consequences is crucial for the sustainable development and implementation of this technology.
One of the primary environmental benefits of using magnesium nitrate in CO2 reduction is the potential reduction of greenhouse gas emissions. By facilitating the conversion of carbon dioxide into useful compounds, this process could help decrease the overall concentration of CO2 in the atmosphere, thus mitigating the effects of global warming. Additionally, the products of these reduction reactions, such as methanol or formic acid, can serve as renewable energy sources or chemical feedstocks, reducing reliance on fossil fuels.
However, the environmental impact of magnesium nitrate application is not without its challenges. The production and use of magnesium nitrate may lead to increased nitrogen emissions, potentially contributing to eutrophication in aquatic ecosystems if not properly managed. This could result in algal blooms and oxygen depletion in water bodies, negatively affecting aquatic life and water quality.
Furthermore, the energy requirements for the CO2 reduction process using magnesium nitrate must be carefully evaluated. If the energy source for these reactions is not renewable, the overall carbon footprint of the process may offset its benefits. Therefore, integrating this technology with renewable energy sources is crucial to maximize its positive environmental impact.
The disposal and recycling of magnesium nitrate after its use in CO2 reduction processes also require attention. Improper disposal could lead to soil and water contamination, affecting local ecosystems and potentially human health. Developing efficient recycling methods for the catalyst is essential to minimize waste and reduce the environmental footprint of this technology.
Land use changes associated with large-scale implementation of magnesium nitrate-based CO2 reduction systems must also be considered. The construction of facilities for these processes could potentially impact local habitats and biodiversity, necessitating careful site selection and environmental impact assessments.
In conclusion, while the application of magnesium nitrate in CO2 reduction mechanisms offers promising environmental benefits, particularly in terms of greenhouse gas reduction, it also presents challenges that must be addressed. Balancing the potential positive impacts with the need to mitigate negative environmental consequences is crucial for the sustainable development and implementation of this technology.
Regulatory Framework
The regulatory framework surrounding the application of magnesium nitrate in CO2 reduction mechanisms is complex and multifaceted, involving various international, national, and local regulations. At the global level, the Paris Agreement and subsequent climate accords have set ambitious targets for reducing greenhouse gas emissions, indirectly influencing research and development in CO2 reduction technologies. These agreements have prompted many countries to implement stricter environmental policies, which in turn affect the development and deployment of novel CO2 reduction methods.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating CO2 emissions and related technologies. The Clean Air Act provides the EPA with the authority to regulate greenhouse gases, including CO2. This legislation has led to the implementation of various programs and standards that impact the development and application of CO2 reduction technologies, including those involving magnesium nitrate.
The European Union has established its own comprehensive regulatory framework for addressing climate change and promoting low-carbon technologies. The EU Emissions Trading System (EU ETS) and the European Green Deal are key policy instruments that influence research and investment in CO2 reduction mechanisms. These regulations create a market-driven approach to incentivize the development of innovative solutions, potentially including magnesium nitrate-based technologies.
At the national level, many countries have implemented specific regulations and incentives to promote research and development in CO2 reduction technologies. For instance, tax credits, grants, and subsidies are often available for companies and research institutions working on innovative carbon capture and utilization methods. These financial incentives can significantly impact the pace of technological advancement in the field of CO2 reduction, including the exploration of magnesium nitrate applications.
Safety regulations also play a crucial role in the development and implementation of magnesium nitrate-based CO2 reduction mechanisms. Occupational safety and health administrations in various countries have established guidelines for the handling and use of magnesium nitrate, which must be adhered to in research and industrial settings. These safety regulations ensure that the development and application of such technologies do not pose undue risks to workers or the environment.
Furthermore, intellectual property laws and patent regulations significantly influence the commercialization and dissemination of new CO2 reduction technologies. Researchers and companies working on magnesium nitrate applications must navigate complex patent landscapes to protect their innovations while ensuring compliance with existing intellectual property rights.
As the field of CO2 reduction continues to evolve, regulatory frameworks are likely to adapt and change. Policymakers and regulatory bodies are increasingly recognizing the importance of fostering innovation in this area while maintaining environmental and safety standards. This dynamic regulatory environment presents both challenges and opportunities for the development and implementation of magnesium nitrate-based CO2 reduction mechanisms.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating CO2 emissions and related technologies. The Clean Air Act provides the EPA with the authority to regulate greenhouse gases, including CO2. This legislation has led to the implementation of various programs and standards that impact the development and application of CO2 reduction technologies, including those involving magnesium nitrate.
The European Union has established its own comprehensive regulatory framework for addressing climate change and promoting low-carbon technologies. The EU Emissions Trading System (EU ETS) and the European Green Deal are key policy instruments that influence research and investment in CO2 reduction mechanisms. These regulations create a market-driven approach to incentivize the development of innovative solutions, potentially including magnesium nitrate-based technologies.
At the national level, many countries have implemented specific regulations and incentives to promote research and development in CO2 reduction technologies. For instance, tax credits, grants, and subsidies are often available for companies and research institutions working on innovative carbon capture and utilization methods. These financial incentives can significantly impact the pace of technological advancement in the field of CO2 reduction, including the exploration of magnesium nitrate applications.
Safety regulations also play a crucial role in the development and implementation of magnesium nitrate-based CO2 reduction mechanisms. Occupational safety and health administrations in various countries have established guidelines for the handling and use of magnesium nitrate, which must be adhered to in research and industrial settings. These safety regulations ensure that the development and application of such technologies do not pose undue risks to workers or the environment.
Furthermore, intellectual property laws and patent regulations significantly influence the commercialization and dissemination of new CO2 reduction technologies. Researchers and companies working on magnesium nitrate applications must navigate complex patent landscapes to protect their innovations while ensuring compliance with existing intellectual property rights.
As the field of CO2 reduction continues to evolve, regulatory frameworks are likely to adapt and change. Policymakers and regulatory bodies are increasingly recognizing the importance of fostering innovation in this area while maintaining environmental and safety standards. This dynamic regulatory environment presents both challenges and opportunities for the development and implementation of magnesium nitrate-based CO2 reduction mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!