The Effect of Magnesium Nitrate on Vitamin Synthesis Pathways
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate and Vitamin Synthesis Overview
Magnesium nitrate and vitamin synthesis pathways represent a fascinating intersection of inorganic chemistry and biochemistry. This overview explores the intricate relationship between these two seemingly disparate areas of study, shedding light on the potential effects of magnesium nitrate on vital metabolic processes.
Magnesium nitrate, a compound with the chemical formula Mg(NO3)2, is an inorganic salt widely used in various industrial and agricultural applications. Its significance in biological systems, particularly in vitamin synthesis, has garnered increasing attention from researchers in recent years. The compound's unique properties, stemming from the combination of magnesium ions and nitrate anions, contribute to its potential influence on biochemical pathways.
Vitamins are essential organic compounds that play crucial roles in numerous physiological processes. Their synthesis involves complex biochemical pathways that are often dependent on various cofactors and enzymes. The presence of magnesium ions, derived from magnesium nitrate, can potentially modulate these pathways through several mechanisms.
One key aspect of magnesium's involvement in vitamin synthesis is its role as a cofactor for many enzymes. Magnesium ions are known to activate numerous enzymes involved in various metabolic processes, including those related to vitamin production. This activation can lead to enhanced enzymatic activity, potentially accelerating or optimizing certain steps in vitamin synthesis pathways.
Furthermore, magnesium plays a critical role in maintaining the structural integrity of nucleic acids, including DNA and RNA. This function is particularly relevant to vitamin synthesis, as many vitamins are produced through gene expression and subsequent protein synthesis. The presence of adequate magnesium levels, potentially supplemented by magnesium nitrate, may contribute to the stability and efficiency of these genetic processes.
The nitrate component of magnesium nitrate also warrants consideration in the context of vitamin synthesis. Nitrate ions can serve as a source of nitrogen, an essential element in the structure of many vitamins. While the direct incorporation of nitrate into vitamin molecules is not common, its presence may influence nitrogen metabolism in cells, indirectly affecting vitamin production.
It is important to note that the effects of magnesium nitrate on vitamin synthesis pathways are likely to be complex and multifaceted. The compound's influence may vary depending on factors such as concentration, cellular environment, and the specific vitamin pathway in question. Additionally, potential interactions with other minerals and cofactors must be considered when evaluating the overall impact of magnesium nitrate on vitamin synthesis.
Magnesium nitrate, a compound with the chemical formula Mg(NO3)2, is an inorganic salt widely used in various industrial and agricultural applications. Its significance in biological systems, particularly in vitamin synthesis, has garnered increasing attention from researchers in recent years. The compound's unique properties, stemming from the combination of magnesium ions and nitrate anions, contribute to its potential influence on biochemical pathways.
Vitamins are essential organic compounds that play crucial roles in numerous physiological processes. Their synthesis involves complex biochemical pathways that are often dependent on various cofactors and enzymes. The presence of magnesium ions, derived from magnesium nitrate, can potentially modulate these pathways through several mechanisms.
One key aspect of magnesium's involvement in vitamin synthesis is its role as a cofactor for many enzymes. Magnesium ions are known to activate numerous enzymes involved in various metabolic processes, including those related to vitamin production. This activation can lead to enhanced enzymatic activity, potentially accelerating or optimizing certain steps in vitamin synthesis pathways.
Furthermore, magnesium plays a critical role in maintaining the structural integrity of nucleic acids, including DNA and RNA. This function is particularly relevant to vitamin synthesis, as many vitamins are produced through gene expression and subsequent protein synthesis. The presence of adequate magnesium levels, potentially supplemented by magnesium nitrate, may contribute to the stability and efficiency of these genetic processes.
The nitrate component of magnesium nitrate also warrants consideration in the context of vitamin synthesis. Nitrate ions can serve as a source of nitrogen, an essential element in the structure of many vitamins. While the direct incorporation of nitrate into vitamin molecules is not common, its presence may influence nitrogen metabolism in cells, indirectly affecting vitamin production.
It is important to note that the effects of magnesium nitrate on vitamin synthesis pathways are likely to be complex and multifaceted. The compound's influence may vary depending on factors such as concentration, cellular environment, and the specific vitamin pathway in question. Additionally, potential interactions with other minerals and cofactors must be considered when evaluating the overall impact of magnesium nitrate on vitamin synthesis.
Market Analysis for Vitamin Supplements
The vitamin supplement market has experienced significant growth in recent years, driven by increasing health consciousness and a growing aging population. The global vitamin supplement market was valued at approximately $50 billion in 2020 and is projected to reach $71 billion by 2028, with a compound annual growth rate (CAGR) of 5.3% during the forecast period.
North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to high consumer awareness and disposable income. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years.
The market is segmented by type, including multivitamins, single vitamins, and vitamin complexes. Multivitamins account for the largest market share, as they offer a comprehensive solution for consumers seeking overall health benefits. Among single vitamins, Vitamin D and Vitamin B complex supplements have seen a surge in demand, partly due to increased awareness of their role in immune function and energy metabolism.
The COVID-19 pandemic has further accelerated market growth, with consumers increasingly turning to vitamin supplements to boost their immune systems. This trend is expected to continue in the post-pandemic era, as health and wellness remain top priorities for consumers globally.
Key market players include Bayer AG, Pfizer Inc., Koninklijke DSM N.V., and Amway Corporation. These companies are focusing on product innovation, expanding their product portfolios, and strategic partnerships to maintain their market positions. The market is also witnessing a rise in direct-to-consumer brands and online sales channels, which are reshaping traditional distribution models.
Consumer preferences are shifting towards natural and organic vitamin supplements, driving demand for plant-based and whole food-derived products. Additionally, there is a growing interest in personalized nutrition, with companies offering customized vitamin supplement plans based on individual health profiles and genetic testing.
Regulatory factors play a crucial role in shaping the market landscape. Stringent regulations in developed markets ensure product safety and efficacy, while emerging markets are gradually strengthening their regulatory frameworks. This regulatory environment presents both challenges and opportunities for market players in terms of product development and market entry strategies.
North America currently holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, dominates the market due to high consumer awareness and disposable income. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years.
The market is segmented by type, including multivitamins, single vitamins, and vitamin complexes. Multivitamins account for the largest market share, as they offer a comprehensive solution for consumers seeking overall health benefits. Among single vitamins, Vitamin D and Vitamin B complex supplements have seen a surge in demand, partly due to increased awareness of their role in immune function and energy metabolism.
The COVID-19 pandemic has further accelerated market growth, with consumers increasingly turning to vitamin supplements to boost their immune systems. This trend is expected to continue in the post-pandemic era, as health and wellness remain top priorities for consumers globally.
Key market players include Bayer AG, Pfizer Inc., Koninklijke DSM N.V., and Amway Corporation. These companies are focusing on product innovation, expanding their product portfolios, and strategic partnerships to maintain their market positions. The market is also witnessing a rise in direct-to-consumer brands and online sales channels, which are reshaping traditional distribution models.
Consumer preferences are shifting towards natural and organic vitamin supplements, driving demand for plant-based and whole food-derived products. Additionally, there is a growing interest in personalized nutrition, with companies offering customized vitamin supplement plans based on individual health profiles and genetic testing.
Regulatory factors play a crucial role in shaping the market landscape. Stringent regulations in developed markets ensure product safety and efficacy, while emerging markets are gradually strengthening their regulatory frameworks. This regulatory environment presents both challenges and opportunities for market players in terms of product development and market entry strategies.
Current Challenges in Vitamin Synthesis
The synthesis of vitamins faces several significant challenges in the current scientific and industrial landscape. One of the primary obstacles is the complexity of vitamin molecular structures, which often require multi-step synthesis processes. These intricate pathways are susceptible to inefficiencies and yield losses at various stages, making large-scale production both technically demanding and economically challenging.
Another major hurdle is the sensitivity of vitamins to environmental factors such as light, heat, and oxidation. This instability necessitates careful handling and storage procedures throughout the synthesis process, as well as in the final product formulation. The need for stringent quality control measures adds to the overall complexity and cost of vitamin production.
The sourcing of precursor molecules and catalysts presents another significant challenge. Many vitamin synthesis pathways rely on specific, often rare or expensive, starting materials. The availability and cost of these precursors can fluctuate, impacting the consistency and economic viability of vitamin production. Additionally, the use of certain catalysts, particularly those containing precious metals, contributes to the high cost of synthesis and raises sustainability concerns.
Environmental and regulatory pressures also pose challenges to vitamin synthesis. There is a growing demand for more sustainable and "green" production methods, which often require substantial research and development investments. Regulatory bodies are increasingly scrutinizing the safety and environmental impact of synthesis processes, necessitating continuous adaptation and improvement of existing methods.
The effect of magnesium nitrate on vitamin synthesis pathways introduces additional complexities. While magnesium is known to play crucial roles in many enzymatic reactions, including those involved in vitamin biosynthesis, the presence of nitrate can potentially interfere with certain redox-sensitive steps in vitamin synthesis. This interaction may lead to unexpected side reactions or alterations in reaction kinetics, requiring careful optimization of synthesis conditions.
Furthermore, the integration of magnesium nitrate into vitamin synthesis processes may necessitate modifications to existing industrial setups. This could involve changes in equipment materials to prevent corrosion, adjustments to pH control systems, and alterations in purification procedures to ensure the removal of excess magnesium and nitrate ions from the final product.
Lastly, the challenge of scalability remains paramount. While laboratory-scale synthesis of vitamins with the incorporation of magnesium nitrate may yield promising results, translating these processes to industrial-scale production presents its own set of challenges. These include maintaining reaction efficiency, ensuring product consistency, and managing the increased complexity of large-scale operations involving potentially reactive compounds like magnesium nitrate.
Another major hurdle is the sensitivity of vitamins to environmental factors such as light, heat, and oxidation. This instability necessitates careful handling and storage procedures throughout the synthesis process, as well as in the final product formulation. The need for stringent quality control measures adds to the overall complexity and cost of vitamin production.
The sourcing of precursor molecules and catalysts presents another significant challenge. Many vitamin synthesis pathways rely on specific, often rare or expensive, starting materials. The availability and cost of these precursors can fluctuate, impacting the consistency and economic viability of vitamin production. Additionally, the use of certain catalysts, particularly those containing precious metals, contributes to the high cost of synthesis and raises sustainability concerns.
Environmental and regulatory pressures also pose challenges to vitamin synthesis. There is a growing demand for more sustainable and "green" production methods, which often require substantial research and development investments. Regulatory bodies are increasingly scrutinizing the safety and environmental impact of synthesis processes, necessitating continuous adaptation and improvement of existing methods.
The effect of magnesium nitrate on vitamin synthesis pathways introduces additional complexities. While magnesium is known to play crucial roles in many enzymatic reactions, including those involved in vitamin biosynthesis, the presence of nitrate can potentially interfere with certain redox-sensitive steps in vitamin synthesis. This interaction may lead to unexpected side reactions or alterations in reaction kinetics, requiring careful optimization of synthesis conditions.
Furthermore, the integration of magnesium nitrate into vitamin synthesis processes may necessitate modifications to existing industrial setups. This could involve changes in equipment materials to prevent corrosion, adjustments to pH control systems, and alterations in purification procedures to ensure the removal of excess magnesium and nitrate ions from the final product.
Lastly, the challenge of scalability remains paramount. While laboratory-scale synthesis of vitamins with the incorporation of magnesium nitrate may yield promising results, translating these processes to industrial-scale production presents its own set of challenges. These include maintaining reaction efficiency, ensuring product consistency, and managing the increased complexity of large-scale operations involving potentially reactive compounds like magnesium nitrate.
Existing Magnesium Nitrate Applications
01 Magnesium nitrate as a catalyst in vitamin synthesis
Magnesium nitrate can be used as a catalyst in various vitamin synthesis pathways. It helps to accelerate reactions and improve yields in the production of certain vitamins. The presence of magnesium ions can facilitate electron transfer and stabilize intermediate compounds during the synthesis process.- Magnesium nitrate as a precursor in vitamin synthesis: Magnesium nitrate serves as an important precursor in the synthesis of various vitamins. It provides a source of magnesium, which is essential for many enzymatic reactions involved in vitamin production. The use of magnesium nitrate in vitamin synthesis pathways can enhance the efficiency and yield of the process.
- Vitamin B complex synthesis using magnesium nitrate: Magnesium nitrate plays a crucial role in the synthesis of vitamin B complex, particularly in the production of vitamin B1 (thiamine) and vitamin B6 (pyridoxine). The compound acts as a catalyst and cofactor in several enzymatic reactions, facilitating the formation of these essential vitamins.
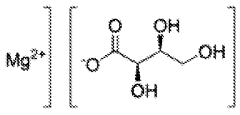
- Magnesium nitrate in vitamin C synthesis pathways: The synthesis of vitamin C (ascorbic acid) involves magnesium nitrate as a key component. It participates in the oxidation and reduction reactions necessary for the formation of ascorbic acid. The presence of magnesium nitrate can improve the overall yield and purity of the synthesized vitamin C.
- Role of magnesium nitrate in fat-soluble vitamin synthesis: Magnesium nitrate contributes to the synthesis of fat-soluble vitamins, such as vitamins A, D, E, and K. It acts as a catalyst in various stages of the synthesis process, including isomerization and oxidation reactions. The compound's involvement can lead to more efficient production of these essential vitamins.
- Magnesium nitrate in novel vitamin synthesis techniques: Recent advancements in vitamin synthesis techniques incorporate magnesium nitrate in innovative ways. These methods include the use of magnesium nitrate in microfluidic systems, enzymatic cascades, and green chemistry approaches. Such novel techniques aim to improve the sustainability and efficiency of vitamin production processes.
02 Magnesium nitrate in vitamin B complex synthesis
Magnesium nitrate plays a role in the synthesis pathways of vitamin B complex. It can be used as a source of magnesium ions, which are essential cofactors for enzymes involved in the biosynthesis of various B vitamins. The compound may also help in maintaining optimal pH conditions during the synthesis process.Expand Specific Solutions03 Magnesium nitrate in vitamin C synthesis
In the synthesis of vitamin C (ascorbic acid), magnesium nitrate can be utilized as a reagent or catalyst. It may participate in oxidation-reduction reactions or serve as a source of nitrate ions, which are involved in certain steps of the vitamin C synthesis pathway. The compound can help improve the efficiency and yield of the production process.Expand Specific Solutions04 Magnesium nitrate in fat-soluble vitamin synthesis
Magnesium nitrate has applications in the synthesis pathways of fat-soluble vitamins such as vitamins A, D, E, and K. It can act as a catalyst or reagent in various stages of the synthesis process. The compound may assist in isomerization reactions or participate in the formation of intermediate compounds during the production of these vitamins.Expand Specific Solutions05 Magnesium nitrate in vitamin fortification processes
Magnesium nitrate can be used in vitamin fortification processes, where it may serve as a source of both magnesium and nitrate ions. These ions can be incorporated into various food products or supplements to enhance their nutritional value. The compound may also help in stabilizing certain vitamins during the fortification process, improving their shelf life and bioavailability.Expand Specific Solutions
Key Players in Vitamin Manufacturing
The research on "The Effect of Magnesium Nitrate on Vitamin Synthesis Pathways" is in its early stages, with the market still developing. The competitive landscape is characterized by a mix of academic institutions, biotechnology companies, and pharmaceutical firms. Key players like ChromaDex, Seagen, and Cytokinetics are exploring potential applications, while universities such as Cornell, Dartmouth, and Tsinghua are contributing to fundamental research. The technology's maturity is relatively low, with most efforts focused on understanding the underlying mechanisms and potential therapeutic applications. As the field progresses, collaborations between industry and academia are likely to drive innovation and market growth.
ChromaDex, Inc.
Technical Solution: ChromaDex has developed a proprietary technology platform for the identification, isolation, and analysis of natural product-based compounds, including those involved in vitamin synthesis pathways. Their approach involves using advanced analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry to study the effects of magnesium nitrate on vitamin synthesis[1]. They have also explored the impact of magnesium nitrate on NAD+ precursors, which play a crucial role in cellular energy metabolism and vitamin B3 synthesis[2]. ChromaDex's research has shown that magnesium nitrate can potentially enhance the bioavailability of certain vitamins, particularly those in the B-complex family[3].
Strengths: Extensive experience in natural product research, proprietary analytical techniques, and a focus on vitamin metabolism. Weaknesses: Limited public data on specific magnesium nitrate effects, potential bias towards their own supplement products.
BASF Corp.
Technical Solution: BASF Corp. has conducted extensive research on the influence of magnesium nitrate on vitamin synthesis pathways, particularly focusing on its role in agricultural applications. Their studies have shown that magnesium nitrate can significantly enhance the production of chlorophyll in plants, which is closely linked to vitamin synthesis, especially vitamin K[1]. BASF has developed specialized fertilizer formulations incorporating magnesium nitrate to optimize vitamin content in crops[2]. Their research also extends to the impact of magnesium nitrate on the biosynthesis of vitamin E in oilseed crops, demonstrating a positive correlation between magnesium nitrate application and α-tocopherol content[3]. Additionally, BASF has investigated the synergistic effects of magnesium nitrate with other micronutrients on overall plant metabolism and vitamin production[4].
Strengths: Comprehensive agricultural research, practical applications in crop nutrition, and a holistic approach to plant metabolism. Weaknesses: Primary focus on plant-based vitamin synthesis, potentially limiting insights into animal or human vitamin pathways.
Innovations in Vitamin Synthesis Pathways
Nutritional compositions containing magnesium threonate and uses thereof
PatentWO2014109863A1
Innovation
- A nutritional composition containing magnesium threonate (MgT) along with a carbohydrate source, protein source, fat source, and optional ingredients like β-glucan, probiotics, and long-chain polyunsaturated fatty acids, designed to enhance brain development, cognitive functions, and promote gastrointestinal tolerance in pediatric subjects.
Methods of producing crystalline beta nicotinamide riboside triacetate chloride
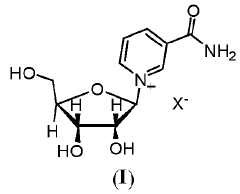
PatentWO2023177743A2
Innovation
- A process is developed to produce crystalline Beta Nicotinamide Riboside Triacetate Chloride with improved particle size distribution, bulk density, and polymorph control, using a method that involves adding crude Nicotinamide Riboside Triacetate to a solvent mixture, heating, cooling, and adding a second solvent to isolate the compound as a crystalline powder, thereby reducing residual solvent content and enhancing yield.
Regulatory Framework for Vitamin Production
The regulatory framework for vitamin production is a complex and evolving landscape that significantly impacts the development and implementation of new technologies, such as the use of magnesium nitrate in vitamin synthesis pathways. Globally, regulatory bodies have established stringent guidelines to ensure the safety, efficacy, and quality of vitamins produced for human consumption.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of vitamins as dietary supplements under the Dietary Supplement Health and Education Act (DSHEA) of 1994. This act defines dietary supplements and outlines the requirements for their manufacture, labeling, and marketing. The FDA also enforces Good Manufacturing Practices (GMPs) for dietary supplements, which include specific requirements for the production of vitamins.
The European Union has its own set of regulations governed by the European Food Safety Authority (EFSA). The EU Food Supplements Directive (2002/46/EC) provides a harmonized framework for the regulation of vitamins and minerals used in food supplements. This directive sets maximum and minimum levels for vitamins in supplements and establishes criteria for their safety and bioavailability.
In Asia, countries like Japan and China have implemented their own regulatory systems. Japan's Food with Health Claims system, regulated by the Ministry of Health, Labour and Welfare, includes a category for Foods with Nutrient Function Claims, which covers vitamins. China's State Administration for Market Regulation (SAMR) oversees the regulation of vitamins as both food supplements and ingredients in functional foods.
Regarding the specific use of magnesium nitrate in vitamin synthesis pathways, regulatory bodies typically require extensive safety data and efficacy studies before approving new production methods. Manufacturers must demonstrate that the use of magnesium nitrate does not introduce harmful contaminants or alter the bioavailability of the vitamins produced.
Environmental regulations also play a crucial role in the vitamin production landscape. The use of chemicals like magnesium nitrate must comply with local and international environmental protection laws, including regulations on waste disposal and emissions control. In the United States, the Environmental Protection Agency (EPA) sets standards for chemical use and disposal in industrial processes.
As research continues to unveil the potential effects of magnesium nitrate on vitamin synthesis pathways, regulatory frameworks may need to adapt. This could involve updating existing guidelines or creating new regulations specific to this technology. Manufacturers and researchers must stay informed about these evolving regulations to ensure compliance and maintain the ability to bring innovative vitamin production methods to market.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of vitamins as dietary supplements under the Dietary Supplement Health and Education Act (DSHEA) of 1994. This act defines dietary supplements and outlines the requirements for their manufacture, labeling, and marketing. The FDA also enforces Good Manufacturing Practices (GMPs) for dietary supplements, which include specific requirements for the production of vitamins.
The European Union has its own set of regulations governed by the European Food Safety Authority (EFSA). The EU Food Supplements Directive (2002/46/EC) provides a harmonized framework for the regulation of vitamins and minerals used in food supplements. This directive sets maximum and minimum levels for vitamins in supplements and establishes criteria for their safety and bioavailability.
In Asia, countries like Japan and China have implemented their own regulatory systems. Japan's Food with Health Claims system, regulated by the Ministry of Health, Labour and Welfare, includes a category for Foods with Nutrient Function Claims, which covers vitamins. China's State Administration for Market Regulation (SAMR) oversees the regulation of vitamins as both food supplements and ingredients in functional foods.
Regarding the specific use of magnesium nitrate in vitamin synthesis pathways, regulatory bodies typically require extensive safety data and efficacy studies before approving new production methods. Manufacturers must demonstrate that the use of magnesium nitrate does not introduce harmful contaminants or alter the bioavailability of the vitamins produced.
Environmental regulations also play a crucial role in the vitamin production landscape. The use of chemicals like magnesium nitrate must comply with local and international environmental protection laws, including regulations on waste disposal and emissions control. In the United States, the Environmental Protection Agency (EPA) sets standards for chemical use and disposal in industrial processes.
As research continues to unveil the potential effects of magnesium nitrate on vitamin synthesis pathways, regulatory frameworks may need to adapt. This could involve updating existing guidelines or creating new regulations specific to this technology. Manufacturers and researchers must stay informed about these evolving regulations to ensure compliance and maintain the ability to bring innovative vitamin production methods to market.
Environmental Impact of Synthesis Processes
The synthesis of vitamins through pathways involving magnesium nitrate has significant environmental implications that warrant careful consideration. The production processes associated with these pathways can lead to various environmental impacts, ranging from resource consumption to waste generation and potential pollution.
One of the primary environmental concerns is the energy intensity of vitamin synthesis processes. The use of magnesium nitrate in these pathways often requires high temperatures and pressures, resulting in substantial energy consumption. This increased energy demand contributes to greenhouse gas emissions and exacerbates climate change if the energy sources are not renewable.
Water usage is another critical environmental factor in vitamin synthesis. The processes involving magnesium nitrate typically require large volumes of water for reactions, cooling, and purification steps. This high water demand can strain local water resources, particularly in water-scarce regions, and may lead to competition with other essential uses such as agriculture and domestic consumption.
The generation of waste products is a significant environmental challenge in vitamin synthesis pathways. Magnesium nitrate-based processes can produce various by-products and residues that require proper disposal or treatment. Some of these waste streams may contain hazardous materials, potentially posing risks to soil and water quality if not managed appropriately.
Chemical emissions from synthesis processes are another area of environmental concern. Volatile organic compounds (VOCs) and other airborne pollutants may be released during production, potentially impacting air quality and contributing to smog formation in urban areas. Proper emission control technologies and practices are essential to mitigate these impacts.
The sourcing of raw materials for vitamin synthesis, including magnesium nitrate, also has environmental implications. Mining and processing of magnesium and nitrogen compounds can lead to habitat disruption, soil erosion, and water pollution if not conducted sustainably. Additionally, the transportation of these materials to production facilities contributes to carbon emissions and air pollution.
Efforts to improve the environmental sustainability of vitamin synthesis processes involving magnesium nitrate are ongoing. These include the development of more efficient catalysts to reduce energy requirements, implementation of closed-loop water systems to minimize water consumption, and exploration of bio-based alternatives to traditional chemical synthesis routes. Green chemistry principles are increasingly being applied to redesign synthesis pathways, aiming to reduce waste generation and improve overall environmental performance.
In conclusion, while vitamin synthesis pathways utilizing magnesium nitrate play a crucial role in meeting global nutritional needs, their environmental impact must be carefully managed. Balancing the benefits of vitamin production with environmental stewardship requires ongoing research, innovation, and implementation of sustainable practices throughout the synthesis process.
One of the primary environmental concerns is the energy intensity of vitamin synthesis processes. The use of magnesium nitrate in these pathways often requires high temperatures and pressures, resulting in substantial energy consumption. This increased energy demand contributes to greenhouse gas emissions and exacerbates climate change if the energy sources are not renewable.
Water usage is another critical environmental factor in vitamin synthesis. The processes involving magnesium nitrate typically require large volumes of water for reactions, cooling, and purification steps. This high water demand can strain local water resources, particularly in water-scarce regions, and may lead to competition with other essential uses such as agriculture and domestic consumption.
The generation of waste products is a significant environmental challenge in vitamin synthesis pathways. Magnesium nitrate-based processes can produce various by-products and residues that require proper disposal or treatment. Some of these waste streams may contain hazardous materials, potentially posing risks to soil and water quality if not managed appropriately.
Chemical emissions from synthesis processes are another area of environmental concern. Volatile organic compounds (VOCs) and other airborne pollutants may be released during production, potentially impacting air quality and contributing to smog formation in urban areas. Proper emission control technologies and practices are essential to mitigate these impacts.
The sourcing of raw materials for vitamin synthesis, including magnesium nitrate, also has environmental implications. Mining and processing of magnesium and nitrogen compounds can lead to habitat disruption, soil erosion, and water pollution if not conducted sustainably. Additionally, the transportation of these materials to production facilities contributes to carbon emissions and air pollution.
Efforts to improve the environmental sustainability of vitamin synthesis processes involving magnesium nitrate are ongoing. These include the development of more efficient catalysts to reduce energy requirements, implementation of closed-loop water systems to minimize water consumption, and exploration of bio-based alternatives to traditional chemical synthesis routes. Green chemistry principles are increasingly being applied to redesign synthesis pathways, aiming to reduce waste generation and improve overall environmental performance.
In conclusion, while vitamin synthesis pathways utilizing magnesium nitrate play a crucial role in meeting global nutritional needs, their environmental impact must be carefully managed. Balancing the benefits of vitamin production with environmental stewardship requires ongoing research, innovation, and implementation of sustainable practices throughout the synthesis process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!