The Impact of Magnesium Nitrate on Potassium Uptake in Plants
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 and K+ Uptake: Background and Objectives
The study of plant nutrition and mineral uptake has been a cornerstone of agricultural research for decades. Among the various essential nutrients, potassium (K+) plays a crucial role in plant growth, development, and stress tolerance. However, the complex interactions between different nutrients in the soil and their impact on plant uptake mechanisms have led to ongoing research in this field. One such area of interest is the influence of magnesium nitrate [Mg(NO3)2] on potassium uptake in plants.
Magnesium and potassium are both essential macronutrients for plants, each with distinct roles in plant physiology. Magnesium is a central component of chlorophyll and plays a vital role in photosynthesis, while potassium is involved in various physiological processes, including osmotic regulation, enzyme activation, and stomatal function. The interplay between these two nutrients and their uptake mechanisms has become an important focus of research in recent years.
The objective of this technical research report is to explore the impact of magnesium nitrate on potassium uptake in plants, with the aim of enhancing our understanding of plant nutrition dynamics and potentially improving agricultural practices. This investigation is driven by the need to optimize nutrient management strategies in crop production systems, particularly in the face of increasing global food demand and environmental challenges.
The evolution of this research area can be traced back to early studies on plant mineral nutrition in the 19th and 20th centuries. However, recent advancements in analytical techniques, molecular biology, and imaging technologies have allowed for more detailed investigations into the mechanisms of nutrient uptake and transport within plants. These technological developments have paved the way for a deeper understanding of the complex interactions between different nutrients in the soil-plant system.
Current research trends in this field focus on elucidating the molecular mechanisms underlying nutrient uptake, transport, and utilization in plants. This includes the study of ion channels, transporters, and their regulation at the genetic and physiological levels. Additionally, there is growing interest in understanding how environmental factors, such as soil pH, temperature, and water availability, influence nutrient uptake and interactions.
The expected outcomes of this research include a comprehensive understanding of how magnesium nitrate affects potassium uptake in plants, potential mechanisms involved in this interaction, and the implications for plant growth and development. This knowledge could lead to the development of more efficient fertilization strategies, improved crop varieties with enhanced nutrient use efficiency, and ultimately, more sustainable agricultural practices.
Magnesium and potassium are both essential macronutrients for plants, each with distinct roles in plant physiology. Magnesium is a central component of chlorophyll and plays a vital role in photosynthesis, while potassium is involved in various physiological processes, including osmotic regulation, enzyme activation, and stomatal function. The interplay between these two nutrients and their uptake mechanisms has become an important focus of research in recent years.
The objective of this technical research report is to explore the impact of magnesium nitrate on potassium uptake in plants, with the aim of enhancing our understanding of plant nutrition dynamics and potentially improving agricultural practices. This investigation is driven by the need to optimize nutrient management strategies in crop production systems, particularly in the face of increasing global food demand and environmental challenges.
The evolution of this research area can be traced back to early studies on plant mineral nutrition in the 19th and 20th centuries. However, recent advancements in analytical techniques, molecular biology, and imaging technologies have allowed for more detailed investigations into the mechanisms of nutrient uptake and transport within plants. These technological developments have paved the way for a deeper understanding of the complex interactions between different nutrients in the soil-plant system.
Current research trends in this field focus on elucidating the molecular mechanisms underlying nutrient uptake, transport, and utilization in plants. This includes the study of ion channels, transporters, and their regulation at the genetic and physiological levels. Additionally, there is growing interest in understanding how environmental factors, such as soil pH, temperature, and water availability, influence nutrient uptake and interactions.
The expected outcomes of this research include a comprehensive understanding of how magnesium nitrate affects potassium uptake in plants, potential mechanisms involved in this interaction, and the implications for plant growth and development. This knowledge could lead to the development of more efficient fertilization strategies, improved crop varieties with enhanced nutrient use efficiency, and ultimately, more sustainable agricultural practices.
Agricultural Market Demand Analysis
The agricultural market for magnesium nitrate and potassium-based fertilizers has shown significant growth in recent years, driven by the increasing demand for high-yield crops and sustainable farming practices. Farmers and agricultural businesses are increasingly recognizing the importance of balanced nutrient management, particularly the role of magnesium and potassium in plant growth and development.
The global fertilizer market, including magnesium nitrate and potassium-based products, is projected to reach substantial values in the coming years. This growth is attributed to the rising global population, shrinking arable land, and the need for improved crop productivity. Specifically, the demand for magnesium nitrate has been steadily increasing due to its dual benefits of providing both magnesium and nitrogen to plants.
In the context of potassium uptake, there is a growing awareness among farmers about the potential impact of magnesium nitrate on this essential process. This has led to an increased interest in research and development of fertilizer formulations that optimize the balance between magnesium and potassium. Agricultural businesses are responding to this demand by developing and marketing specialized fertilizer blends that address the complex interactions between these nutrients.
The market for precision agriculture technologies that can accurately measure and manage nutrient levels in soil and plants is also expanding. This includes sensors, soil testing equipment, and data analytics platforms that help farmers make informed decisions about fertilizer application, taking into account the potential effects of magnesium nitrate on potassium uptake.
Regionally, emerging economies in Asia-Pacific and Latin America are showing the highest growth rates in demand for advanced fertilizers, including those containing magnesium nitrate. This is due to the modernization of agricultural practices and increasing awareness of the benefits of balanced nutrient management. In more mature markets like North America and Europe, the focus is shifting towards sustainable and environmentally friendly fertilizer solutions that minimize nutrient runoff while maximizing crop yields.
The organic farming sector is also influencing the market, with a growing demand for natural and organic sources of magnesium and potassium. This has led to the development of new products and formulations that cater to this niche but expanding market segment. As a result, companies are investing in research to understand how organic sources of magnesium, such as dolomite, interact with potassium uptake in plants.
Overall, the agricultural market demand for products and technologies related to magnesium nitrate and its impact on potassium uptake in plants is robust and diverse. It encompasses traditional fertilizers, precision agriculture technologies, and organic alternatives, reflecting the complex needs of modern agriculture and the growing emphasis on sustainable, high-yield farming practices.
The global fertilizer market, including magnesium nitrate and potassium-based products, is projected to reach substantial values in the coming years. This growth is attributed to the rising global population, shrinking arable land, and the need for improved crop productivity. Specifically, the demand for magnesium nitrate has been steadily increasing due to its dual benefits of providing both magnesium and nitrogen to plants.
In the context of potassium uptake, there is a growing awareness among farmers about the potential impact of magnesium nitrate on this essential process. This has led to an increased interest in research and development of fertilizer formulations that optimize the balance between magnesium and potassium. Agricultural businesses are responding to this demand by developing and marketing specialized fertilizer blends that address the complex interactions between these nutrients.
The market for precision agriculture technologies that can accurately measure and manage nutrient levels in soil and plants is also expanding. This includes sensors, soil testing equipment, and data analytics platforms that help farmers make informed decisions about fertilizer application, taking into account the potential effects of magnesium nitrate on potassium uptake.
Regionally, emerging economies in Asia-Pacific and Latin America are showing the highest growth rates in demand for advanced fertilizers, including those containing magnesium nitrate. This is due to the modernization of agricultural practices and increasing awareness of the benefits of balanced nutrient management. In more mature markets like North America and Europe, the focus is shifting towards sustainable and environmentally friendly fertilizer solutions that minimize nutrient runoff while maximizing crop yields.
The organic farming sector is also influencing the market, with a growing demand for natural and organic sources of magnesium and potassium. This has led to the development of new products and formulations that cater to this niche but expanding market segment. As a result, companies are investing in research to understand how organic sources of magnesium, such as dolomite, interact with potassium uptake in plants.
Overall, the agricultural market demand for products and technologies related to magnesium nitrate and its impact on potassium uptake in plants is robust and diverse. It encompasses traditional fertilizers, precision agriculture technologies, and organic alternatives, reflecting the complex needs of modern agriculture and the growing emphasis on sustainable, high-yield farming practices.
Current Understanding and Challenges
The current understanding of the impact of magnesium nitrate on potassium uptake in plants is multifaceted and presents several challenges for researchers and agronomists. Magnesium and potassium are both essential macronutrients for plant growth and development, playing crucial roles in various physiological processes. However, their interaction, particularly when magnesium is applied as magnesium nitrate, is complex and not fully elucidated.
Recent studies have shown that magnesium nitrate application can significantly influence potassium uptake in plants, but the effects are not uniform across all plant species and growing conditions. In some cases, magnesium nitrate has been observed to enhance potassium uptake, potentially due to improved root growth and increased soil solution conductivity. Conversely, other research has indicated that excessive magnesium nitrate can lead to antagonistic effects, reducing potassium absorption and translocation within the plant.
One of the primary challenges in understanding this interaction is the variability in plant responses across different soil types and environmental conditions. Factors such as soil pH, organic matter content, and the presence of other ions can significantly alter the availability and uptake of both magnesium and potassium. This complexity makes it difficult to establish universal guidelines for magnesium nitrate application in relation to potassium nutrition.
Another significant challenge lies in the molecular mechanisms governing the interaction between magnesium and potassium uptake. While it is known that these cations can compete for uptake sites on root membranes, the specific transporters and channels involved in this process are not fully characterized. Understanding these molecular interactions is crucial for developing targeted strategies to optimize nutrient management in agricultural systems.
The impact of magnesium nitrate on potassium uptake also varies depending on the growth stage of the plant. Research has shown that the effects can be more pronounced during certain developmental phases, such as early vegetative growth or fruit development. This temporal variability adds another layer of complexity to nutrient management strategies and highlights the need for stage-specific recommendations.
Furthermore, the long-term effects of magnesium nitrate application on soil potassium dynamics and plant uptake patterns remain unclear. There is a need for extended field trials to assess how repeated applications of magnesium nitrate might alter soil chemistry and microbial communities, which in turn could affect potassium availability and plant uptake over time.
In conclusion, while significant progress has been made in understanding the impact of magnesium nitrate on potassium uptake in plants, numerous challenges persist. These include the need for more comprehensive studies across diverse plant species and environmental conditions, elucidation of molecular mechanisms, and development of precise, context-specific nutrient management strategies. Addressing these challenges will be crucial for optimizing crop nutrition and enhancing agricultural productivity in the face of growing global food demands.
Recent studies have shown that magnesium nitrate application can significantly influence potassium uptake in plants, but the effects are not uniform across all plant species and growing conditions. In some cases, magnesium nitrate has been observed to enhance potassium uptake, potentially due to improved root growth and increased soil solution conductivity. Conversely, other research has indicated that excessive magnesium nitrate can lead to antagonistic effects, reducing potassium absorption and translocation within the plant.
One of the primary challenges in understanding this interaction is the variability in plant responses across different soil types and environmental conditions. Factors such as soil pH, organic matter content, and the presence of other ions can significantly alter the availability and uptake of both magnesium and potassium. This complexity makes it difficult to establish universal guidelines for magnesium nitrate application in relation to potassium nutrition.
Another significant challenge lies in the molecular mechanisms governing the interaction between magnesium and potassium uptake. While it is known that these cations can compete for uptake sites on root membranes, the specific transporters and channels involved in this process are not fully characterized. Understanding these molecular interactions is crucial for developing targeted strategies to optimize nutrient management in agricultural systems.
The impact of magnesium nitrate on potassium uptake also varies depending on the growth stage of the plant. Research has shown that the effects can be more pronounced during certain developmental phases, such as early vegetative growth or fruit development. This temporal variability adds another layer of complexity to nutrient management strategies and highlights the need for stage-specific recommendations.
Furthermore, the long-term effects of magnesium nitrate application on soil potassium dynamics and plant uptake patterns remain unclear. There is a need for extended field trials to assess how repeated applications of magnesium nitrate might alter soil chemistry and microbial communities, which in turn could affect potassium availability and plant uptake over time.
In conclusion, while significant progress has been made in understanding the impact of magnesium nitrate on potassium uptake in plants, numerous challenges persist. These include the need for more comprehensive studies across diverse plant species and environmental conditions, elucidation of molecular mechanisms, and development of precise, context-specific nutrient management strategies. Addressing these challenges will be crucial for optimizing crop nutrition and enhancing agricultural productivity in the face of growing global food demands.
Existing Strategies for Optimizing Nutrient Uptake
01 Magnesium nitrate as a fertilizer component
Magnesium nitrate is used as a component in fertilizer formulations to enhance potassium uptake in plants. It provides both magnesium and nitrogen, which are essential nutrients for plant growth and development. The presence of magnesium nitrate can improve the overall nutrient balance and promote better potassium absorption by plant roots.- Magnesium nitrate as a fertilizer component: Magnesium nitrate is used as a component in fertilizer formulations to enhance potassium uptake in plants. It provides both magnesium and nitrogen, which are essential nutrients for plant growth and can improve the efficiency of potassium absorption by roots.
- Foliar application of magnesium nitrate: Foliar application of magnesium nitrate solutions can improve potassium uptake in plants. This method allows for quick absorption of nutrients through the leaves, bypassing potential soil limitations and enhancing overall nutrient utilization, including potassium.
- Synergistic effects with other nutrients: Combining magnesium nitrate with other nutrients or compounds can create synergistic effects that enhance potassium uptake. These combinations may include specific ratios of magnesium, nitrogen, and potassium, or the addition of organic compounds to improve nutrient absorption.
- Soil conditioning for improved potassium uptake: Magnesium nitrate can be used to condition soil, improving its structure and chemical composition. This can lead to better potassium availability and uptake by plants. The treatment may involve adjusting soil pH or improving cation exchange capacity.
- Controlled-release formulations: Developing controlled-release formulations containing magnesium nitrate can optimize potassium uptake over time. These formulations may use encapsulation techniques or chemical modifications to regulate the release of nutrients, ensuring a steady supply of magnesium and improved potassium absorption throughout the growing season.
02 Foliar application of magnesium nitrate
Foliar application of magnesium nitrate solutions can enhance potassium uptake in plants. This method allows for direct absorption of nutrients through the leaves, bypassing potential soil limitations. Foliar sprays containing magnesium nitrate can be particularly effective in addressing nutrient deficiencies and improving overall plant health and potassium utilization.Expand Specific Solutions03 Synergistic effects with other nutrients
Combining magnesium nitrate with other nutrients can create synergistic effects that enhance potassium uptake. For example, formulations that include magnesium nitrate along with potassium sources and other micronutrients can improve overall nutrient absorption and utilization by plants, leading to better growth and yield outcomes.Expand Specific Solutions04 Soil conditioning for improved potassium uptake
Magnesium nitrate can be used to condition soil and improve its structure, which indirectly enhances potassium uptake. By improving soil pH and reducing soil compaction, magnesium nitrate can create a more favorable environment for root growth and nutrient absorption, including potassium.Expand Specific Solutions05 Controlled-release formulations
Controlled-release formulations containing magnesium nitrate can provide a steady supply of nutrients to plants, promoting consistent potassium uptake over time. These formulations can be designed to release nutrients gradually, matching the plant's growth stages and nutritional needs, thereby optimizing potassium utilization and overall plant nutrition.Expand Specific Solutions
Key Players in Agrochemical Industry
The research on "The Impact of Magnesium Nitrate on Potassium Uptake in Plants" is in a developing stage, with growing interest due to its potential implications for agricultural productivity. The market for related technologies and products is expanding, driven by the need for improved crop nutrition strategies. While the technology is still evolving, several key players are contributing to its advancement. Companies like Pioneer Hi-Bred International, Inc. and Syngenta Participations AG are likely at the forefront of commercial applications, while academic institutions such as Nanjing Agricultural University and University of Guelph are conducting fundamental research. The involvement of diverse organizations indicates a competitive landscape with opportunities for innovation and market growth in plant nutrition and fertilizer technologies.
Nanjing Agricultural University
Technical Solution: Nanjing Agricultural University has developed a novel approach to studying the impact of magnesium nitrate on potassium uptake in plants. Their research utilizes advanced imaging techniques, including X-ray fluorescence microscopy, to visualize the distribution and movement of potassium ions within plant tissues[1]. The university has also implemented isotope tracing methods to accurately track potassium uptake rates under varying magnesium nitrate concentrations[3]. Additionally, they have developed a sophisticated hydroponic system that allows precise control of nutrient solutions, enabling researchers to isolate the effects of magnesium nitrate on potassium uptake[5].
Strengths: Cutting-edge imaging technology and isotope tracing methods provide highly accurate data. The controlled hydroponic system allows for precise experimentation. Weaknesses: The sophisticated equipment may limit large-scale field applications, and the controlled environment might not fully represent real-world agricultural conditions.
The Regents of the University of California
Technical Solution: The University of California has pioneered a comprehensive approach to studying the impact of magnesium nitrate on potassium uptake in plants. Their research combines molecular biology techniques with field studies to provide a holistic understanding of the interaction. They have developed transgenic plant lines with modified potassium transporters to investigate the specific mechanisms by which magnesium nitrate affects potassium uptake[2]. The university has also implemented long-term field trials across various soil types to assess the practical implications of magnesium nitrate application on crop potassium nutrition[4]. Furthermore, they have utilized advanced metabolomics approaches to analyze changes in plant metabolic profiles in response to varying magnesium nitrate and potassium levels[6].
Strengths: Comprehensive approach combining molecular techniques with field studies provides both mechanistic insights and practical applications. Long-term field trials offer valuable real-world data. Weaknesses: The development of transgenic plants may face regulatory hurdles and public acceptance issues in some regions.
Innovative Approaches in Mg-K Interaction Studies
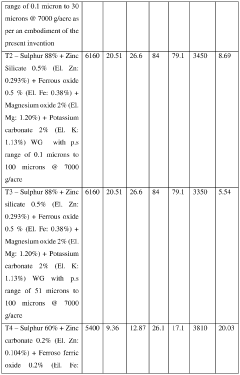
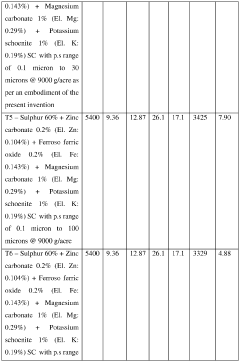
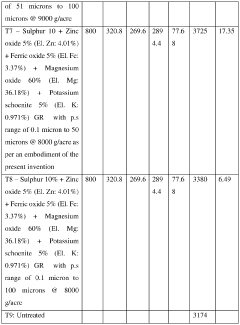
Crop nutrition composition
PatentActiveIN202427038517A
Innovation
- A crop nutrition composition in the form of water dispersible granules or aqueous suspension comprising water insoluble Magnesium salts and water soluble Potassium salts, with a surfactant, formulated to have elemental Magnesium and Potassium content in the range of 1% to 50% by weight and particle size of 0.1 to 30 microns, ensuring quick and balanced nutrient availability.
Crop nutrition and fortification composition
PatentWO2024142110A1
Innovation
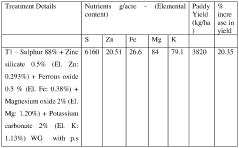
- A crop nutrition and fortification composition comprising elemental sulphur, magnesium, potassium, iron, and zinc in specific proportions, formulated as water dispersible or disintegrable granules or liquid suspension, ensuring balanced nutrient uptake and reducing the need for excessive NPK fertilizers.
Environmental Impact of Fertilizer Use
The use of fertilizers has significantly transformed agricultural practices, leading to increased crop yields and food production. However, this widespread application has also resulted in substantial environmental consequences. The environmental impact of fertilizer use is a critical concern that encompasses various aspects of ecosystem health and sustainability.
Fertilizer runoff from agricultural lands is a primary contributor to water pollution. Excess nutrients, particularly nitrogen and phosphorus, can leach into groundwater or be carried by surface runoff into nearby water bodies. This nutrient enrichment often leads to eutrophication, causing algal blooms that deplete oxygen levels in aquatic ecosystems. The resulting hypoxic zones can devastate aquatic life and disrupt entire food chains.
Soil degradation is another significant environmental issue associated with fertilizer use. Excessive application of chemical fertilizers can alter soil pH, disrupt beneficial microbial communities, and lead to the accumulation of heavy metals. Over time, this can result in reduced soil fertility, increased erosion, and decreased water retention capacity. The long-term consequences include diminished agricultural productivity and increased vulnerability to drought.
Fertilizer production and use contribute to greenhouse gas emissions, exacerbating climate change. The manufacturing process of synthetic fertilizers, particularly nitrogen-based ones, is energy-intensive and relies heavily on fossil fuels. Additionally, the application of nitrogen fertilizers to soil can lead to the release of nitrous oxide, a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide.
The impact on biodiversity is another crucial aspect of fertilizer use. Nutrient runoff can lead to changes in plant community composition, favoring fast-growing species that outcompete native flora. This alteration in vegetation can have cascading effects on local fauna, disrupting established ecosystems and potentially leading to the loss of sensitive species.
Air quality is also affected by fertilizer use, primarily through the emission of ammonia. Volatilization of ammonia from urea-based fertilizers contributes to the formation of particulate matter, which can have adverse effects on human health and visibility. Furthermore, ammonia emissions can lead to acid rain, impacting both terrestrial and aquatic ecosystems far from the source.
Addressing the environmental impact of fertilizer use requires a multifaceted approach. Precision agriculture techniques, such as variable rate application and soil testing, can optimize fertilizer use and minimize excess. The development and adoption of slow-release fertilizers and nitrification inhibitors can reduce nutrient leaching and gaseous losses. Additionally, promoting organic farming practices and the use of cover crops can enhance soil health and reduce reliance on synthetic fertilizers.
Fertilizer runoff from agricultural lands is a primary contributor to water pollution. Excess nutrients, particularly nitrogen and phosphorus, can leach into groundwater or be carried by surface runoff into nearby water bodies. This nutrient enrichment often leads to eutrophication, causing algal blooms that deplete oxygen levels in aquatic ecosystems. The resulting hypoxic zones can devastate aquatic life and disrupt entire food chains.
Soil degradation is another significant environmental issue associated with fertilizer use. Excessive application of chemical fertilizers can alter soil pH, disrupt beneficial microbial communities, and lead to the accumulation of heavy metals. Over time, this can result in reduced soil fertility, increased erosion, and decreased water retention capacity. The long-term consequences include diminished agricultural productivity and increased vulnerability to drought.
Fertilizer production and use contribute to greenhouse gas emissions, exacerbating climate change. The manufacturing process of synthetic fertilizers, particularly nitrogen-based ones, is energy-intensive and relies heavily on fossil fuels. Additionally, the application of nitrogen fertilizers to soil can lead to the release of nitrous oxide, a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide.
The impact on biodiversity is another crucial aspect of fertilizer use. Nutrient runoff can lead to changes in plant community composition, favoring fast-growing species that outcompete native flora. This alteration in vegetation can have cascading effects on local fauna, disrupting established ecosystems and potentially leading to the loss of sensitive species.
Air quality is also affected by fertilizer use, primarily through the emission of ammonia. Volatilization of ammonia from urea-based fertilizers contributes to the formation of particulate matter, which can have adverse effects on human health and visibility. Furthermore, ammonia emissions can lead to acid rain, impacting both terrestrial and aquatic ecosystems far from the source.
Addressing the environmental impact of fertilizer use requires a multifaceted approach. Precision agriculture techniques, such as variable rate application and soil testing, can optimize fertilizer use and minimize excess. The development and adoption of slow-release fertilizers and nitrification inhibitors can reduce nutrient leaching and gaseous losses. Additionally, promoting organic farming practices and the use of cover crops can enhance soil health and reduce reliance on synthetic fertilizers.
Regulatory Framework for Fertilizer Application
The regulatory framework for fertilizer application plays a crucial role in managing the use of magnesium nitrate and its impact on potassium uptake in plants. This framework encompasses a complex set of rules, guidelines, and standards designed to ensure the safe and effective use of fertilizers while minimizing potential environmental and health risks.
At the international level, organizations such as the Food and Agriculture Organization (FAO) of the United Nations provide overarching guidelines for fertilizer use. These guidelines often serve as a basis for national and regional regulations, emphasizing the importance of balanced nutrient management and sustainable agricultural practices.
In the United States, the Environmental Protection Agency (EPA) and the Department of Agriculture (USDA) are the primary federal agencies responsible for regulating fertilizer use. The EPA focuses on environmental protection, setting limits on nutrient runoff and groundwater contamination. The USDA, through its Natural Resources Conservation Service, provides technical assistance and guidelines for optimal fertilizer application.
The European Union has implemented stringent regulations through its Fertilizing Products Regulation (EU) 2019/1009. This regulation sets harmonized rules for the marketing of fertilizing products, including specific provisions for inorganic macronutrient fertilizers like magnesium nitrate. It also establishes limits on contaminants and emphasizes the need for efficient nutrient use to reduce environmental impact.
Many countries have developed their own regulatory frameworks tailored to their specific agricultural needs and environmental conditions. For instance, China's Ministry of Agriculture and Rural Affairs has implemented strict controls on fertilizer use to address issues of soil degradation and water pollution. These regulations include limits on application rates and promotion of precision agriculture techniques.
Regulatory bodies often require detailed labeling of fertilizer products, including information on nutrient content, application rates, and potential environmental hazards. This ensures that farmers and agricultural professionals can make informed decisions about fertilizer use, particularly when considering the balance between magnesium and potassium in plant nutrition.
Compliance with these regulations is typically enforced through a combination of regular inspections, product testing, and penalties for non-compliance. Many jurisdictions also require fertilizer manufacturers and distributors to obtain licenses or permits, ensuring that only approved products enter the market.
As research continues to reveal the complex interactions between different nutrients in plant systems, regulatory frameworks are evolving to incorporate new scientific findings. This includes ongoing assessments of the impact of magnesium nitrate on potassium uptake and overall plant health, potentially leading to more nuanced guidelines for fertilizer application in the future.
At the international level, organizations such as the Food and Agriculture Organization (FAO) of the United Nations provide overarching guidelines for fertilizer use. These guidelines often serve as a basis for national and regional regulations, emphasizing the importance of balanced nutrient management and sustainable agricultural practices.
In the United States, the Environmental Protection Agency (EPA) and the Department of Agriculture (USDA) are the primary federal agencies responsible for regulating fertilizer use. The EPA focuses on environmental protection, setting limits on nutrient runoff and groundwater contamination. The USDA, through its Natural Resources Conservation Service, provides technical assistance and guidelines for optimal fertilizer application.
The European Union has implemented stringent regulations through its Fertilizing Products Regulation (EU) 2019/1009. This regulation sets harmonized rules for the marketing of fertilizing products, including specific provisions for inorganic macronutrient fertilizers like magnesium nitrate. It also establishes limits on contaminants and emphasizes the need for efficient nutrient use to reduce environmental impact.
Many countries have developed their own regulatory frameworks tailored to their specific agricultural needs and environmental conditions. For instance, China's Ministry of Agriculture and Rural Affairs has implemented strict controls on fertilizer use to address issues of soil degradation and water pollution. These regulations include limits on application rates and promotion of precision agriculture techniques.
Regulatory bodies often require detailed labeling of fertilizer products, including information on nutrient content, application rates, and potential environmental hazards. This ensures that farmers and agricultural professionals can make informed decisions about fertilizer use, particularly when considering the balance between magnesium and potassium in plant nutrition.
Compliance with these regulations is typically enforced through a combination of regular inspections, product testing, and penalties for non-compliance. Many jurisdictions also require fertilizer manufacturers and distributors to obtain licenses or permits, ensuring that only approved products enter the market.
As research continues to reveal the complex interactions between different nutrients in plant systems, regulatory frameworks are evolving to incorporate new scientific findings. This includes ongoing assessments of the impact of magnesium nitrate on potassium uptake and overall plant health, potentially leading to more nuanced guidelines for fertilizer application in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!