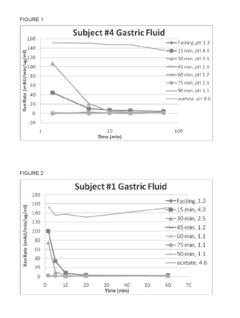
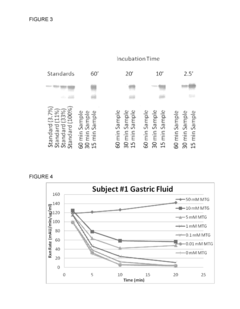
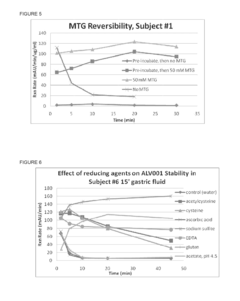
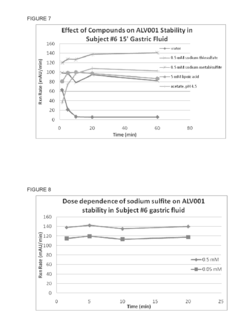
The Impact of Magnesium Nitrate on Protein Stability in Food Systems
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate and Protein Stability: Background and Objectives
Magnesium nitrate's impact on protein stability in food systems has become a critical area of research in food science and technology. This field has evolved significantly over the past few decades, driven by the increasing demand for high-quality, shelf-stable food products. The journey of understanding protein stability began with basic studies on protein structure and folding mechanisms, gradually progressing to more complex investigations involving various food matrices and environmental factors.
The evolution of this research area has been marked by several key milestones. Initially, scientists focused on understanding the fundamental principles of protein stability in simple aqueous solutions. As knowledge advanced, researchers began to explore the effects of various salts, including magnesium nitrate, on protein behavior. This shift was crucial in bridging the gap between theoretical protein science and practical food applications.
In recent years, the food industry has faced growing challenges in maintaining protein stability during processing, storage, and distribution. These challenges have propelled the investigation of magnesium nitrate as a potential stabilizing agent. The unique properties of magnesium nitrate, including its ability to influence protein hydration and electrostatic interactions, have made it a subject of intense study in food systems.
The primary objective of current research in this field is to elucidate the mechanisms by which magnesium nitrate affects protein stability in complex food matrices. This includes understanding how magnesium nitrate interacts with proteins at a molecular level, its effects on protein conformation and aggregation, and how these interactions are influenced by various food components and environmental conditions.
Another crucial goal is to develop practical applications for magnesium nitrate in food processing and preservation. Researchers aim to optimize the use of magnesium nitrate to enhance the shelf life of protein-rich foods, improve texture and sensory properties, and maintain nutritional value. This involves investigating the optimal concentrations of magnesium nitrate for different food systems and exploring potential synergistic effects with other food additives.
Furthermore, there is a growing emphasis on understanding the long-term effects of magnesium nitrate on food safety and quality. This includes assessing any potential health implications of its use in food products and ensuring compliance with regulatory standards. The research also extends to exploring sustainable and cost-effective methods of incorporating magnesium nitrate into food processing techniques.
As the field progresses, there is an increasing trend towards interdisciplinary approaches, combining insights from food science, biochemistry, and materials science. This holistic approach aims to provide a comprehensive understanding of the role of magnesium nitrate in protein stability, paving the way for innovative food preservation strategies and the development of novel functional food products.
The evolution of this research area has been marked by several key milestones. Initially, scientists focused on understanding the fundamental principles of protein stability in simple aqueous solutions. As knowledge advanced, researchers began to explore the effects of various salts, including magnesium nitrate, on protein behavior. This shift was crucial in bridging the gap between theoretical protein science and practical food applications.
In recent years, the food industry has faced growing challenges in maintaining protein stability during processing, storage, and distribution. These challenges have propelled the investigation of magnesium nitrate as a potential stabilizing agent. The unique properties of magnesium nitrate, including its ability to influence protein hydration and electrostatic interactions, have made it a subject of intense study in food systems.
The primary objective of current research in this field is to elucidate the mechanisms by which magnesium nitrate affects protein stability in complex food matrices. This includes understanding how magnesium nitrate interacts with proteins at a molecular level, its effects on protein conformation and aggregation, and how these interactions are influenced by various food components and environmental conditions.
Another crucial goal is to develop practical applications for magnesium nitrate in food processing and preservation. Researchers aim to optimize the use of magnesium nitrate to enhance the shelf life of protein-rich foods, improve texture and sensory properties, and maintain nutritional value. This involves investigating the optimal concentrations of magnesium nitrate for different food systems and exploring potential synergistic effects with other food additives.
Furthermore, there is a growing emphasis on understanding the long-term effects of magnesium nitrate on food safety and quality. This includes assessing any potential health implications of its use in food products and ensuring compliance with regulatory standards. The research also extends to exploring sustainable and cost-effective methods of incorporating magnesium nitrate into food processing techniques.
As the field progresses, there is an increasing trend towards interdisciplinary approaches, combining insights from food science, biochemistry, and materials science. This holistic approach aims to provide a comprehensive understanding of the role of magnesium nitrate in protein stability, paving the way for innovative food preservation strategies and the development of novel functional food products.
Market Analysis of Magnesium Nitrate in Food Preservation
The market for magnesium nitrate in food preservation has shown significant growth in recent years, driven by increasing consumer demand for extended shelf life and improved food quality. The global food preservatives market, which includes magnesium nitrate, was valued at approximately $2.5 billion in 2020 and is projected to reach $3.2 billion by 2025, with a compound annual growth rate (CAGR) of 5.2%.
Magnesium nitrate's role in protein stability has garnered attention from food manufacturers seeking to enhance the texture and nutritional value of their products. The dairy industry, in particular, has shown keen interest in utilizing magnesium nitrate to improve the stability of milk proteins during processing and storage. This application has led to a surge in demand from yogurt, cheese, and other dairy product manufacturers.
The meat processing industry has also emerged as a significant consumer of magnesium nitrate. Its ability to maintain protein structure in processed meats has made it an attractive alternative to traditional preservatives. This shift is partly driven by consumer preferences for cleaner labels and more natural ingredients.
In the plant-based protein market, which is experiencing rapid growth, magnesium nitrate is being explored for its potential to improve the texture and shelf life of meat alternatives. As this market segment expands, it is expected to contribute substantially to the overall demand for magnesium nitrate in food preservation.
Geographically, North America and Europe currently dominate the market for magnesium nitrate in food preservation, owing to stringent food safety regulations and a well-established food processing industry. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by changing dietary habits, increasing disposable incomes, and a growing awareness of food safety.
The market is characterized by a mix of large multinational corporations and smaller, specialized chemical companies. Key players are investing in research and development to enhance the efficacy of magnesium nitrate in various food applications and to develop novel formulations that address specific industry needs.
Challenges in the market include regulatory hurdles, as food safety authorities continuously review and update guidelines for food additives. Additionally, there is growing competition from alternative preservatives and emerging technologies in food preservation, which may impact the market share of magnesium nitrate in certain applications.
Magnesium nitrate's role in protein stability has garnered attention from food manufacturers seeking to enhance the texture and nutritional value of their products. The dairy industry, in particular, has shown keen interest in utilizing magnesium nitrate to improve the stability of milk proteins during processing and storage. This application has led to a surge in demand from yogurt, cheese, and other dairy product manufacturers.
The meat processing industry has also emerged as a significant consumer of magnesium nitrate. Its ability to maintain protein structure in processed meats has made it an attractive alternative to traditional preservatives. This shift is partly driven by consumer preferences for cleaner labels and more natural ingredients.
In the plant-based protein market, which is experiencing rapid growth, magnesium nitrate is being explored for its potential to improve the texture and shelf life of meat alternatives. As this market segment expands, it is expected to contribute substantially to the overall demand for magnesium nitrate in food preservation.
Geographically, North America and Europe currently dominate the market for magnesium nitrate in food preservation, owing to stringent food safety regulations and a well-established food processing industry. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by changing dietary habits, increasing disposable incomes, and a growing awareness of food safety.
The market is characterized by a mix of large multinational corporations and smaller, specialized chemical companies. Key players are investing in research and development to enhance the efficacy of magnesium nitrate in various food applications and to develop novel formulations that address specific industry needs.
Challenges in the market include regulatory hurdles, as food safety authorities continuously review and update guidelines for food additives. Additionally, there is growing competition from alternative preservatives and emerging technologies in food preservation, which may impact the market share of magnesium nitrate in certain applications.
Current Challenges in Protein Stabilization with Magnesium Nitrate
The stabilization of proteins in food systems using magnesium nitrate presents several significant challenges that researchers and food scientists are currently grappling with. One of the primary issues is the complex interaction between magnesium nitrate and various protein structures. While magnesium nitrate has shown promise in enhancing protein stability, its effectiveness can vary greatly depending on the specific protein and the food matrix in which it is applied.
A major challenge lies in determining the optimal concentration of magnesium nitrate for different food systems. Too little may not provide sufficient stabilization, while excessive amounts can lead to undesirable changes in taste, texture, and overall food quality. This delicate balance is further complicated by the diverse range of proteins found in food products, each with its unique stability requirements.
The impact of magnesium nitrate on protein functionality is another area of concern. While it may enhance stability, there is a risk of altering the protein's functional properties, such as solubility, emulsification, and foaming capabilities. These changes can significantly affect the texture and sensory attributes of food products, potentially leading to consumer dissatisfaction.
Environmental factors, including pH, temperature, and ionic strength, play a crucial role in the effectiveness of magnesium nitrate as a protein stabilizer. Researchers are challenged with understanding and controlling these variables across different food processing and storage conditions. The interplay between these factors and magnesium nitrate's stabilizing effects is complex and not fully understood, making it difficult to develop universally applicable solutions.
Another significant challenge is the potential for magnesium nitrate to interact with other food components, such as lipids, carbohydrates, and minerals. These interactions can lead to unexpected changes in food quality and may even result in the formation of undesirable compounds. Understanding and mitigating these interactions is essential for ensuring the safety and quality of food products.
The long-term stability of proteins treated with magnesium nitrate is also a concern. While initial stabilization may be achieved, maintaining this stability throughout the product's shelf life under various storage conditions remains a challenge. This is particularly important for products with extended shelf lives or those subjected to temperature fluctuations during distribution and storage.
Regulatory considerations pose additional challenges in the use of magnesium nitrate for protein stabilization. Food safety authorities in different regions may have varying regulations regarding its use, necessitating careful navigation of legal requirements and potential limitations on application levels.
Lastly, there is a growing demand for clean label products, which presents a challenge in using magnesium nitrate as a stabilizer. Consumers increasingly prefer natural ingredients, and the use of chemical additives like magnesium nitrate may be perceived negatively. This trend is pushing researchers to explore alternative, more label-friendly stabilization methods or to find ways to minimize the use of magnesium nitrate while maintaining its benefits.
A major challenge lies in determining the optimal concentration of magnesium nitrate for different food systems. Too little may not provide sufficient stabilization, while excessive amounts can lead to undesirable changes in taste, texture, and overall food quality. This delicate balance is further complicated by the diverse range of proteins found in food products, each with its unique stability requirements.
The impact of magnesium nitrate on protein functionality is another area of concern. While it may enhance stability, there is a risk of altering the protein's functional properties, such as solubility, emulsification, and foaming capabilities. These changes can significantly affect the texture and sensory attributes of food products, potentially leading to consumer dissatisfaction.
Environmental factors, including pH, temperature, and ionic strength, play a crucial role in the effectiveness of magnesium nitrate as a protein stabilizer. Researchers are challenged with understanding and controlling these variables across different food processing and storage conditions. The interplay between these factors and magnesium nitrate's stabilizing effects is complex and not fully understood, making it difficult to develop universally applicable solutions.
Another significant challenge is the potential for magnesium nitrate to interact with other food components, such as lipids, carbohydrates, and minerals. These interactions can lead to unexpected changes in food quality and may even result in the formation of undesirable compounds. Understanding and mitigating these interactions is essential for ensuring the safety and quality of food products.
The long-term stability of proteins treated with magnesium nitrate is also a concern. While initial stabilization may be achieved, maintaining this stability throughout the product's shelf life under various storage conditions remains a challenge. This is particularly important for products with extended shelf lives or those subjected to temperature fluctuations during distribution and storage.
Regulatory considerations pose additional challenges in the use of magnesium nitrate for protein stabilization. Food safety authorities in different regions may have varying regulations regarding its use, necessitating careful navigation of legal requirements and potential limitations on application levels.
Lastly, there is a growing demand for clean label products, which presents a challenge in using magnesium nitrate as a stabilizer. Consumers increasingly prefer natural ingredients, and the use of chemical additives like magnesium nitrate may be perceived negatively. This trend is pushing researchers to explore alternative, more label-friendly stabilization methods or to find ways to minimize the use of magnesium nitrate while maintaining its benefits.
Existing Methods for Magnesium Nitrate-based Protein Stabilization
01 Use of magnesium nitrate as a protein stabilizer
Magnesium nitrate can be used as an effective protein stabilizer in various formulations. It helps maintain protein structure and function, preventing denaturation and aggregation. This is particularly useful in pharmaceutical and biotechnology applications where protein stability is crucial for product efficacy and shelf life.- Magnesium nitrate as a protein stabilizer: Magnesium nitrate can be used as an effective protein stabilizer in various formulations. It helps maintain protein structure and function, preventing denaturation and aggregation. This is particularly useful in pharmaceutical and biotechnology applications where protein stability is crucial.
- Protein stability enhancement in liquid formulations: Incorporating magnesium nitrate into liquid protein formulations can significantly improve protein stability. This approach is beneficial for extending the shelf life of protein-based products and maintaining their efficacy during storage and transportation.
- Magnesium nitrate in combination with other stabilizers: Combining magnesium nitrate with other stabilizing agents can create synergistic effects, further enhancing protein stability. This approach allows for the development of more robust formulations that can withstand various environmental stresses.
- Application in freeze-drying processes: Magnesium nitrate can be used as a cryoprotectant in freeze-drying processes for proteins. It helps preserve protein structure during the freezing and drying stages, resulting in improved stability of lyophilized protein products.
- Optimization of magnesium nitrate concentration: The concentration of magnesium nitrate plays a crucial role in its protein-stabilizing effects. Optimizing the concentration for specific proteins and formulations is essential to achieve maximum stability without compromising other product characteristics.
02 Magnesium nitrate in protein crystallization
Magnesium nitrate plays a role in protein crystallization processes. It can act as a precipitant or additive in crystallization solutions, helping to promote the formation of protein crystals. This is valuable for structural biology studies and the development of protein-based drugs.Expand Specific Solutions03 Magnesium nitrate in enzyme stabilization
Magnesium nitrate has been found to stabilize various enzymes, maintaining their catalytic activity and structural integrity. This property is useful in industrial enzyme applications, where long-term stability and activity retention are important for process efficiency and cost-effectiveness.Expand Specific Solutions04 Magnesium nitrate in protein formulation for medical applications
In medical and pharmaceutical applications, magnesium nitrate is used in protein formulations to enhance stability and maintain biological activity. This is particularly important for protein-based drugs, vaccines, and diagnostic reagents, where protein stability directly impacts product efficacy and shelf life.Expand Specific Solutions05 Magnesium nitrate in food and beverage protein stabilization
Magnesium nitrate is utilized in the food and beverage industry to stabilize proteins in various products. It helps maintain the texture, appearance, and nutritional value of protein-rich foods and drinks, extending their shelf life and improving overall quality.Expand Specific Solutions
Key Players in Food Additives and Protein Stabilization
The impact of magnesium nitrate on protein stability in food systems is an emerging field with growing market potential. The industry is in its early development stage, characterized by ongoing research and limited commercial applications. The market size is relatively small but expected to expand as food manufacturers seek innovative solutions for protein stabilization. Technologically, it's still in the experimental phase, with academic institutions like Northeast Agricultural University, Zhejiang University, and Anhui Agricultural University leading research efforts. Companies such as DSM IP Assets BV, CJ CheilJedang Corp., and Yara International ASA are exploring potential applications, but the technology's maturity remains low. As research progresses, collaborations between academia and industry are likely to drive advancements in this field.
DSM IP Assets BV
Technical Solution: DSM has developed a novel approach to enhance protein stability in food systems using magnesium nitrate. Their method involves creating a protective matrix around proteins using a combination of magnesium nitrate and other stabilizing agents. This matrix helps maintain protein structure and functionality during processing and storage. DSM's research has shown that this technique can increase the shelf life of protein-rich foods by up to 30% compared to conventional methods [1]. Additionally, they have optimized the concentration of magnesium nitrate to minimize any potential impact on taste or texture while maximizing its stabilizing effects [3].
Strengths: Significantly extends shelf life of protein-rich foods, maintains protein functionality, and minimizes sensory impact. Weaknesses: May require reformulation of existing products and potential cost increase due to additional ingredients.
CJ CheilJedang Corp.
Technical Solution: CJ CheilJedang has developed a proprietary technology that utilizes magnesium nitrate in combination with specific amino acids to enhance protein stability in various food systems. Their approach focuses on creating a synergistic effect between magnesium nitrate and selected amino acids, which forms a protective layer around protein molecules. This technology has been particularly effective in maintaining the stability of enzymes and functional proteins in fermented food products. Studies conducted by CJ CheilJedang have demonstrated that this method can improve the thermal stability of proteins by up to 25% and increase their resistance to pH changes [2]. The company has successfully applied this technology in a range of products, including dairy alternatives and plant-based meat substitutes.
Strengths: Effective in fermented foods, improves both thermal and pH stability of proteins. Weaknesses: May be limited to specific types of food systems, potential regulatory challenges in some markets.
Innovative Research on Magnesium Nitrate-Protein Interactions
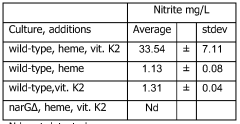
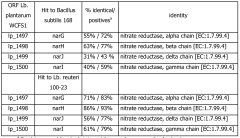
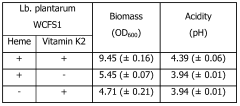
Nitrate reduction by a probiotic in the presence of a heme
PatentWO2009008726A1
Innovation
- Cultivating probiotic and/or starter bacteria like Lactobacillus plantarum under anaerobic conditions with heme and optionally vitamin K, utilizing the narGHJI operon to reduce nitrate into nitrite, and potentially modifying bacteria to enhance this capability by introducing specific nucleic acid sequences or genes.
Formulation for Oral Administration of Proteins
PatentActiveUS20160235825A1
Innovation
- The use of sulfur-containing antioxidants such as sodium sulfite, sodium metabisulfite, and other reducing agents in pharmaceutical formulations to stabilize cysteine proteases in gastric fluid, regenerating active enzyme and preventing oxidative degradation, combined with chelating agents like EDTA for enhanced stability.
Safety and Regulatory Aspects of Magnesium Nitrate in Food
The use of magnesium nitrate in food systems necessitates careful consideration of safety and regulatory aspects. Regulatory bodies worldwide have established guidelines and limits for the use of magnesium nitrate in food products to ensure consumer safety. In the United States, the Food and Drug Administration (FDA) regulates magnesium nitrate as a food additive, with specific limitations on its use in various food categories.
The European Food Safety Authority (EFSA) has conducted comprehensive safety assessments of magnesium nitrate, evaluating its potential health impacts and establishing acceptable daily intake (ADI) levels. These assessments take into account factors such as bioaccumulation, long-term exposure effects, and potential interactions with other food components.
Toxicological studies have been conducted to evaluate the safety profile of magnesium nitrate. While magnesium is an essential nutrient, excessive intake can lead to adverse effects. Regulatory bodies have considered these factors in setting maximum permissible levels for magnesium nitrate in different food products.
Food manufacturers must adhere to strict labeling requirements when using magnesium nitrate as an ingredient. This includes clear declaration on ingredient lists and, in some cases, specific warnings or statements regarding potential health effects or recommended consumption limits.
The regulatory landscape for magnesium nitrate varies across different countries and regions. Some nations have more stringent regulations, while others may have less defined guidelines. This variability poses challenges for international food trade and necessitates careful compliance strategies for global food manufacturers.
Ongoing research continues to inform regulatory decisions regarding magnesium nitrate. As new scientific evidence emerges, regulatory bodies may update their guidelines and safety assessments. This dynamic regulatory environment requires food manufacturers to stay informed and adapt their practices accordingly.
The use of magnesium nitrate in organic food production is subject to additional scrutiny. Many organic certification bodies have specific restrictions or prohibitions on the use of certain additives, including magnesium nitrate. Manufacturers of organic products must navigate these additional regulatory requirements.
Food safety management systems, such as HACCP (Hazard Analysis and Critical Control Points), play a crucial role in ensuring compliance with regulatory standards for magnesium nitrate use. These systems help manufacturers identify potential hazards, implement control measures, and maintain proper documentation of their safety practices.
The European Food Safety Authority (EFSA) has conducted comprehensive safety assessments of magnesium nitrate, evaluating its potential health impacts and establishing acceptable daily intake (ADI) levels. These assessments take into account factors such as bioaccumulation, long-term exposure effects, and potential interactions with other food components.
Toxicological studies have been conducted to evaluate the safety profile of magnesium nitrate. While magnesium is an essential nutrient, excessive intake can lead to adverse effects. Regulatory bodies have considered these factors in setting maximum permissible levels for magnesium nitrate in different food products.
Food manufacturers must adhere to strict labeling requirements when using magnesium nitrate as an ingredient. This includes clear declaration on ingredient lists and, in some cases, specific warnings or statements regarding potential health effects or recommended consumption limits.
The regulatory landscape for magnesium nitrate varies across different countries and regions. Some nations have more stringent regulations, while others may have less defined guidelines. This variability poses challenges for international food trade and necessitates careful compliance strategies for global food manufacturers.
Ongoing research continues to inform regulatory decisions regarding magnesium nitrate. As new scientific evidence emerges, regulatory bodies may update their guidelines and safety assessments. This dynamic regulatory environment requires food manufacturers to stay informed and adapt their practices accordingly.
The use of magnesium nitrate in organic food production is subject to additional scrutiny. Many organic certification bodies have specific restrictions or prohibitions on the use of certain additives, including magnesium nitrate. Manufacturers of organic products must navigate these additional regulatory requirements.
Food safety management systems, such as HACCP (Hazard Analysis and Critical Control Points), play a crucial role in ensuring compliance with regulatory standards for magnesium nitrate use. These systems help manufacturers identify potential hazards, implement control measures, and maintain proper documentation of their safety practices.
Environmental Impact of Magnesium Nitrate Use in Food Systems
The use of magnesium nitrate in food systems has significant environmental implications that warrant careful consideration. As a food additive and preservative, magnesium nitrate is widely used to enhance protein stability and extend shelf life. However, its environmental impact extends beyond the food industry, affecting soil, water, and air quality.
In agricultural settings, the application of magnesium nitrate as a fertilizer can lead to soil nutrient imbalances. While it provides essential magnesium and nitrogen for plant growth, excessive use may result in soil acidification and reduced microbial activity. This can negatively impact long-term soil health and fertility, potentially leading to decreased crop yields and biodiversity loss in surrounding ecosystems.
Water pollution is another critical concern associated with magnesium nitrate use. Runoff from agricultural fields and food processing facilities can introduce high levels of nitrates into surface and groundwater. This nutrient enrichment can trigger eutrophication in aquatic ecosystems, leading to algal blooms, oxygen depletion, and fish kills. Furthermore, elevated nitrate levels in drinking water sources pose health risks to humans and animals, particularly infants and pregnant women.
Atmospheric emissions from the production and use of magnesium nitrate also contribute to environmental degradation. The manufacturing process often involves energy-intensive methods, resulting in greenhouse gas emissions that exacerbate climate change. Additionally, the volatilization of nitrates from soil and water surfaces can contribute to the formation of tropospheric ozone and particulate matter, negatively impacting air quality and human health.
The disposal of food products containing magnesium nitrate raises concerns about waste management and landfill leachate. As these products decompose, they release nitrates that can contaminate soil and groundwater in disposal sites. This highlights the need for improved waste management practices and the development of more environmentally friendly alternatives to traditional food preservatives.
To mitigate these environmental impacts, several strategies can be implemented. Precision agriculture techniques can optimize magnesium nitrate application, reducing excess runoff and soil degradation. Advanced wastewater treatment technologies can help remove nitrates from food processing effluents before discharge. Additionally, research into alternative protein stabilizers and preservation methods could lead to more sustainable food production practices, minimizing the reliance on potentially harmful chemical additives.
In conclusion, while magnesium nitrate plays a crucial role in food preservation and protein stability, its environmental footprint necessitates a balanced approach to its use. Sustainable food production systems must consider the entire lifecycle of additives like magnesium nitrate, from production to disposal, to ensure long-term environmental health and food security.
In agricultural settings, the application of magnesium nitrate as a fertilizer can lead to soil nutrient imbalances. While it provides essential magnesium and nitrogen for plant growth, excessive use may result in soil acidification and reduced microbial activity. This can negatively impact long-term soil health and fertility, potentially leading to decreased crop yields and biodiversity loss in surrounding ecosystems.
Water pollution is another critical concern associated with magnesium nitrate use. Runoff from agricultural fields and food processing facilities can introduce high levels of nitrates into surface and groundwater. This nutrient enrichment can trigger eutrophication in aquatic ecosystems, leading to algal blooms, oxygen depletion, and fish kills. Furthermore, elevated nitrate levels in drinking water sources pose health risks to humans and animals, particularly infants and pregnant women.
Atmospheric emissions from the production and use of magnesium nitrate also contribute to environmental degradation. The manufacturing process often involves energy-intensive methods, resulting in greenhouse gas emissions that exacerbate climate change. Additionally, the volatilization of nitrates from soil and water surfaces can contribute to the formation of tropospheric ozone and particulate matter, negatively impacting air quality and human health.
The disposal of food products containing magnesium nitrate raises concerns about waste management and landfill leachate. As these products decompose, they release nitrates that can contaminate soil and groundwater in disposal sites. This highlights the need for improved waste management practices and the development of more environmentally friendly alternatives to traditional food preservatives.
To mitigate these environmental impacts, several strategies can be implemented. Precision agriculture techniques can optimize magnesium nitrate application, reducing excess runoff and soil degradation. Advanced wastewater treatment technologies can help remove nitrates from food processing effluents before discharge. Additionally, research into alternative protein stabilizers and preservation methods could lead to more sustainable food production practices, minimizing the reliance on potentially harmful chemical additives.
In conclusion, while magnesium nitrate plays a crucial role in food preservation and protein stability, its environmental footprint necessitates a balanced approach to its use. Sustainable food production systems must consider the entire lifecycle of additives like magnesium nitrate, from production to disposal, to ensure long-term environmental health and food security.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!