The Role of Magnesium Nitrate in Advanced Oxidation Processes
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate in AOP: Background and Objectives
Advanced Oxidation Processes (AOPs) have emerged as a promising technology for water and wastewater treatment, capable of degrading a wide range of recalcitrant organic pollutants. Within this context, magnesium nitrate has garnered significant attention for its potential role in enhancing the efficiency and effectiveness of AOPs. The evolution of this technology can be traced back to the 1970s when the concept of using hydroxyl radicals for oxidation was first introduced.
Over the past few decades, researchers and industry professionals have been exploring various ways to improve the performance of AOPs, with a particular focus on developing more efficient catalysts and oxidants. Magnesium nitrate, a compound traditionally known for its applications in agriculture and pyrotechnics, has recently emerged as a promising candidate for AOP enhancement. Its unique properties and potential synergistic effects with other components in AOPs have sparked interest in the scientific community.
The primary objective of investigating magnesium nitrate in AOPs is to address the limitations of conventional treatment methods, particularly in dealing with persistent organic pollutants. These contaminants, which are resistant to traditional biological and chemical treatments, pose significant challenges to water quality and environmental health. By incorporating magnesium nitrate into AOP systems, researchers aim to achieve higher degradation rates, lower energy consumption, and improved overall treatment efficiency.
Another crucial goal is to expand the applicability of AOPs across various industrial sectors. While AOPs have shown promise in treating municipal wastewater and certain industrial effluents, there is a growing need to adapt these processes for more complex waste streams. Magnesium nitrate's potential to enhance the oxidation capacity and stability of AOP systems could pave the way for broader industrial applications, including in the pharmaceutical, textile, and petrochemical industries.
Furthermore, the integration of magnesium nitrate in AOPs aligns with the global trend towards developing more sustainable and environmentally friendly treatment technologies. As regulatory standards for water quality become increasingly stringent, there is a pressing need for innovative solutions that can effectively remove emerging contaminants while minimizing the environmental footprint of treatment processes. The exploration of magnesium nitrate in this context represents a step towards achieving these sustainability goals.
In conclusion, the investigation of magnesium nitrate's role in AOPs is driven by the need for more efficient, versatile, and sustainable water treatment solutions. By understanding the fundamental mechanisms and optimizing the use of magnesium nitrate in these processes, researchers and industry professionals aim to overcome current limitations and unlock new possibilities in water and wastewater treatment technologies.
Over the past few decades, researchers and industry professionals have been exploring various ways to improve the performance of AOPs, with a particular focus on developing more efficient catalysts and oxidants. Magnesium nitrate, a compound traditionally known for its applications in agriculture and pyrotechnics, has recently emerged as a promising candidate for AOP enhancement. Its unique properties and potential synergistic effects with other components in AOPs have sparked interest in the scientific community.
The primary objective of investigating magnesium nitrate in AOPs is to address the limitations of conventional treatment methods, particularly in dealing with persistent organic pollutants. These contaminants, which are resistant to traditional biological and chemical treatments, pose significant challenges to water quality and environmental health. By incorporating magnesium nitrate into AOP systems, researchers aim to achieve higher degradation rates, lower energy consumption, and improved overall treatment efficiency.
Another crucial goal is to expand the applicability of AOPs across various industrial sectors. While AOPs have shown promise in treating municipal wastewater and certain industrial effluents, there is a growing need to adapt these processes for more complex waste streams. Magnesium nitrate's potential to enhance the oxidation capacity and stability of AOP systems could pave the way for broader industrial applications, including in the pharmaceutical, textile, and petrochemical industries.
Furthermore, the integration of magnesium nitrate in AOPs aligns with the global trend towards developing more sustainable and environmentally friendly treatment technologies. As regulatory standards for water quality become increasingly stringent, there is a pressing need for innovative solutions that can effectively remove emerging contaminants while minimizing the environmental footprint of treatment processes. The exploration of magnesium nitrate in this context represents a step towards achieving these sustainability goals.
In conclusion, the investigation of magnesium nitrate's role in AOPs is driven by the need for more efficient, versatile, and sustainable water treatment solutions. By understanding the fundamental mechanisms and optimizing the use of magnesium nitrate in these processes, researchers and industry professionals aim to overcome current limitations and unlock new possibilities in water and wastewater treatment technologies.
Market Demand for AOP Technologies
The market demand for Advanced Oxidation Process (AOP) technologies has been steadily increasing in recent years, driven by growing environmental concerns and stringent regulations on water and air quality. AOPs are gaining traction across various industries due to their effectiveness in treating recalcitrant pollutants and emerging contaminants that conventional treatment methods struggle to address.
In the water treatment sector, AOPs are becoming increasingly popular for both industrial and municipal applications. The global water and wastewater treatment market is expected to reach significant growth in the coming years, with AOPs playing a crucial role in this expansion. Industries such as pharmaceuticals, textiles, and petrochemicals are adopting AOP technologies to meet discharge standards and reduce their environmental footprint.
The demand for AOPs in air pollution control is also on the rise, particularly in regions with severe air quality issues. As governments worldwide implement stricter air quality regulations, industries are turning to advanced oxidation techniques to reduce emissions of volatile organic compounds (VOCs) and other harmful pollutants.
The role of magnesium nitrate in AOPs is gaining attention within this growing market. Magnesium nitrate's potential as a catalyst or oxidant enhancer in certain AOP applications is driving research and development efforts. Its ability to potentially improve the efficiency and cost-effectiveness of AOP systems could further stimulate market demand.
Emerging applications of AOPs in soil remediation and food processing are opening new market opportunities. The agriculture sector is showing interest in AOPs for pesticide degradation and soil decontamination, while the food industry is exploring these technologies for disinfection and shelf-life extension of products.
The market for AOP technologies is characterized by a growing demand for more sustainable and energy-efficient solutions. This trend is pushing manufacturers to develop innovative AOP systems that minimize energy consumption and chemical usage while maximizing treatment efficiency. The integration of AOPs with other treatment technologies, such as membrane filtration or biological processes, is another area of market growth.
Geographically, North America and Europe currently lead the AOP technology market due to stringent environmental regulations and high adoption rates in industrial sectors. However, rapid industrialization and urbanization in Asia-Pacific and Latin America are creating significant growth opportunities for AOP technologies in these regions.
In the water treatment sector, AOPs are becoming increasingly popular for both industrial and municipal applications. The global water and wastewater treatment market is expected to reach significant growth in the coming years, with AOPs playing a crucial role in this expansion. Industries such as pharmaceuticals, textiles, and petrochemicals are adopting AOP technologies to meet discharge standards and reduce their environmental footprint.
The demand for AOPs in air pollution control is also on the rise, particularly in regions with severe air quality issues. As governments worldwide implement stricter air quality regulations, industries are turning to advanced oxidation techniques to reduce emissions of volatile organic compounds (VOCs) and other harmful pollutants.
The role of magnesium nitrate in AOPs is gaining attention within this growing market. Magnesium nitrate's potential as a catalyst or oxidant enhancer in certain AOP applications is driving research and development efforts. Its ability to potentially improve the efficiency and cost-effectiveness of AOP systems could further stimulate market demand.
Emerging applications of AOPs in soil remediation and food processing are opening new market opportunities. The agriculture sector is showing interest in AOPs for pesticide degradation and soil decontamination, while the food industry is exploring these technologies for disinfection and shelf-life extension of products.
The market for AOP technologies is characterized by a growing demand for more sustainable and energy-efficient solutions. This trend is pushing manufacturers to develop innovative AOP systems that minimize energy consumption and chemical usage while maximizing treatment efficiency. The integration of AOPs with other treatment technologies, such as membrane filtration or biological processes, is another area of market growth.
Geographically, North America and Europe currently lead the AOP technology market due to stringent environmental regulations and high adoption rates in industrial sectors. However, rapid industrialization and urbanization in Asia-Pacific and Latin America are creating significant growth opportunities for AOP technologies in these regions.
Current State of Magnesium Nitrate in AOP
Magnesium nitrate has emerged as a significant component in Advanced Oxidation Processes (AOPs), playing a crucial role in enhancing the efficiency of water treatment and environmental remediation. The current state of magnesium nitrate in AOPs reflects a growing interest in its application due to its unique properties and synergistic effects with other oxidizing agents.
In recent years, researchers have extensively investigated the use of magnesium nitrate as a catalyst and promoter in various AOP systems. Its primary function is to enhance the generation of reactive oxygen species (ROS), particularly hydroxyl radicals, which are the key oxidizing agents in AOPs. The presence of magnesium nitrate has been shown to significantly improve the degradation rates of recalcitrant organic pollutants in water and wastewater treatment processes.
One of the most promising applications of magnesium nitrate in AOPs is its integration with photocatalytic systems. Studies have demonstrated that the addition of magnesium nitrate to titanium dioxide (TiO2) photocatalysts can substantially increase their photocatalytic activity. This enhancement is attributed to the formation of magnesium-doped TiO2 structures, which exhibit improved charge separation and extended light absorption range, leading to more efficient pollutant degradation under both UV and visible light irradiation.
Furthermore, magnesium nitrate has shown remarkable potential in Fenton-like processes, where it acts as a catalyst to promote the decomposition of hydrogen peroxide into hydroxyl radicals. The magnesium ions from magnesium nitrate can activate hydrogen peroxide through a similar mechanism to iron-based Fenton reactions, but with the added advantage of being effective over a wider pH range. This characteristic makes magnesium nitrate-based AOPs particularly suitable for treating alkaline wastewaters without the need for pH adjustment.
In the field of electrochemical AOPs, magnesium nitrate has been explored as an electrolyte and supporting agent. Its presence in the electrolyte solution has been found to enhance the generation of reactive species at the electrode surface, leading to improved degradation efficiency of organic contaminants. Additionally, the magnesium ions can contribute to the formation of beneficial reactive intermediates, further accelerating the oxidation processes.
Recent advancements in nanotechnology have opened new avenues for the application of magnesium nitrate in AOPs. Nanostructured magnesium-based materials, synthesized using magnesium nitrate as a precursor, have shown exceptional catalytic properties in various oxidation reactions. These nanomaterials offer increased surface area and improved reactivity, resulting in enhanced pollutant removal rates and reduced treatment times.
Despite the promising results, challenges remain in fully understanding the mechanisms by which magnesium nitrate enhances AOP performance. Ongoing research is focused on elucidating the complex interactions between magnesium nitrate and other components of AOP systems, as well as optimizing its concentration and application methods for maximum efficiency. As environmental regulations become more stringent, the role of magnesium nitrate in AOPs is expected to grow, driving further innovation in water treatment technologies.
In recent years, researchers have extensively investigated the use of magnesium nitrate as a catalyst and promoter in various AOP systems. Its primary function is to enhance the generation of reactive oxygen species (ROS), particularly hydroxyl radicals, which are the key oxidizing agents in AOPs. The presence of magnesium nitrate has been shown to significantly improve the degradation rates of recalcitrant organic pollutants in water and wastewater treatment processes.
One of the most promising applications of magnesium nitrate in AOPs is its integration with photocatalytic systems. Studies have demonstrated that the addition of magnesium nitrate to titanium dioxide (TiO2) photocatalysts can substantially increase their photocatalytic activity. This enhancement is attributed to the formation of magnesium-doped TiO2 structures, which exhibit improved charge separation and extended light absorption range, leading to more efficient pollutant degradation under both UV and visible light irradiation.
Furthermore, magnesium nitrate has shown remarkable potential in Fenton-like processes, where it acts as a catalyst to promote the decomposition of hydrogen peroxide into hydroxyl radicals. The magnesium ions from magnesium nitrate can activate hydrogen peroxide through a similar mechanism to iron-based Fenton reactions, but with the added advantage of being effective over a wider pH range. This characteristic makes magnesium nitrate-based AOPs particularly suitable for treating alkaline wastewaters without the need for pH adjustment.
In the field of electrochemical AOPs, magnesium nitrate has been explored as an electrolyte and supporting agent. Its presence in the electrolyte solution has been found to enhance the generation of reactive species at the electrode surface, leading to improved degradation efficiency of organic contaminants. Additionally, the magnesium ions can contribute to the formation of beneficial reactive intermediates, further accelerating the oxidation processes.
Recent advancements in nanotechnology have opened new avenues for the application of magnesium nitrate in AOPs. Nanostructured magnesium-based materials, synthesized using magnesium nitrate as a precursor, have shown exceptional catalytic properties in various oxidation reactions. These nanomaterials offer increased surface area and improved reactivity, resulting in enhanced pollutant removal rates and reduced treatment times.
Despite the promising results, challenges remain in fully understanding the mechanisms by which magnesium nitrate enhances AOP performance. Ongoing research is focused on elucidating the complex interactions between magnesium nitrate and other components of AOP systems, as well as optimizing its concentration and application methods for maximum efficiency. As environmental regulations become more stringent, the role of magnesium nitrate in AOPs is expected to grow, driving further innovation in water treatment technologies.
Existing AOP Solutions with Magnesium Nitrate
01 Magnesium nitrate in fertilizer compositions
Magnesium nitrate is used in fertilizer compositions to provide both magnesium and nitrogen nutrients to plants. These compositions can be formulated as liquid or solid fertilizers, often combined with other nutrients to create balanced plant nutrition solutions.- Magnesium nitrate in fertilizer compositions: Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer compositions can be tailored for specific crops or soil conditions.
- Magnesium nitrate in energy storage applications: Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its ability to absorb and release heat during phase transitions. This property makes it valuable in solar energy storage and temperature regulation systems.
- Magnesium nitrate in flame retardant formulations: Magnesium nitrate is incorporated into flame retardant formulations for various materials, including textiles, plastics, and wood products. It acts as an effective fire suppressant by releasing non-flammable gases when exposed to high temperatures, thereby inhibiting the spread of flames.
- Magnesium nitrate in water treatment processes: Magnesium nitrate is employed in water treatment processes for its ability to remove contaminants and improve water quality. It can be used in the treatment of wastewater, groundwater, and industrial effluents to remove heavy metals, phosphates, and other pollutants through precipitation and ion exchange mechanisms.
- Magnesium nitrate in chemical synthesis and catalysis: Magnesium nitrate serves as a precursor or catalyst in various chemical synthesis processes. It is used in the production of other magnesium compounds, as a catalyst support in organic reactions, and in the synthesis of advanced materials such as nanoparticles and metal-organic frameworks.
02 Magnesium nitrate in energy storage applications
Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage materials. It can be incorporated into phase change materials or used in heat transfer fluids due to its thermal properties and ability to store and release heat energy efficiently.Expand Specific Solutions03 Magnesium nitrate in flame retardant compositions
Magnesium nitrate is employed in flame retardant formulations for various materials. It can enhance fire resistance properties when incorporated into coatings, textiles, or polymer composites, often synergizing with other flame retardant compounds.Expand Specific Solutions04 Magnesium nitrate in water treatment processes
Magnesium nitrate finds applications in water treatment processes, including wastewater treatment and desalination. It can be used for removing contaminants, adjusting water hardness, or as part of membrane treatment systems.Expand Specific Solutions05 Magnesium nitrate in chemical synthesis and catalysis
Magnesium nitrate serves as a precursor or catalyst in various chemical synthesis processes. It can be used in the production of other magnesium compounds, as a component in catalytic systems, or in the synthesis of advanced materials such as nanoparticles or metal-organic frameworks.Expand Specific Solutions
Key Players in AOP Industry
The advanced oxidation processes (AOPs) market is in a growth phase, driven by increasing environmental concerns and stringent regulations. The global AOP market size is projected to expand significantly in the coming years, with a growing emphasis on water and wastewater treatment applications. The technology's maturity varies across different AOP methods, with some well-established techniques and others still emerging. Companies like Sharp Corp., BASF Corp., and Koninklijke Philips NV are actively involved in AOP research and development, focusing on improving efficiency and expanding applications. Academic institutions such as the University of Strasbourg and Nanjing University are contributing to the field through fundamental research and innovative approaches, while research organizations like CNRS and Fraunhofer-Gesellschaft are bridging the gap between academia and industry, accelerating the technology's advancement and commercialization.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has been at the forefront of research on the role of magnesium nitrate in advanced oxidation processes (AOPs). Their approach involves utilizing magnesium nitrate as a catalyst enhancer in Fenton-like reactions, significantly improving the efficiency of pollutant degradation in wastewater treatment[1]. The CNRS team has developed a novel method combining magnesium nitrate with iron-based catalysts, which has shown remarkable results in degrading recalcitrant organic compounds. This synergistic effect leads to enhanced production of hydroxyl radicals, the key oxidizing agents in AOPs[2]. Furthermore, their research has demonstrated that magnesium nitrate can stabilize the catalytic activity of iron species, prolonging the effective lifespan of the treatment system and reducing overall operational costs[3].
Strengths: Improved efficiency in pollutant degradation, enhanced production of hydroxyl radicals, and prolonged catalyst lifespan. Weaknesses: Potential for increased treatment costs due to the addition of magnesium nitrate, and possible pH sensitivity of the process.
Consiglio Nazionale delle Ricerche
Technical Solution: The Consiglio Nazionale delle Ricerche (CNR) has made significant strides in understanding the role of magnesium nitrate in advanced oxidation processes. Their research focuses on the application of magnesium nitrate as a promoter in photocatalytic AOPs, particularly in the degradation of emerging contaminants in water[1]. CNR scientists have developed a novel nanocomposite material incorporating magnesium nitrate, which exhibits enhanced photocatalytic activity under visible light irradiation[2]. This innovation addresses one of the key limitations of traditional photocatalysts, which often require UV light for activation. Additionally, the CNR team has explored the use of magnesium nitrate in electrochemical AOPs, where it serves as an electrolyte enhancer, improving the conductivity and overall efficiency of the treatment process[3].
Strengths: Enhanced photocatalytic activity under visible light, improved efficiency in electrochemical AOPs. Weaknesses: Potential for magnesium accumulation in treated water, and possible scaling issues in long-term applications.
Core Innovations in Magnesium Nitrate-based AOP
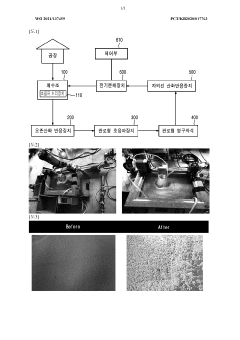
Advanced oxidation treatment apparatus comprising electrolysis apparatus for molecular destruction, and advanced oxidation treatment method using same
PatentWO2021137459A1
Innovation
- A cyclic advanced oxidation treatment device and method combining an ozone oxidation reaction device, ultraviolet oxidation reaction device, ultrasonic treatment, and electrolysis device with a control unit for polarity conversion, which generates OH radicals and applies magnetic fields to efficiently decompose pollutants in a wastewater tank, allowing for simultaneous dissociation of ionic and covalent substances.
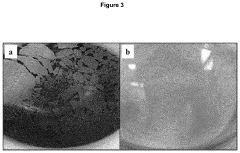
Preparation of magnetite nanoparticles and uses thereof
PatentPendingUS20220371912A1
Innovation
- A method involving a gas-liquid reaction interface between an ammonia gas headspace and an aqueous ferrous and ferric iron salts solution, without agitation, to synthesize magnetite nanoparticles with high yield and phase purity, using a total iron salts concentration of 1-10 mM, and varying ammonia gas concentrations to control the reaction.
Environmental Impact of Magnesium Nitrate in AOP
The environmental impact of magnesium nitrate in Advanced Oxidation Processes (AOPs) is a critical consideration for the implementation and sustainability of these water treatment technologies. Magnesium nitrate, when used in AOPs, can have both positive and negative effects on the environment, necessitating a careful evaluation of its role in these processes.
One of the primary environmental benefits of using magnesium nitrate in AOPs is its potential to enhance the efficiency of pollutant degradation. By acting as a catalyst or electron acceptor, magnesium nitrate can accelerate the production of highly reactive species such as hydroxyl radicals, which are essential for the breakdown of recalcitrant organic compounds. This increased efficiency can lead to reduced treatment times and energy consumption, ultimately minimizing the overall environmental footprint of the water treatment process.
However, the introduction of magnesium nitrate into aquatic ecosystems through treated effluents may pose certain risks. Excess nitrate levels in water bodies can contribute to eutrophication, a process that leads to algal blooms and subsequent oxygen depletion. This can have detrimental effects on aquatic life and overall ecosystem health. Therefore, careful monitoring and control of magnesium nitrate dosages in AOPs are crucial to prevent unintended environmental consequences.
The fate and transport of magnesium nitrate in the environment after its use in AOPs is another important aspect to consider. While magnesium is generally considered non-toxic and is an essential nutrient for plants, excessive concentrations in soil or water can disrupt the natural balance of ecosystems. The potential for magnesium accumulation in sediments and its impact on benthic organisms should be thoroughly investigated to ensure long-term environmental sustainability.
From a life cycle perspective, the production and transportation of magnesium nitrate for use in AOPs also contribute to the overall environmental impact. The energy and resources required for manufacturing and distributing this chemical compound should be weighed against the environmental benefits gained from its application in water treatment processes. Efforts to develop more sustainable production methods and optimize transportation logistics can help mitigate these upstream environmental impacts.
In terms of regulatory compliance, the use of magnesium nitrate in AOPs must adhere to environmental standards and discharge limits set by local and national authorities. Continuous monitoring of treated effluents and receiving water bodies is essential to ensure that the application of magnesium nitrate does not lead to violations of water quality standards or cause unintended ecological disturbances.
Research into alternative compounds or process modifications that could reduce or eliminate the need for magnesium nitrate in AOPs while maintaining treatment efficacy is ongoing. These efforts aim to develop more environmentally friendly approaches that minimize potential negative impacts while maximizing the benefits of advanced oxidation technologies in water treatment.
One of the primary environmental benefits of using magnesium nitrate in AOPs is its potential to enhance the efficiency of pollutant degradation. By acting as a catalyst or electron acceptor, magnesium nitrate can accelerate the production of highly reactive species such as hydroxyl radicals, which are essential for the breakdown of recalcitrant organic compounds. This increased efficiency can lead to reduced treatment times and energy consumption, ultimately minimizing the overall environmental footprint of the water treatment process.
However, the introduction of magnesium nitrate into aquatic ecosystems through treated effluents may pose certain risks. Excess nitrate levels in water bodies can contribute to eutrophication, a process that leads to algal blooms and subsequent oxygen depletion. This can have detrimental effects on aquatic life and overall ecosystem health. Therefore, careful monitoring and control of magnesium nitrate dosages in AOPs are crucial to prevent unintended environmental consequences.
The fate and transport of magnesium nitrate in the environment after its use in AOPs is another important aspect to consider. While magnesium is generally considered non-toxic and is an essential nutrient for plants, excessive concentrations in soil or water can disrupt the natural balance of ecosystems. The potential for magnesium accumulation in sediments and its impact on benthic organisms should be thoroughly investigated to ensure long-term environmental sustainability.
From a life cycle perspective, the production and transportation of magnesium nitrate for use in AOPs also contribute to the overall environmental impact. The energy and resources required for manufacturing and distributing this chemical compound should be weighed against the environmental benefits gained from its application in water treatment processes. Efforts to develop more sustainable production methods and optimize transportation logistics can help mitigate these upstream environmental impacts.
In terms of regulatory compliance, the use of magnesium nitrate in AOPs must adhere to environmental standards and discharge limits set by local and national authorities. Continuous monitoring of treated effluents and receiving water bodies is essential to ensure that the application of magnesium nitrate does not lead to violations of water quality standards or cause unintended ecological disturbances.
Research into alternative compounds or process modifications that could reduce or eliminate the need for magnesium nitrate in AOPs while maintaining treatment efficacy is ongoing. These efforts aim to develop more environmentally friendly approaches that minimize potential negative impacts while maximizing the benefits of advanced oxidation technologies in water treatment.
Cost-Benefit Analysis of Magnesium Nitrate in AOP
The cost-benefit analysis of magnesium nitrate in Advanced Oxidation Processes (AOPs) reveals a complex interplay of economic and environmental factors. Initial investment costs for implementing magnesium nitrate-based AOPs are generally moderate, with the chemical itself being relatively inexpensive and readily available. However, the true value lies in its potential to enhance the efficiency of oxidation reactions, potentially reducing overall treatment times and energy consumption.
One of the primary benefits of using magnesium nitrate in AOPs is its ability to act as a catalyst, accelerating the generation of hydroxyl radicals. This increased reaction rate can lead to faster degradation of pollutants, potentially reducing the operational time of treatment systems. Consequently, this may result in lower energy costs and increased throughput capacity, which can be particularly advantageous for large-scale water treatment facilities.
Furthermore, magnesium nitrate's role in AOPs can lead to improved removal efficiencies for a wide range of contaminants, including recalcitrant organic compounds. This enhanced performance may reduce the need for additional treatment steps or the use of more expensive oxidizing agents, thereby offering potential cost savings in the long term. The versatility of magnesium nitrate-enhanced AOPs also allows for the treatment of various water matrices, from industrial wastewater to drinking water, expanding its potential applications and economic benefits.
However, the cost-benefit analysis must also consider potential drawbacks. The use of magnesium nitrate may increase the total dissolved solids (TDS) in the treated water, potentially necessitating additional post-treatment processes in some applications. This could offset some of the initial cost savings, especially in scenarios where stringent effluent quality standards must be met.
Environmental considerations also play a crucial role in the cost-benefit analysis. While magnesium nitrate itself is not considered highly toxic, its use in AOPs may lead to the formation of nitrate by-products. Depending on the application and regulatory requirements, additional denitrification steps may be necessary, adding to the overall treatment costs. However, this should be weighed against the environmental benefits of more effective pollutant removal and potentially reduced chemical usage compared to conventional treatment methods.
In terms of operational costs, the dosage requirements for magnesium nitrate are generally lower compared to some other catalysts or oxidants used in AOPs. This can lead to reduced chemical handling and storage costs. Additionally, the stability of magnesium nitrate solutions allows for easier on-site storage and dosing, potentially simplifying operational procedures and reducing associated labor costs.
One of the primary benefits of using magnesium nitrate in AOPs is its ability to act as a catalyst, accelerating the generation of hydroxyl radicals. This increased reaction rate can lead to faster degradation of pollutants, potentially reducing the operational time of treatment systems. Consequently, this may result in lower energy costs and increased throughput capacity, which can be particularly advantageous for large-scale water treatment facilities.
Furthermore, magnesium nitrate's role in AOPs can lead to improved removal efficiencies for a wide range of contaminants, including recalcitrant organic compounds. This enhanced performance may reduce the need for additional treatment steps or the use of more expensive oxidizing agents, thereby offering potential cost savings in the long term. The versatility of magnesium nitrate-enhanced AOPs also allows for the treatment of various water matrices, from industrial wastewater to drinking water, expanding its potential applications and economic benefits.
However, the cost-benefit analysis must also consider potential drawbacks. The use of magnesium nitrate may increase the total dissolved solids (TDS) in the treated water, potentially necessitating additional post-treatment processes in some applications. This could offset some of the initial cost savings, especially in scenarios where stringent effluent quality standards must be met.
Environmental considerations also play a crucial role in the cost-benefit analysis. While magnesium nitrate itself is not considered highly toxic, its use in AOPs may lead to the formation of nitrate by-products. Depending on the application and regulatory requirements, additional denitrification steps may be necessary, adding to the overall treatment costs. However, this should be weighed against the environmental benefits of more effective pollutant removal and potentially reduced chemical usage compared to conventional treatment methods.
In terms of operational costs, the dosage requirements for magnesium nitrate are generally lower compared to some other catalysts or oxidants used in AOPs. This can lead to reduced chemical handling and storage costs. Additionally, the stability of magnesium nitrate solutions allows for easier on-site storage and dosing, potentially simplifying operational procedures and reducing associated labor costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!