The Role of Magnesium Nitrate in Biotechnological Fermentation
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate in Fermentation: Background and Objectives
Magnesium nitrate has emerged as a crucial component in biotechnological fermentation processes, playing a significant role in enhancing microbial growth and metabolic activities. The evolution of this technology can be traced back to the early 20th century when researchers first recognized the importance of mineral nutrients in microbial cultivation. Over the decades, the understanding of magnesium's role in cellular functions has deepened, leading to its widespread use in fermentation media.
The progression of magnesium nitrate utilization in fermentation has been marked by several key milestones. Initially, it was primarily used as a simple nitrogen source. However, as biochemical knowledge advanced, its multifaceted benefits became apparent. Magnesium's role as a cofactor for numerous enzymes and its involvement in cell membrane stability have been extensively studied, revealing its critical importance in various metabolic pathways.
Recent trends in fermentation technology have focused on optimizing nutrient compositions to maximize product yields and improve process efficiency. This has led to a renewed interest in understanding the precise mechanisms by which magnesium nitrate influences microbial physiology and fermentation outcomes. The advent of systems biology and metabolic engineering has further accelerated research in this area, enabling more targeted approaches to nutrient supplementation.
The primary objective of current research in this field is to elucidate the optimal concentrations and delivery methods of magnesium nitrate in different fermentation processes. This includes investigating its effects on various microbial strains and its interactions with other media components. Researchers aim to develop tailored nutrient formulations that can enhance specific metabolic pathways, leading to improved production of desired compounds such as enzymes, biofuels, and pharmaceuticals.
Another key goal is to understand the broader implications of magnesium nitrate supplementation on industrial-scale fermentations. This involves studying its impact on process parameters like pH regulation, foam control, and product recovery. Additionally, there is growing interest in exploring sustainable sources of magnesium nitrate and developing eco-friendly fermentation processes that align with the principles of green chemistry.
As the biotechnology industry continues to expand, the role of magnesium nitrate in fermentation is expected to gain even more prominence. Future research directions may include the development of smart fermentation systems that can dynamically adjust magnesium nitrate levels based on real-time monitoring of microbial metabolic states. This could lead to more efficient and cost-effective bioprocesses, ultimately contributing to advancements in various sectors, from food and beverage production to pharmaceutical manufacturing.
The progression of magnesium nitrate utilization in fermentation has been marked by several key milestones. Initially, it was primarily used as a simple nitrogen source. However, as biochemical knowledge advanced, its multifaceted benefits became apparent. Magnesium's role as a cofactor for numerous enzymes and its involvement in cell membrane stability have been extensively studied, revealing its critical importance in various metabolic pathways.
Recent trends in fermentation technology have focused on optimizing nutrient compositions to maximize product yields and improve process efficiency. This has led to a renewed interest in understanding the precise mechanisms by which magnesium nitrate influences microbial physiology and fermentation outcomes. The advent of systems biology and metabolic engineering has further accelerated research in this area, enabling more targeted approaches to nutrient supplementation.
The primary objective of current research in this field is to elucidate the optimal concentrations and delivery methods of magnesium nitrate in different fermentation processes. This includes investigating its effects on various microbial strains and its interactions with other media components. Researchers aim to develop tailored nutrient formulations that can enhance specific metabolic pathways, leading to improved production of desired compounds such as enzymes, biofuels, and pharmaceuticals.
Another key goal is to understand the broader implications of magnesium nitrate supplementation on industrial-scale fermentations. This involves studying its impact on process parameters like pH regulation, foam control, and product recovery. Additionally, there is growing interest in exploring sustainable sources of magnesium nitrate and developing eco-friendly fermentation processes that align with the principles of green chemistry.
As the biotechnology industry continues to expand, the role of magnesium nitrate in fermentation is expected to gain even more prominence. Future research directions may include the development of smart fermentation systems that can dynamically adjust magnesium nitrate levels based on real-time monitoring of microbial metabolic states. This could lead to more efficient and cost-effective bioprocesses, ultimately contributing to advancements in various sectors, from food and beverage production to pharmaceutical manufacturing.
Market Analysis of Magnesium Nitrate in Biotech Industry
The market for magnesium nitrate in the biotechnology industry has been experiencing steady growth, driven by its crucial role in fermentation processes. This compound serves as an essential nutrient for microorganisms, promoting cell growth and enhancing metabolic activities during fermentation. The increasing demand for bio-based products, such as enzymes, amino acids, and organic acids, has significantly contributed to the expansion of the magnesium nitrate market in biotech applications.
In recent years, the global biotechnology industry has witnessed substantial growth, with a particular focus on sustainable and eco-friendly production methods. This trend has led to a surge in demand for fermentation-based processes, consequently boosting the market for magnesium nitrate. The compound's ability to improve yield and product quality in various fermentation applications has made it a preferred choice among biotech companies.
The pharmaceutical and food industries are major consumers of magnesium nitrate in biotechnological fermentation. In pharmaceutical applications, it is used in the production of antibiotics, vitamins, and other therapeutic compounds. The food industry utilizes magnesium nitrate in the fermentation of probiotics, flavoring agents, and food additives. These sectors are expected to continue driving the demand for magnesium nitrate in the coming years.
Geographically, North America and Europe currently dominate the market for magnesium nitrate in biotech applications. This is primarily due to the presence of well-established biotechnology companies and research institutions in these regions. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing investments in biotechnology research and development, particularly in countries like China and India.
The market is characterized by a mix of large multinational chemical companies and specialized suppliers. Key players are focusing on product innovation and strategic partnerships to maintain their competitive edge. There is also a growing emphasis on developing high-purity grades of magnesium nitrate to meet the stringent quality requirements of the biotech industry.
Despite the positive outlook, the market faces challenges such as price volatility of raw materials and environmental concerns associated with nitrate compounds. However, ongoing research into sustainable production methods and the development of bio-based alternatives may help address these issues in the long term.
In conclusion, the market for magnesium nitrate in the biotechnology industry shows promising growth potential. As the biotech sector continues to expand and diversify, the demand for magnesium nitrate in fermentation processes is expected to increase, creating new opportunities for market players and driving further innovation in this space.
In recent years, the global biotechnology industry has witnessed substantial growth, with a particular focus on sustainable and eco-friendly production methods. This trend has led to a surge in demand for fermentation-based processes, consequently boosting the market for magnesium nitrate. The compound's ability to improve yield and product quality in various fermentation applications has made it a preferred choice among biotech companies.
The pharmaceutical and food industries are major consumers of magnesium nitrate in biotechnological fermentation. In pharmaceutical applications, it is used in the production of antibiotics, vitamins, and other therapeutic compounds. The food industry utilizes magnesium nitrate in the fermentation of probiotics, flavoring agents, and food additives. These sectors are expected to continue driving the demand for magnesium nitrate in the coming years.
Geographically, North America and Europe currently dominate the market for magnesium nitrate in biotech applications. This is primarily due to the presence of well-established biotechnology companies and research institutions in these regions. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing investments in biotechnology research and development, particularly in countries like China and India.
The market is characterized by a mix of large multinational chemical companies and specialized suppliers. Key players are focusing on product innovation and strategic partnerships to maintain their competitive edge. There is also a growing emphasis on developing high-purity grades of magnesium nitrate to meet the stringent quality requirements of the biotech industry.
Despite the positive outlook, the market faces challenges such as price volatility of raw materials and environmental concerns associated with nitrate compounds. However, ongoing research into sustainable production methods and the development of bio-based alternatives may help address these issues in the long term.
In conclusion, the market for magnesium nitrate in the biotechnology industry shows promising growth potential. As the biotech sector continues to expand and diversify, the demand for magnesium nitrate in fermentation processes is expected to increase, creating new opportunities for market players and driving further innovation in this space.
Current Challenges in Magnesium Nitrate Utilization
Despite the significant potential of magnesium nitrate in biotechnological fermentation, several challenges currently hinder its widespread adoption and optimal utilization. One of the primary obstacles is the precise control of magnesium nitrate concentration in fermentation media. Excessive levels can lead to osmotic stress and inhibit microbial growth, while insufficient amounts may result in suboptimal enzyme activity and metabolic processes.
Another challenge lies in the complex interactions between magnesium nitrate and other media components. The presence of certain ions or organic compounds can affect the bioavailability of magnesium, potentially leading to unexpected fermentation outcomes. This necessitates extensive optimization studies for each specific fermentation process, which can be time-consuming and resource-intensive.
The stability of magnesium nitrate in fermentation broths poses an additional challenge. Under certain conditions, such as high temperatures or extreme pH levels, magnesium nitrate may undergo chemical transformations or precipitate out of solution. This instability can lead to inconsistent fermentation results and difficulties in maintaining steady-state conditions throughout the bioprocess.
Furthermore, the impact of magnesium nitrate on downstream processing and product recovery has not been fully elucidated. In some cases, residual magnesium ions may interfere with purification steps or affect the final product quality, necessitating additional separation or treatment processes.
The environmental implications of using magnesium nitrate in large-scale fermentations also present challenges. The discharge of nitrate-rich effluents can contribute to eutrophication in aquatic ecosystems, requiring careful waste management strategies and potentially increasing operational costs.
Additionally, there is a lack of standardized protocols for incorporating magnesium nitrate into various fermentation processes. This absence of established guidelines makes it difficult for researchers and industry professionals to effectively compare results across different studies or implement optimized conditions in diverse fermentation systems.
Lastly, the economic feasibility of using magnesium nitrate in industrial-scale fermentations remains a concern. While its benefits in enhancing microbial growth and product yields are recognized, the cost-effectiveness of magnesium nitrate supplementation compared to alternative nutrient sources or process optimizations needs to be thoroughly evaluated on a case-by-case basis.
Another challenge lies in the complex interactions between magnesium nitrate and other media components. The presence of certain ions or organic compounds can affect the bioavailability of magnesium, potentially leading to unexpected fermentation outcomes. This necessitates extensive optimization studies for each specific fermentation process, which can be time-consuming and resource-intensive.
The stability of magnesium nitrate in fermentation broths poses an additional challenge. Under certain conditions, such as high temperatures or extreme pH levels, magnesium nitrate may undergo chemical transformations or precipitate out of solution. This instability can lead to inconsistent fermentation results and difficulties in maintaining steady-state conditions throughout the bioprocess.
Furthermore, the impact of magnesium nitrate on downstream processing and product recovery has not been fully elucidated. In some cases, residual magnesium ions may interfere with purification steps or affect the final product quality, necessitating additional separation or treatment processes.
The environmental implications of using magnesium nitrate in large-scale fermentations also present challenges. The discharge of nitrate-rich effluents can contribute to eutrophication in aquatic ecosystems, requiring careful waste management strategies and potentially increasing operational costs.
Additionally, there is a lack of standardized protocols for incorporating magnesium nitrate into various fermentation processes. This absence of established guidelines makes it difficult for researchers and industry professionals to effectively compare results across different studies or implement optimized conditions in diverse fermentation systems.
Lastly, the economic feasibility of using magnesium nitrate in industrial-scale fermentations remains a concern. While its benefits in enhancing microbial growth and product yields are recognized, the cost-effectiveness of magnesium nitrate supplementation compared to alternative nutrient sources or process optimizations needs to be thoroughly evaluated on a case-by-case basis.
Existing Applications of Magnesium Nitrate in Fermentation
01 Magnesium nitrate in fertilizer compositions
Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer compositions can be tailored for specific crops or soil conditions.- Magnesium nitrate in fertilizer compositions: Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer compositions can be tailored for specific crops or soil conditions.
- Magnesium nitrate in energy storage applications: Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its ability to absorb and release heat during phase transitions. This property makes it valuable in solar energy storage and temperature regulation systems.
- Magnesium nitrate in flame retardant formulations: Magnesium nitrate is incorporated into flame retardant formulations for various materials. It acts as an effective fire suppressant by releasing non-flammable gases when exposed to high temperatures. These formulations can be applied to textiles, plastics, and other combustible materials to enhance their fire resistance properties.
- Magnesium nitrate in water treatment processes: Magnesium nitrate is employed in water treatment processes for its ability to remove contaminants and improve water quality. It can be used in the treatment of wastewater, industrial effluents, and drinking water. The compound helps in the removal of phosphates, heavy metals, and other pollutants through precipitation and coagulation mechanisms.
- Magnesium nitrate in chemical synthesis: Magnesium nitrate serves as a versatile reagent in various chemical synthesis processes. It is used as a precursor for the production of other magnesium compounds, catalysts, and specialty chemicals. The compound's high solubility and reactivity make it valuable in organic and inorganic synthesis reactions across different industries.
02 Magnesium nitrate in energy storage applications
Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its heat absorption and release properties during melting and solidification. This makes it valuable for solar energy storage and temperature regulation in buildings.Expand Specific Solutions03 Magnesium nitrate in flame retardant formulations
Magnesium nitrate is incorporated into flame retardant formulations for various materials, including textiles, plastics, and wood products. It acts as an effective fire suppressant by releasing nitrogen and forming a protective layer when exposed to high temperatures, thus improving the fire resistance of treated materials.Expand Specific Solutions04 Magnesium nitrate in water treatment processes
Magnesium nitrate is employed in water treatment processes for various purposes. It can be used to remove contaminants, adjust water hardness, or as a coagulant aid in wastewater treatment. Its application helps improve water quality and meets environmental standards for both industrial and municipal water systems.Expand Specific Solutions05 Magnesium nitrate in chemical synthesis and catalysis
Magnesium nitrate serves as a precursor or catalyst in various chemical synthesis processes. It is used in the production of other magnesium compounds, as a catalyst support in organic reactions, and in the synthesis of advanced materials such as nanoparticles or metal-organic frameworks. Its versatility makes it valuable in both research and industrial applications.Expand Specific Solutions
Key Players in Biotechnological Fermentation Industry
The biotechnological fermentation industry, particularly in the context of magnesium nitrate's role, is in a growth phase with increasing market size and technological advancements. The global fermentation market is expanding rapidly, driven by demand in various sectors including food, pharmaceuticals, and biofuels. Companies like Ginkgo Bioworks and Neimenggu Fufeng Biotechnologies are at the forefront, developing innovative fermentation processes. Academic institutions such as China Agricultural University and Zhejiang University are contributing to research and development. The technology's maturity is advancing, with firms like MIKLENS BIO and DuPont de Nemours pushing boundaries in fermentation techniques and applications, indicating a competitive and evolving landscape in this field.
China Agricultural University
Technical Solution: As a leading agricultural research institution, China Agricultural University likely approaches the role of magnesium nitrate in biotechnological fermentation from both fundamental and applied perspectives. Their research may focus on elucidating the molecular mechanisms by which magnesium and nitrate ions influence microbial metabolism and gene expression during fermentation[9]. The university might be developing novel fermentation strategies that optimize magnesium nitrate utilization for the production of various agricultural products, such as bio-fertilizers, biopesticides, or animal feed additives. Additionally, they may be exploring the potential of magnesium nitrate to enhance the production of valuable secondary metabolites in plant cell cultures or microbial fermentations[10].
Strengths: Strong research capabilities, access to diverse microbial and plant resources, and potential for interdisciplinary collaborations. Weaknesses: Possible challenges in commercializing research findings and competing with industry-led R&D efforts.
Ginkgo Bioworks, Inc.
Technical Solution: Ginkgo Bioworks has developed a platform for engineering microorganisms to produce various compounds through fermentation. Their approach likely involves optimizing the use of magnesium nitrate as a nutrient source in fermentation media. The company utilizes high-throughput screening and machine learning algorithms to identify optimal conditions for fermentation, including the concentration and timing of magnesium nitrate addition[1]. Their proprietary strain engineering techniques may also focus on enhancing microbial uptake and utilization of magnesium and nitrate ions, potentially improving overall fermentation efficiency and product yields[2].
Strengths: Advanced synthetic biology platform, high-throughput optimization capabilities, and expertise in strain engineering. Weaknesses: May face challenges in scaling up processes and competing with established fermentation industry players.
Innovative Research on Magnesium Nitrate in Bioprocesses
Method of producing target substance from starting substance via NADH-accumulating reaction pathway
PatentWO2020084713A1
Innovation
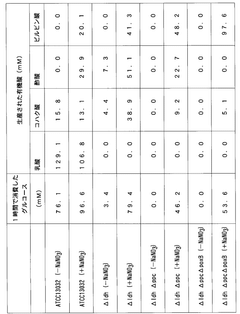
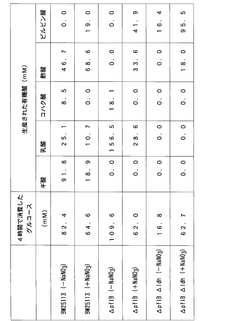
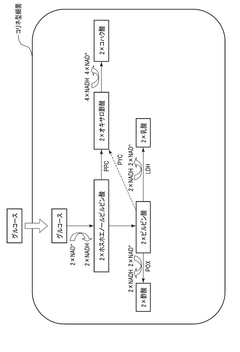
- Incorporating nitrate ions into the fermentation process allows bacteria to consume NADH through nitric acid respiration, eliminating accumulation and restoring enzyme activity, thereby enhancing the production efficiency of target substances like pyruvate and its derivatives by switching between aerobic and anaerobic conditions.
Microbial material for reducing soil/water quality contamination, restricting warming gas generation, and improving plant function, and method for manufacturing fermentation product
PatentActiveUS20200017821A1
Innovation
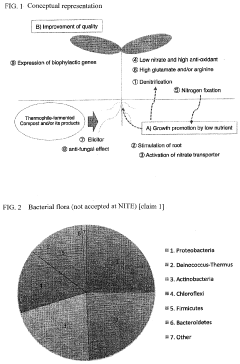
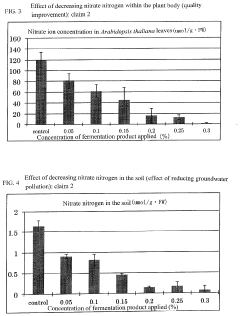
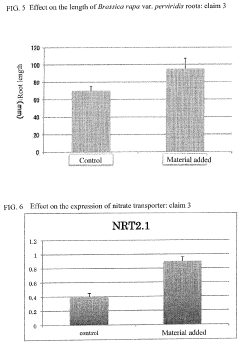
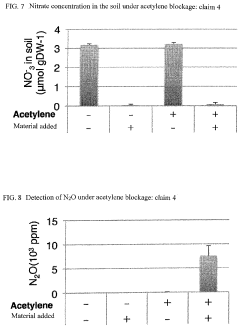
- A microbial material produced through fermentation using a mixture of thermophilic microorganisms, including Bacillus brevis and Bacillus stearothermophilus, which reduces nitrate ions in soil, promotes denitrification, and enhances plant functionality by activating nitrate transporters and producing antioxidants, thereby improving soil health and plant resistance.
Environmental Impact of Magnesium Nitrate in Bioprocesses
The environmental impact of magnesium nitrate in bioprocesses is a critical consideration for sustainable biotechnological fermentation practices. Magnesium nitrate, while essential for microbial growth and metabolism, can have significant effects on the surrounding ecosystem if not properly managed.
One of the primary environmental concerns is the potential for eutrophication in aquatic systems. When magnesium nitrate-rich effluents from fermentation processes are discharged into water bodies, they can lead to excessive algal growth. This proliferation of algae can deplete oxygen levels, causing harm to aquatic life and disrupting the ecological balance of affected water systems.
Soil quality is another area of environmental impact. The accumulation of magnesium nitrate in soil can alter its chemical composition, potentially affecting pH levels and nutrient availability for plants. This may lead to changes in local vegetation patterns and soil microbial communities, which are crucial for maintaining healthy ecosystems.
Atmospheric emissions during the production and use of magnesium nitrate in bioprocesses can contribute to air pollution. The release of nitrogen oxides, a byproduct of nitrate-based compounds, can contribute to the formation of smog and acid rain, impacting both urban and rural environments.
The energy-intensive nature of magnesium nitrate production also raises concerns about carbon footprint. The manufacturing process often relies on fossil fuels, contributing to greenhouse gas emissions and climate change. As the biotechnology industry expands, the cumulative environmental impact of increased magnesium nitrate usage becomes more significant.
Waste management is a critical aspect of mitigating the environmental impact of magnesium nitrate in bioprocesses. Proper treatment and disposal of fermentation byproducts and spent media containing magnesium nitrate are essential to prevent contamination of soil and water resources. Advanced wastewater treatment technologies, such as ion exchange and membrane filtration, can help reduce the release of nitrates into the environment.
Efforts to minimize the environmental footprint of magnesium nitrate in biotechnological applications are ongoing. Research into alternative nutrient sources, optimized fermentation processes that require less magnesium nitrate, and closed-loop systems that recycle nutrients are promising avenues for reducing environmental impact. Additionally, the development of more efficient production methods for magnesium nitrate itself could help decrease the overall ecological burden associated with its use in bioprocesses.
One of the primary environmental concerns is the potential for eutrophication in aquatic systems. When magnesium nitrate-rich effluents from fermentation processes are discharged into water bodies, they can lead to excessive algal growth. This proliferation of algae can deplete oxygen levels, causing harm to aquatic life and disrupting the ecological balance of affected water systems.
Soil quality is another area of environmental impact. The accumulation of magnesium nitrate in soil can alter its chemical composition, potentially affecting pH levels and nutrient availability for plants. This may lead to changes in local vegetation patterns and soil microbial communities, which are crucial for maintaining healthy ecosystems.
Atmospheric emissions during the production and use of magnesium nitrate in bioprocesses can contribute to air pollution. The release of nitrogen oxides, a byproduct of nitrate-based compounds, can contribute to the formation of smog and acid rain, impacting both urban and rural environments.
The energy-intensive nature of magnesium nitrate production also raises concerns about carbon footprint. The manufacturing process often relies on fossil fuels, contributing to greenhouse gas emissions and climate change. As the biotechnology industry expands, the cumulative environmental impact of increased magnesium nitrate usage becomes more significant.
Waste management is a critical aspect of mitigating the environmental impact of magnesium nitrate in bioprocesses. Proper treatment and disposal of fermentation byproducts and spent media containing magnesium nitrate are essential to prevent contamination of soil and water resources. Advanced wastewater treatment technologies, such as ion exchange and membrane filtration, can help reduce the release of nitrates into the environment.
Efforts to minimize the environmental footprint of magnesium nitrate in biotechnological applications are ongoing. Research into alternative nutrient sources, optimized fermentation processes that require less magnesium nitrate, and closed-loop systems that recycle nutrients are promising avenues for reducing environmental impact. Additionally, the development of more efficient production methods for magnesium nitrate itself could help decrease the overall ecological burden associated with its use in bioprocesses.
Regulatory Framework for Fermentation Additives
The regulatory framework for fermentation additives plays a crucial role in ensuring the safety and efficacy of biotechnological processes, including those involving magnesium nitrate. In the context of fermentation, regulatory bodies worldwide have established guidelines and standards to govern the use of additives, including inorganic salts like magnesium nitrate.
The United States Food and Drug Administration (FDA) has set forth regulations under the Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act. These regulations specify the conditions under which fermentation additives can be used in food production. Magnesium nitrate, when used as a fermentation aid, must comply with the Generally Recognized as Safe (GRAS) status or obtain specific approval through a food additive petition.
In the European Union, the European Food Safety Authority (EFSA) oversees the regulation of fermentation additives. The EFSA has established a comprehensive framework for evaluating the safety of food additives, including those used in fermentation processes. Magnesium nitrate, when employed in biotechnological fermentation, must adhere to the guidelines set forth in Regulation (EC) No 1333/2008 on food additives.
The regulatory landscape also extends to other major markets, such as China and Japan. The National Medical Products Administration (NMPA) in China and the Ministry of Health, Labour and Welfare in Japan have their own sets of regulations governing the use of fermentation additives. These regulations often require extensive safety assessments and documentation before approving the use of additives like magnesium nitrate in fermentation processes.
International organizations, such as the Joint FAO/WHO Expert Committee on Food Additives (JECFA), provide additional guidance on the safety evaluation of fermentation additives. Their recommendations often influence national regulatory frameworks and help harmonize global standards for the use of additives in biotechnological processes.
Compliance with these regulatory frameworks is essential for companies utilizing magnesium nitrate in fermentation processes. This involves maintaining detailed records of the additive's use, conducting regular quality control checks, and ensuring that the final products meet all safety and purity standards. Failure to comply with these regulations can result in significant penalties and market access restrictions.
As the field of biotechnology continues to evolve, regulatory frameworks are also adapting to address new challenges and innovations. This dynamic regulatory environment requires companies to stay informed about changes in legislation and be prepared to adjust their processes accordingly to maintain compliance and ensure the safety of their fermentation products.
The United States Food and Drug Administration (FDA) has set forth regulations under the Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act. These regulations specify the conditions under which fermentation additives can be used in food production. Magnesium nitrate, when used as a fermentation aid, must comply with the Generally Recognized as Safe (GRAS) status or obtain specific approval through a food additive petition.
In the European Union, the European Food Safety Authority (EFSA) oversees the regulation of fermentation additives. The EFSA has established a comprehensive framework for evaluating the safety of food additives, including those used in fermentation processes. Magnesium nitrate, when employed in biotechnological fermentation, must adhere to the guidelines set forth in Regulation (EC) No 1333/2008 on food additives.
The regulatory landscape also extends to other major markets, such as China and Japan. The National Medical Products Administration (NMPA) in China and the Ministry of Health, Labour and Welfare in Japan have their own sets of regulations governing the use of fermentation additives. These regulations often require extensive safety assessments and documentation before approving the use of additives like magnesium nitrate in fermentation processes.
International organizations, such as the Joint FAO/WHO Expert Committee on Food Additives (JECFA), provide additional guidance on the safety evaluation of fermentation additives. Their recommendations often influence national regulatory frameworks and help harmonize global standards for the use of additives in biotechnological processes.
Compliance with these regulatory frameworks is essential for companies utilizing magnesium nitrate in fermentation processes. This involves maintaining detailed records of the additive's use, conducting regular quality control checks, and ensuring that the final products meet all safety and purity standards. Failure to comply with these regulations can result in significant penalties and market access restrictions.
As the field of biotechnology continues to evolve, regulatory frameworks are also adapting to address new challenges and innovations. This dynamic regulatory environment requires companies to stay informed about changes in legislation and be prepared to adjust their processes accordingly to maintain compliance and ensure the safety of their fermentation products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!