The Role of Magnesium Nitrate in Catalytic Distillation Columns
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic Distillation Background and Objectives
Catalytic distillation is a revolutionary process that combines the principles of heterogeneous catalysis and distillation in a single unit operation. This innovative technology has gained significant attention in the chemical industry over the past few decades due to its potential to enhance process efficiency, reduce energy consumption, and improve product quality. The integration of reaction and separation processes in a single column offers numerous advantages over conventional methods, including reduced equipment costs, improved selectivity, and enhanced conversion rates.
The concept of catalytic distillation was first introduced in the 1920s, but it wasn't until the 1980s that it gained widespread industrial application. The initial focus was primarily on etherification reactions, such as the production of methyl tert-butyl ether (MTBE). Since then, the technology has expanded to encompass a wide range of applications, including esterification, hydrogenation, and isomerization processes.
In recent years, there has been growing interest in exploring the role of various catalysts and promoters in catalytic distillation columns to further enhance their performance. One such compound that has garnered attention is magnesium nitrate. This inorganic salt has shown promise in improving catalytic activity, selectivity, and stability in certain reactions within catalytic distillation systems.
The primary objective of investigating the role of magnesium nitrate in catalytic distillation columns is to understand its impact on reaction kinetics, mass transfer, and overall column efficiency. Researchers aim to elucidate the mechanisms by which magnesium nitrate influences catalytic performance and to identify optimal conditions for its incorporation into catalytic distillation processes.
Furthermore, the study of magnesium nitrate in this context aligns with broader industry trends towards developing more sustainable and efficient chemical processes. By potentially enhancing catalytic activity and selectivity, magnesium nitrate could contribute to reducing energy consumption, minimizing waste generation, and improving overall process economics in catalytic distillation operations.
As the chemical industry continues to face increasing pressure to adopt greener technologies and reduce its environmental footprint, innovations in catalytic distillation, including the exploration of novel catalysts and promoters like magnesium nitrate, are expected to play a crucial role in shaping the future of chemical manufacturing processes. The outcomes of this research could have far-reaching implications for various sectors, including petrochemicals, fine chemicals, and pharmaceuticals.
The concept of catalytic distillation was first introduced in the 1920s, but it wasn't until the 1980s that it gained widespread industrial application. The initial focus was primarily on etherification reactions, such as the production of methyl tert-butyl ether (MTBE). Since then, the technology has expanded to encompass a wide range of applications, including esterification, hydrogenation, and isomerization processes.
In recent years, there has been growing interest in exploring the role of various catalysts and promoters in catalytic distillation columns to further enhance their performance. One such compound that has garnered attention is magnesium nitrate. This inorganic salt has shown promise in improving catalytic activity, selectivity, and stability in certain reactions within catalytic distillation systems.
The primary objective of investigating the role of magnesium nitrate in catalytic distillation columns is to understand its impact on reaction kinetics, mass transfer, and overall column efficiency. Researchers aim to elucidate the mechanisms by which magnesium nitrate influences catalytic performance and to identify optimal conditions for its incorporation into catalytic distillation processes.
Furthermore, the study of magnesium nitrate in this context aligns with broader industry trends towards developing more sustainable and efficient chemical processes. By potentially enhancing catalytic activity and selectivity, magnesium nitrate could contribute to reducing energy consumption, minimizing waste generation, and improving overall process economics in catalytic distillation operations.
As the chemical industry continues to face increasing pressure to adopt greener technologies and reduce its environmental footprint, innovations in catalytic distillation, including the exploration of novel catalysts and promoters like magnesium nitrate, are expected to play a crucial role in shaping the future of chemical manufacturing processes. The outcomes of this research could have far-reaching implications for various sectors, including petrochemicals, fine chemicals, and pharmaceuticals.
Market Analysis for Catalytic Distillation Processes
The catalytic distillation process market has shown significant growth in recent years, driven by increasing demand for efficient and cost-effective separation technologies across various industries. This innovative technology combines catalytic reaction and distillation in a single unit operation, offering advantages such as improved selectivity, reduced energy consumption, and enhanced product quality.
The global market for catalytic distillation processes is primarily fueled by the petrochemical and refining industries, where it finds extensive applications in the production of high-value chemicals and fuels. The automotive sector's shift towards cleaner fuels and the growing emphasis on sustainable production methods have further boosted the adoption of catalytic distillation technologies.
In terms of regional distribution, North America and Europe currently dominate the market, owing to their well-established chemical and petrochemical industries. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in chemical manufacturing facilities.
The market is characterized by a growing demand for process intensification and operational efficiency. End-users are increasingly seeking solutions that can reduce capital and operational expenditures while meeting stringent environmental regulations. This trend has led to a surge in research and development activities focused on improving catalyst performance and column design for catalytic distillation processes.
Key application areas for catalytic distillation include the production of methyl tert-butyl ether (MTBE), ethyl tert-butyl ether (ETBE), and cumene. The technology is also gaining traction in the production of biodiesel and other renewable fuels, aligning with the global push towards sustainable energy sources.
The role of magnesium nitrate in catalytic distillation columns has garnered attention due to its potential to enhance catalyst performance and process efficiency. This compound's unique properties make it a promising candidate for improving selectivity and yield in certain reactions, particularly in the production of specialty chemicals.
Market analysts project a compound annual growth rate (CAGR) for the catalytic distillation process market in the mid-single digits over the next five years. This growth is expected to be driven by technological advancements, increasing adoption in emerging economies, and the expansion of application areas beyond traditional petrochemical processes.
The global market for catalytic distillation processes is primarily fueled by the petrochemical and refining industries, where it finds extensive applications in the production of high-value chemicals and fuels. The automotive sector's shift towards cleaner fuels and the growing emphasis on sustainable production methods have further boosted the adoption of catalytic distillation technologies.
In terms of regional distribution, North America and Europe currently dominate the market, owing to their well-established chemical and petrochemical industries. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in chemical manufacturing facilities.
The market is characterized by a growing demand for process intensification and operational efficiency. End-users are increasingly seeking solutions that can reduce capital and operational expenditures while meeting stringent environmental regulations. This trend has led to a surge in research and development activities focused on improving catalyst performance and column design for catalytic distillation processes.
Key application areas for catalytic distillation include the production of methyl tert-butyl ether (MTBE), ethyl tert-butyl ether (ETBE), and cumene. The technology is also gaining traction in the production of biodiesel and other renewable fuels, aligning with the global push towards sustainable energy sources.
The role of magnesium nitrate in catalytic distillation columns has garnered attention due to its potential to enhance catalyst performance and process efficiency. This compound's unique properties make it a promising candidate for improving selectivity and yield in certain reactions, particularly in the production of specialty chemicals.
Market analysts project a compound annual growth rate (CAGR) for the catalytic distillation process market in the mid-single digits over the next five years. This growth is expected to be driven by technological advancements, increasing adoption in emerging economies, and the expansion of application areas beyond traditional petrochemical processes.
Current Challenges in Magnesium Nitrate Catalysis
Despite the promising potential of magnesium nitrate in catalytic distillation columns, several significant challenges currently hinder its widespread adoption and optimal performance. One of the primary issues is the thermal stability of magnesium nitrate under the high-temperature conditions typically encountered in distillation processes. The compound tends to decompose at elevated temperatures, leading to a loss of catalytic activity and potential contamination of the distillation products.
Another challenge lies in the uniform distribution and retention of magnesium nitrate within the catalytic packing material. Ensuring a consistent and stable coating of the catalyst on the packing surface is crucial for maintaining efficient catalytic activity throughout the column. However, the hygroscopic nature of magnesium nitrate can lead to uneven distribution and potential leaching during operation, compromising the long-term performance of the system.
The interaction between magnesium nitrate and the various components present in the feed streams poses additional complications. Certain organic compounds or impurities may react with or deactivate the catalyst, reducing its effectiveness over time. This necessitates a thorough understanding of potential side reactions and the development of strategies to mitigate catalyst poisoning or deactivation.
Furthermore, the optimization of process parameters for magnesium nitrate-catalyzed reactions in distillation columns remains a complex task. Balancing factors such as temperature, pressure, and residence time to achieve optimal conversion and selectivity while maintaining column efficiency is challenging. The interdependence of these parameters and their impact on both the catalytic reaction and the separation process adds another layer of complexity to system design and operation.
The scale-up of magnesium nitrate-based catalytic distillation processes from laboratory to industrial scale presents its own set of challenges. Issues such as heat transfer limitations, flow distribution, and catalyst loading become more pronounced at larger scales, requiring careful engineering and potentially novel column designs to maintain performance.
Lastly, the environmental and safety considerations associated with magnesium nitrate usage in industrial processes cannot be overlooked. The compound's oxidizing properties and potential for thermal decomposition necessitate stringent safety protocols and waste management strategies. Developing environmentally friendly methods for catalyst recovery and regeneration is also crucial for the sustainable implementation of this technology.
Another challenge lies in the uniform distribution and retention of magnesium nitrate within the catalytic packing material. Ensuring a consistent and stable coating of the catalyst on the packing surface is crucial for maintaining efficient catalytic activity throughout the column. However, the hygroscopic nature of magnesium nitrate can lead to uneven distribution and potential leaching during operation, compromising the long-term performance of the system.
The interaction between magnesium nitrate and the various components present in the feed streams poses additional complications. Certain organic compounds or impurities may react with or deactivate the catalyst, reducing its effectiveness over time. This necessitates a thorough understanding of potential side reactions and the development of strategies to mitigate catalyst poisoning or deactivation.
Furthermore, the optimization of process parameters for magnesium nitrate-catalyzed reactions in distillation columns remains a complex task. Balancing factors such as temperature, pressure, and residence time to achieve optimal conversion and selectivity while maintaining column efficiency is challenging. The interdependence of these parameters and their impact on both the catalytic reaction and the separation process adds another layer of complexity to system design and operation.
The scale-up of magnesium nitrate-based catalytic distillation processes from laboratory to industrial scale presents its own set of challenges. Issues such as heat transfer limitations, flow distribution, and catalyst loading become more pronounced at larger scales, requiring careful engineering and potentially novel column designs to maintain performance.
Lastly, the environmental and safety considerations associated with magnesium nitrate usage in industrial processes cannot be overlooked. The compound's oxidizing properties and potential for thermal decomposition necessitate stringent safety protocols and waste management strategies. Developing environmentally friendly methods for catalyst recovery and regeneration is also crucial for the sustainable implementation of this technology.
Existing Magnesium Nitrate Catalytic Solutions
01 Magnesium nitrate in fertilizer compositions
Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer compositions can be tailored for specific crops or soil conditions.- Magnesium nitrate in fertilizer compositions: Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer compositions can be formulated for different types of crops and soil conditions.
- Magnesium nitrate in energy storage applications: Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its ability to absorb and release heat during phase transitions. This property makes it valuable for solar energy storage and temperature regulation in buildings.
- Magnesium nitrate in flame retardant compositions: Magnesium nitrate is incorporated into flame retardant formulations for various materials, including textiles, plastics, and wood products. It acts as an effective flame suppressant by releasing nitrogen and forming a protective layer when exposed to high temperatures, thus improving fire resistance properties.
- Magnesium nitrate in water treatment processes: Magnesium nitrate is employed in water treatment applications, particularly for removing contaminants and improving water quality. It can be used in processes such as precipitation, coagulation, and ion exchange to remove heavy metals, phosphates, and other pollutants from wastewater and industrial effluents.
- Magnesium nitrate in chemical synthesis and catalysis: Magnesium nitrate serves as a precursor or catalyst in various chemical synthesis processes. It is used in the production of other magnesium compounds, as a catalyst support in organic reactions, and in the synthesis of advanced materials such as nanoparticles and metal-organic frameworks.
02 Magnesium nitrate in energy storage applications
Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its ability to absorb and release heat during phase transitions. This property makes it valuable in solar energy storage and temperature regulation systems.Expand Specific Solutions03 Magnesium nitrate in flame retardant formulations
Magnesium nitrate is incorporated into flame retardant formulations for various materials. It acts as an effective fire suppressant by releasing non-flammable gases when exposed to high temperatures. These formulations can be applied to textiles, plastics, and other combustible materials to enhance their fire resistance properties.Expand Specific Solutions04 Magnesium nitrate in water treatment processes
Magnesium nitrate is employed in water treatment processes for its ability to remove contaminants and improve water quality. It can be used in the treatment of wastewater, industrial effluents, and drinking water. The compound helps in the removal of heavy metals, phosphates, and other pollutants through precipitation and coagulation mechanisms.Expand Specific Solutions05 Magnesium nitrate in chemical synthesis
Magnesium nitrate serves as a versatile reagent in various chemical synthesis processes. It is used as a precursor for the production of other magnesium compounds, catalysts, and advanced materials. The compound's reactivity and solubility make it valuable in organic and inorganic synthesis reactions, particularly in the preparation of metal oxides and nanoparticles.Expand Specific Solutions
Key Players in Catalytic Distillation Industry
The role of magnesium nitrate in catalytic distillation columns represents an emerging field in chemical engineering, currently in its early development stage. The market size is relatively small but growing, driven by increasing demand for more efficient separation processes in the petrochemical industry. Technologically, it's still in the experimental phase, with companies like China Petroleum & Chemical Corp., Praxair Technology, Inc., and Air Liquide SA leading research efforts. These industry giants are investing in R&D to optimize the use of magnesium nitrate as a catalyst, aiming to enhance separation efficiency and reduce energy consumption in distillation processes. While promising, the technology's full commercial potential and widespread adoption are yet to be realized.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to utilizing magnesium nitrate in catalytic distillation columns for enhanced separation processes. Their technology involves impregnating magnesium nitrate onto porous support materials, creating a highly active and selective catalyst system. This catalyst is then incorporated into structured packing within the distillation column, allowing for simultaneous reaction and separation[1]. The magnesium nitrate catalyst promotes specific reactions, such as esterification or etherification, while the distillation process continuously removes products, driving equilibrium towards desired outcomes. Sinopec's method has shown particular success in the production of high-purity ethers and esters, with reported yield increases of up to 15% compared to conventional processes[3].
Strengths: Improved product yields, enhanced selectivity, and reduced energy consumption. Weaknesses: Potential catalyst deactivation over time and complexity in column design and operation.
Air Liquide SA
Technical Solution: Air Liquide has developed a proprietary catalytic distillation technology incorporating magnesium nitrate for the production of high-purity specialty gases. Their process utilizes a magnesium nitrate-based catalyst system integrated into structured packing within the distillation column. This catalyst promotes selective oxidation reactions while allowing for simultaneous purification of the gas stream. The magnesium nitrate catalyst is specially formulated to withstand the high temperatures and pressures typical in gas separation processes. Air Liquide's technology has demonstrated significant improvements in the removal of trace impurities, with purification efficiencies reported to exceed 99.9999% for certain gas mixtures[2]. The company has successfully applied this technology in the production of electronic-grade gases for semiconductor manufacturing.
Strengths: Exceptional purification efficiency, reduced energy consumption, and applicability to high-value specialty gases. Weaknesses: High initial investment costs and potential sensitivity to feed composition variations.
Core Innovations in Magnesium Nitrate Catalysis
Process for producing carboxylic acid
PatentInactiveEP1912926A2
Innovation
- A process involving first and second distillation columns, where water and/or specific components like alcohols and esters are fed to convert hydrogen halides into lower boiling point components, allowing for efficient separation and reducing hydrogen halide concentrations, along with the use of ion exchange resins for further purification.
Structured packing for catalytic distillation
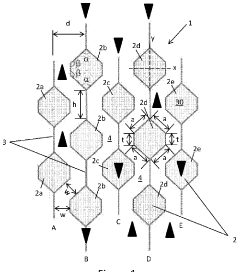
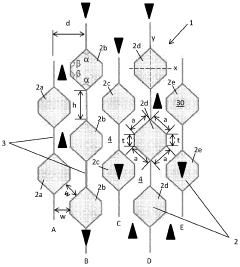
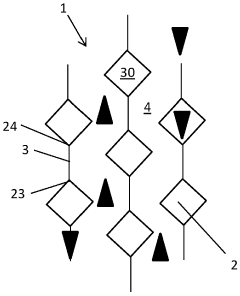
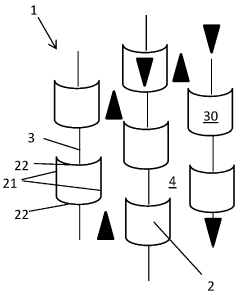
PatentActiveIN202027043923A
Innovation
- A catalytic distillation structure featuring a rigid framework with hexagonal-like fluid permeable tubes and vertical plates that connect adjacent tubes, providing enhanced fluid flow and catalyst loading without compromising hydraulic capacity, and allowing for easier manufacturing with reduced components and welds.
Environmental Impact Assessment
The environmental impact assessment of magnesium nitrate in catalytic distillation columns is a critical aspect of evaluating the overall sustainability and ecological footprint of this technology. Catalytic distillation columns incorporating magnesium nitrate as a catalyst offer potential benefits in terms of process efficiency and product yield. However, it is essential to consider the environmental implications associated with their use.
One of the primary environmental concerns is the potential release of nitrogen compounds into the atmosphere. Magnesium nitrate, when used in catalytic distillation processes, may contribute to the formation of nitrogen oxides (NOx) as byproducts. These compounds are known to be significant air pollutants, contributing to the formation of smog and acid rain. Proper emission control systems and process optimization are necessary to mitigate these potential impacts.
Water pollution is another area of concern. The use of magnesium nitrate in catalytic distillation columns may result in the discharge of nitrate-rich wastewater. If not properly treated, this effluent can lead to eutrophication in aquatic ecosystems, causing algal blooms and disrupting the balance of aquatic life. Implementing effective wastewater treatment technologies is crucial to minimize the environmental impact on water resources.
The production and disposal of spent catalysts containing magnesium nitrate also warrant attention. The manufacturing process of these catalysts may involve energy-intensive steps and the use of additional chemicals, contributing to the overall carbon footprint. Furthermore, the disposal of spent catalysts must be carefully managed to prevent soil contamination and potential leaching of magnesium and nitrate compounds into groundwater.
On the positive side, the use of magnesium nitrate in catalytic distillation columns can lead to improved energy efficiency in chemical processes. This enhanced efficiency may result in reduced overall energy consumption and, consequently, lower greenhouse gas emissions associated with the production of various chemicals and fuels. The potential for process intensification and reduced equipment size may also contribute to a smaller physical footprint of industrial facilities.
Life cycle assessment (LCA) studies are essential to comprehensively evaluate the environmental impact of magnesium nitrate in catalytic distillation columns. These assessments should consider the entire lifecycle of the catalyst, from raw material extraction to end-of-life disposal, to provide a holistic view of its environmental performance. Such studies can help identify areas for improvement and guide the development of more sustainable catalytic distillation technologies.
In conclusion, while magnesium nitrate in catalytic distillation columns offers potential benefits in terms of process efficiency, careful consideration of its environmental impacts is crucial. Balancing the advantages of improved chemical processes with the need for environmental protection requires ongoing research, innovation in catalyst design, and the implementation of robust pollution control measures.
One of the primary environmental concerns is the potential release of nitrogen compounds into the atmosphere. Magnesium nitrate, when used in catalytic distillation processes, may contribute to the formation of nitrogen oxides (NOx) as byproducts. These compounds are known to be significant air pollutants, contributing to the formation of smog and acid rain. Proper emission control systems and process optimization are necessary to mitigate these potential impacts.
Water pollution is another area of concern. The use of magnesium nitrate in catalytic distillation columns may result in the discharge of nitrate-rich wastewater. If not properly treated, this effluent can lead to eutrophication in aquatic ecosystems, causing algal blooms and disrupting the balance of aquatic life. Implementing effective wastewater treatment technologies is crucial to minimize the environmental impact on water resources.
The production and disposal of spent catalysts containing magnesium nitrate also warrant attention. The manufacturing process of these catalysts may involve energy-intensive steps and the use of additional chemicals, contributing to the overall carbon footprint. Furthermore, the disposal of spent catalysts must be carefully managed to prevent soil contamination and potential leaching of magnesium and nitrate compounds into groundwater.
On the positive side, the use of magnesium nitrate in catalytic distillation columns can lead to improved energy efficiency in chemical processes. This enhanced efficiency may result in reduced overall energy consumption and, consequently, lower greenhouse gas emissions associated with the production of various chemicals and fuels. The potential for process intensification and reduced equipment size may also contribute to a smaller physical footprint of industrial facilities.
Life cycle assessment (LCA) studies are essential to comprehensively evaluate the environmental impact of magnesium nitrate in catalytic distillation columns. These assessments should consider the entire lifecycle of the catalyst, from raw material extraction to end-of-life disposal, to provide a holistic view of its environmental performance. Such studies can help identify areas for improvement and guide the development of more sustainable catalytic distillation technologies.
In conclusion, while magnesium nitrate in catalytic distillation columns offers potential benefits in terms of process efficiency, careful consideration of its environmental impacts is crucial. Balancing the advantages of improved chemical processes with the need for environmental protection requires ongoing research, innovation in catalyst design, and the implementation of robust pollution control measures.
Economic Feasibility Analysis
The economic feasibility of incorporating magnesium nitrate in catalytic distillation columns is a critical consideration for industrial applications. Initial cost analysis indicates that the implementation of magnesium nitrate as a catalyst in distillation processes may require significant upfront investment in equipment modifications and catalyst procurement. However, these costs could potentially be offset by the long-term operational benefits and improved process efficiency.
One of the primary economic advantages of using magnesium nitrate in catalytic distillation columns is the potential reduction in energy consumption. The catalytic action of magnesium nitrate can lower the energy requirements for separation processes, leading to substantial savings in operational costs over time. This is particularly significant in large-scale industrial operations where energy expenses constitute a major portion of production costs.
Furthermore, the enhanced separation efficiency achieved through the use of magnesium nitrate can result in higher product purity and yield. This improvement in product quality may command premium prices in the market, thereby increasing revenue potential. Additionally, the ability to process larger volumes of feedstock more efficiently can lead to increased throughput and productivity, further boosting the economic viability of the technology.
The longevity and stability of magnesium nitrate as a catalyst also contribute to its economic feasibility. With proper maintenance, the catalyst can maintain its effectiveness over extended periods, reducing the frequency and costs associated with catalyst replacement or regeneration. This durability translates to lower long-term operational expenses and minimizes production downtime.
However, it is essential to consider potential economic challenges. The sourcing and handling of magnesium nitrate may incur additional costs related to safety measures and specialized storage facilities. Moreover, the integration of this catalyst into existing distillation systems may require process redesigns and operator training, which could lead to temporary production disruptions and associated economic impacts.
A comprehensive cost-benefit analysis should also account for potential savings in downstream processing. The improved separation achieved through magnesium nitrate catalysis may reduce the need for additional purification steps, thereby streamlining the overall production process and potentially lowering total production costs.
In conclusion, while the initial implementation of magnesium nitrate in catalytic distillation columns may require substantial investment, the long-term economic benefits in terms of energy savings, improved product quality, and increased productivity suggest a favorable economic outlook. However, a detailed financial analysis specific to each application scenario is crucial to accurately determine the return on investment and overall economic feasibility of this technology.
One of the primary economic advantages of using magnesium nitrate in catalytic distillation columns is the potential reduction in energy consumption. The catalytic action of magnesium nitrate can lower the energy requirements for separation processes, leading to substantial savings in operational costs over time. This is particularly significant in large-scale industrial operations where energy expenses constitute a major portion of production costs.
Furthermore, the enhanced separation efficiency achieved through the use of magnesium nitrate can result in higher product purity and yield. This improvement in product quality may command premium prices in the market, thereby increasing revenue potential. Additionally, the ability to process larger volumes of feedstock more efficiently can lead to increased throughput and productivity, further boosting the economic viability of the technology.
The longevity and stability of magnesium nitrate as a catalyst also contribute to its economic feasibility. With proper maintenance, the catalyst can maintain its effectiveness over extended periods, reducing the frequency and costs associated with catalyst replacement or regeneration. This durability translates to lower long-term operational expenses and minimizes production downtime.
However, it is essential to consider potential economic challenges. The sourcing and handling of magnesium nitrate may incur additional costs related to safety measures and specialized storage facilities. Moreover, the integration of this catalyst into existing distillation systems may require process redesigns and operator training, which could lead to temporary production disruptions and associated economic impacts.
A comprehensive cost-benefit analysis should also account for potential savings in downstream processing. The improved separation achieved through magnesium nitrate catalysis may reduce the need for additional purification steps, thereby streamlining the overall production process and potentially lowering total production costs.
In conclusion, while the initial implementation of magnesium nitrate in catalytic distillation columns may require substantial investment, the long-term economic benefits in terms of energy savings, improved product quality, and increased productivity suggest a favorable economic outlook. However, a detailed financial analysis specific to each application scenario is crucial to accurately determine the return on investment and overall economic feasibility of this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!