The Role of Magnesium Nitrate in Improving Catalytic Converters
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic Converter Evolution and Magnesium Nitrate Goals
Catalytic converters have undergone significant evolution since their introduction in the 1970s. Initially designed to reduce harmful emissions from internal combustion engines, these devices have become increasingly sophisticated in response to stricter environmental regulations and the need for improved fuel efficiency. The primary goal of catalytic converter technology has been to maximize the conversion of toxic exhaust gases into less harmful substances while minimizing the use of precious metals.
In recent years, the focus has shifted towards enhancing the performance and durability of catalytic converters while reducing their cost and environmental impact. This has led to the exploration of novel materials and designs, including the use of magnesium nitrate as a potential catalyst enhancer. The introduction of magnesium nitrate into catalytic converter technology aims to address several key objectives in the field.
One of the primary goals of incorporating magnesium nitrate is to improve the overall efficiency of the catalytic process. By enhancing the catalytic activity, magnesium nitrate could potentially allow for a reduction in the amount of precious metals required, such as platinum, palladium, and rhodium. This would not only lower production costs but also decrease the environmental impact associated with mining these rare elements.
Another objective is to increase the thermal stability of the catalyst. Magnesium nitrate's potential to form stable compounds at high temperatures could lead to improved longevity of catalytic converters, reducing the need for frequent replacements and minimizing waste. This aligns with the broader industry trend towards developing more durable and sustainable automotive components.
Researchers are also exploring the possibility of using magnesium nitrate to expand the temperature range at which catalytic converters operate effectively. This could result in improved performance during cold starts and under varying engine conditions, addressing one of the long-standing challenges in emission control technology.
Furthermore, the integration of magnesium nitrate into catalytic converters aims to enhance the conversion rates of specific pollutants, particularly nitrogen oxides (NOx). As emission standards become increasingly stringent, the ability to more effectively reduce NOx emissions is crucial for meeting regulatory requirements and improving air quality in urban areas.
In the context of evolving engine technologies, such as hybrid and lean-burn engines, magnesium nitrate could play a role in developing catalytic converters that are better suited to these advanced propulsion systems. This adaptability is essential for ensuring that emission control technologies keep pace with innovations in automotive engineering.
In recent years, the focus has shifted towards enhancing the performance and durability of catalytic converters while reducing their cost and environmental impact. This has led to the exploration of novel materials and designs, including the use of magnesium nitrate as a potential catalyst enhancer. The introduction of magnesium nitrate into catalytic converter technology aims to address several key objectives in the field.
One of the primary goals of incorporating magnesium nitrate is to improve the overall efficiency of the catalytic process. By enhancing the catalytic activity, magnesium nitrate could potentially allow for a reduction in the amount of precious metals required, such as platinum, palladium, and rhodium. This would not only lower production costs but also decrease the environmental impact associated with mining these rare elements.
Another objective is to increase the thermal stability of the catalyst. Magnesium nitrate's potential to form stable compounds at high temperatures could lead to improved longevity of catalytic converters, reducing the need for frequent replacements and minimizing waste. This aligns with the broader industry trend towards developing more durable and sustainable automotive components.
Researchers are also exploring the possibility of using magnesium nitrate to expand the temperature range at which catalytic converters operate effectively. This could result in improved performance during cold starts and under varying engine conditions, addressing one of the long-standing challenges in emission control technology.
Furthermore, the integration of magnesium nitrate into catalytic converters aims to enhance the conversion rates of specific pollutants, particularly nitrogen oxides (NOx). As emission standards become increasingly stringent, the ability to more effectively reduce NOx emissions is crucial for meeting regulatory requirements and improving air quality in urban areas.
In the context of evolving engine technologies, such as hybrid and lean-burn engines, magnesium nitrate could play a role in developing catalytic converters that are better suited to these advanced propulsion systems. This adaptability is essential for ensuring that emission control technologies keep pace with innovations in automotive engineering.
Market Demand for Enhanced Emission Control Systems
The global market for enhanced emission control systems has been experiencing significant growth, driven by increasingly stringent environmental regulations and a growing awareness of air pollution's impact on public health. As governments worldwide implement tighter emission standards for vehicles, the demand for more efficient catalytic converters has surged. This trend is particularly evident in developed markets such as North America, Europe, and Japan, where regulations like Euro 6 and Tier 3 standards have pushed automakers to adopt advanced emission control technologies.
The automotive industry, being the primary consumer of catalytic converters, has been actively seeking innovative solutions to meet these stringent requirements. The market for catalytic converters is projected to expand substantially over the next decade, with a compound annual growth rate (CAGR) expected to exceed the overall automotive market growth. This increased demand is not limited to new vehicles but also extends to the aftermarket segment, as older vehicles are retrofitted with improved emission control systems to comply with updated regulations.
Emerging markets, particularly in Asia-Pacific and Latin America, are also contributing to the growing demand for enhanced emission control systems. As these regions experience rapid industrialization and urbanization, coupled with rising vehicle ownership rates, governments are implementing stricter emission norms to combat deteriorating air quality. This has created new opportunities for catalytic converter manufacturers and suppliers of advanced materials like magnesium nitrate.
The commercial vehicle sector has emerged as a significant driver of demand for improved catalytic converters. With the focus on reducing emissions from heavy-duty trucks and buses, there is a growing market for larger, more efficient catalytic systems capable of handling higher exhaust volumes and temperatures. This segment presents unique challenges and opportunities for innovation in catalyst formulations and converter designs.
Moreover, the shift towards alternative powertrains, such as hybrid and electric vehicles, has not diminished the importance of catalytic converters. Many hybrid vehicles still rely on internal combustion engines and require efficient emission control systems. Additionally, the growing interest in hydrogen fuel cell vehicles has opened up new avenues for catalytic converter applications, as these systems require specialized catalysts for optimal performance.
The market demand for enhanced emission control systems has also spurred research and development efforts in material science. The potential of magnesium nitrate in improving catalytic converter efficiency has garnered attention from both industry players and academic institutions. As a result, there is an increasing investment in R&D activities focused on developing novel catalyst formulations and manufacturing processes that incorporate magnesium nitrate to enhance the performance and durability of catalytic converters.
The automotive industry, being the primary consumer of catalytic converters, has been actively seeking innovative solutions to meet these stringent requirements. The market for catalytic converters is projected to expand substantially over the next decade, with a compound annual growth rate (CAGR) expected to exceed the overall automotive market growth. This increased demand is not limited to new vehicles but also extends to the aftermarket segment, as older vehicles are retrofitted with improved emission control systems to comply with updated regulations.
Emerging markets, particularly in Asia-Pacific and Latin America, are also contributing to the growing demand for enhanced emission control systems. As these regions experience rapid industrialization and urbanization, coupled with rising vehicle ownership rates, governments are implementing stricter emission norms to combat deteriorating air quality. This has created new opportunities for catalytic converter manufacturers and suppliers of advanced materials like magnesium nitrate.
The commercial vehicle sector has emerged as a significant driver of demand for improved catalytic converters. With the focus on reducing emissions from heavy-duty trucks and buses, there is a growing market for larger, more efficient catalytic systems capable of handling higher exhaust volumes and temperatures. This segment presents unique challenges and opportunities for innovation in catalyst formulations and converter designs.
Moreover, the shift towards alternative powertrains, such as hybrid and electric vehicles, has not diminished the importance of catalytic converters. Many hybrid vehicles still rely on internal combustion engines and require efficient emission control systems. Additionally, the growing interest in hydrogen fuel cell vehicles has opened up new avenues for catalytic converter applications, as these systems require specialized catalysts for optimal performance.
The market demand for enhanced emission control systems has also spurred research and development efforts in material science. The potential of magnesium nitrate in improving catalytic converter efficiency has garnered attention from both industry players and academic institutions. As a result, there is an increasing investment in R&D activities focused on developing novel catalyst formulations and manufacturing processes that incorporate magnesium nitrate to enhance the performance and durability of catalytic converters.
Current Challenges in Catalytic Converter Technology
Catalytic converters, while essential for reducing harmful emissions from vehicles, face several significant challenges in their current technological state. One of the primary issues is the degradation of catalyst performance over time, leading to reduced efficiency in converting pollutants. This degradation is often caused by thermal aging, where high exhaust temperatures cause sintering of the precious metal particles, reducing their active surface area.
Another major challenge is the cold-start emissions problem. Catalytic converters require high temperatures to function effectively, typically around 200-300°C. During the first few minutes of engine operation, especially in cold weather, the converter is not hot enough to catalyze reactions efficiently, resulting in higher emissions during this period.
The reliance on precious metals, particularly platinum, palladium, and rhodium, presents both economic and supply chain challenges. These metals are expensive and their limited availability can lead to price volatility and supply disruptions. This dependence also raises concerns about the long-term sustainability of catalytic converter production.
Catalyst poisoning is another significant issue, where certain compounds in the exhaust stream, such as sulfur or lead, can chemically deactivate the catalyst. This is particularly problematic in regions where fuel quality standards are less stringent.
The increasing stringency of emissions regulations worldwide poses a continuous challenge for catalytic converter technology. As standards become more demanding, converters need to achieve higher conversion efficiencies across a broader range of operating conditions and for a wider variety of pollutants.
There's also a growing need for catalytic converters to perform effectively under real-world driving conditions, which can differ significantly from laboratory testing environments. This includes dealing with varying driving patterns, fuel qualities, and environmental conditions.
Lastly, the size and weight of catalytic converters present design challenges for vehicle manufacturers. There's a constant push to develop more compact and lightweight converters without compromising performance, to improve overall vehicle efficiency and meet packaging constraints in modern vehicle designs.
Another major challenge is the cold-start emissions problem. Catalytic converters require high temperatures to function effectively, typically around 200-300°C. During the first few minutes of engine operation, especially in cold weather, the converter is not hot enough to catalyze reactions efficiently, resulting in higher emissions during this period.
The reliance on precious metals, particularly platinum, palladium, and rhodium, presents both economic and supply chain challenges. These metals are expensive and their limited availability can lead to price volatility and supply disruptions. This dependence also raises concerns about the long-term sustainability of catalytic converter production.
Catalyst poisoning is another significant issue, where certain compounds in the exhaust stream, such as sulfur or lead, can chemically deactivate the catalyst. This is particularly problematic in regions where fuel quality standards are less stringent.
The increasing stringency of emissions regulations worldwide poses a continuous challenge for catalytic converter technology. As standards become more demanding, converters need to achieve higher conversion efficiencies across a broader range of operating conditions and for a wider variety of pollutants.
There's also a growing need for catalytic converters to perform effectively under real-world driving conditions, which can differ significantly from laboratory testing environments. This includes dealing with varying driving patterns, fuel qualities, and environmental conditions.
Lastly, the size and weight of catalytic converters present design challenges for vehicle manufacturers. There's a constant push to develop more compact and lightweight converters without compromising performance, to improve overall vehicle efficiency and meet packaging constraints in modern vehicle designs.
Existing Magnesium Nitrate-based Solutions
01 Magnesium nitrate as a catalyst in chemical reactions
Magnesium nitrate exhibits catalytic properties in various chemical reactions, enhancing reaction rates and yields. It is particularly effective in oxidation processes and can be used in combination with other catalysts to improve overall performance.- Magnesium nitrate as a catalyst in chemical reactions: Magnesium nitrate exhibits catalytic properties in various chemical reactions, enhancing reaction rates and yields. It is particularly effective in oxidation processes and can be used in combination with other catalysts to improve overall performance.
- Magnesium nitrate in environmental applications: The catalytic performance of magnesium nitrate is utilized in environmental applications, such as air pollution control and wastewater treatment. It aids in the decomposition of harmful compounds and promotes the removal of contaminants from various media.
- Magnesium nitrate in energy storage and conversion: Magnesium nitrate demonstrates catalytic activity in energy-related applications, including fuel cells, batteries, and hydrogen production. Its performance contributes to improved efficiency and stability in these systems.
- Magnesium nitrate in materials synthesis and modification: The catalytic properties of magnesium nitrate are exploited in the synthesis and modification of various materials, including nanoparticles, polymers, and composite materials. It influences the morphology, size, and properties of the resulting products.
- Magnesium nitrate in organic synthesis: Magnesium nitrate serves as a catalyst in organic synthesis reactions, facilitating the formation of carbon-carbon bonds and promoting selective transformations. It is particularly useful in the synthesis of fine chemicals and pharmaceutical intermediates.
02 Magnesium nitrate in environmental applications
The catalytic performance of magnesium nitrate is utilized in environmental applications, such as air purification and wastewater treatment. It aids in the decomposition of pollutants and harmful substances, contributing to more efficient and eco-friendly processes.Expand Specific Solutions03 Magnesium nitrate in energy storage and conversion
Magnesium nitrate demonstrates catalytic activity in energy-related applications, including fuel cells and batteries. Its performance contributes to improved energy conversion efficiency and storage capacity in these systems.Expand Specific Solutions04 Magnesium nitrate in materials synthesis
The catalytic properties of magnesium nitrate are exploited in the synthesis of various materials, including nanoparticles, ceramics, and advanced composites. It influences the morphology, size, and properties of the resulting materials.Expand Specific Solutions05 Magnesium nitrate in agricultural applications
Magnesium nitrate exhibits catalytic performance in agricultural processes, such as fertilizer production and soil treatment. It enhances nutrient availability and promotes plant growth through its catalytic interactions with other soil components.Expand Specific Solutions
Key Players in Catalytic Converter Industry
The competition landscape for magnesium nitrate in improving catalytic converters is in a growth phase, with increasing market size driven by stringent emission regulations. The technology is maturing but still has room for innovation. Key players like Johnson Matthey, BASF, and Umicore are leading commercial development, while research institutions such as CNRS and universities are advancing fundamental understanding. Chinese companies like Sinopec and Shanghai Hanyu are also entering the market, indicating global interest. The involvement of diverse players from automotive, chemical, and materials sectors suggests a complex and competitive ecosystem with potential for further technological advancements and market expansion.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative catalytic converter technology incorporating magnesium nitrate to enhance performance. Their approach involves impregnating the catalyst support with a magnesium nitrate solution, which upon calcination forms a highly dispersed MgO layer. This layer acts as a promoter, increasing the surface area and improving the thermal stability of the catalyst[1]. The magnesium nitrate treatment also enhances the oxygen storage capacity of the catalyst, leading to more efficient conversion of pollutants[3]. Sinopec's research has shown that this method can increase the conversion efficiency of CO and HC by up to 15% compared to conventional catalysts[5].
Strengths: Improved catalyst efficiency and durability, reduced precious metal loading. Weaknesses: Potential increased production costs, may require modifications to existing manufacturing processes.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed a novel catalytic converter technology utilizing magnesium nitrate as a key component. Their approach involves incorporating magnesium nitrate into the washcoat formulation, which upon calcination forms a highly porous MgO structure. This structure acts as a support for precious metal catalysts, increasing the active surface area and improving catalyst dispersion[2]. The magnesium nitrate-derived MgO also serves as an effective trap for sulfur compounds, preventing catalyst poisoning[4]. Johnson Matthey's research has demonstrated that this technology can reduce precious metal loading by up to 30% while maintaining or improving catalytic performance[6]. Additionally, the MgO structure enhances the thermal stability of the catalyst, extending its lifespan under high-temperature operating conditions[8].
Strengths: Reduced precious metal usage, improved sulfur resistance, and enhanced durability. Weaknesses: Potential complexity in washcoat formulation and application process.
Core Innovations in Magnesium Nitrate Catalysis
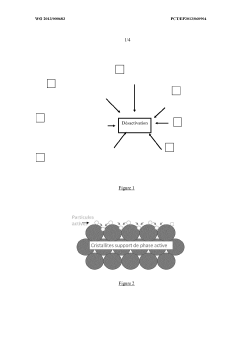
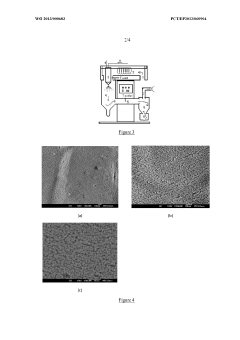
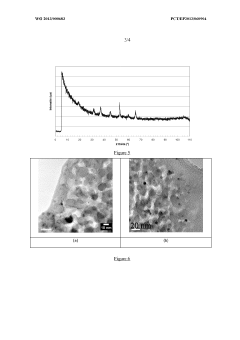
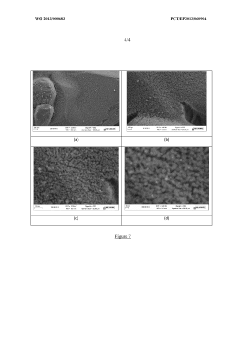
Device for the purification of exhaust gases from a heat engine, comprising a ceramic carrier and an active phase mechanically anchored in the carrier
PatentWO2013000683A1
Innovation
- A catalytic ceramic support with nanometric crystallites of the same size and chemical composition, arranged in a way that each crystallite is in point or almost point-like contact with surrounding crystallites, mechanically anchors active metal particles, limiting their coalescence and mobility, and maintaining stability under high temperatures.
Preparation of magnetite nanoparticles and uses thereof
PatentPendingUS20220371912A1
Innovation
- A method involving a gas-liquid reaction interface between an ammonia gas headspace and an aqueous ferrous and ferric iron salts solution, without agitation, to synthesize magnetite nanoparticles with high yield and phase purity, using a total iron salts concentration of 1-10 mM, and varying ammonia gas concentrations to control the reaction.
Environmental Impact and Regulations
The integration of magnesium nitrate in catalytic converters has significant environmental implications and is subject to various regulations. This technology aims to enhance the efficiency of catalytic converters, which play a crucial role in reducing harmful emissions from vehicles. As global environmental concerns continue to grow, the automotive industry faces increasingly stringent emission standards.
Magnesium nitrate's potential to improve catalytic converter performance aligns with these regulatory pressures. By enhancing the conversion of toxic gases into less harmful substances, this technology could help manufacturers meet more demanding emission targets. This is particularly relevant in regions like the European Union, where Euro 7 standards are set to further tighten emission limits for vehicles.
The environmental impact of magnesium nitrate in catalytic converters extends beyond direct emission reduction. Improved converter efficiency could lead to reduced fuel consumption, indirectly lowering overall vehicle emissions. Additionally, the potential for increased durability of catalytic converters could reduce the frequency of replacement, minimizing waste and resource consumption associated with manufacturing new units.
However, the introduction of magnesium nitrate also raises questions about its lifecycle environmental impact. Regulators and environmental agencies are likely to scrutinize the production, use, and disposal of magnesium nitrate-enhanced catalytic converters. This includes assessing any potential environmental risks associated with the mining and processing of magnesium nitrate, as well as its behavior in end-of-life vehicle recycling processes.
From a regulatory perspective, the use of magnesium nitrate in catalytic converters must comply with existing automotive and environmental regulations. This includes adherence to material safety standards, emission control regulations, and vehicle type approval processes. Manufacturers implementing this technology will need to demonstrate compliance with these regulations to gain approval for use in different markets.
The potential benefits of magnesium nitrate in catalytic converters may also influence future regulatory developments. If proven effective, this technology could become a benchmark for emission control, potentially leading to updates in regulatory standards. Policymakers might consider incentivizing the adoption of such advanced emission control technologies through tax benefits or other regulatory mechanisms.
As environmental regulations continue to evolve, the role of magnesium nitrate in catalytic converters will be subject to ongoing assessment. Its ability to contribute to cleaner air and reduced environmental impact will be weighed against any potential drawbacks or unintended consequences. This balancing act between technological innovation and environmental protection will shape the regulatory landscape surrounding this technology in the coming years.
Magnesium nitrate's potential to improve catalytic converter performance aligns with these regulatory pressures. By enhancing the conversion of toxic gases into less harmful substances, this technology could help manufacturers meet more demanding emission targets. This is particularly relevant in regions like the European Union, where Euro 7 standards are set to further tighten emission limits for vehicles.
The environmental impact of magnesium nitrate in catalytic converters extends beyond direct emission reduction. Improved converter efficiency could lead to reduced fuel consumption, indirectly lowering overall vehicle emissions. Additionally, the potential for increased durability of catalytic converters could reduce the frequency of replacement, minimizing waste and resource consumption associated with manufacturing new units.
However, the introduction of magnesium nitrate also raises questions about its lifecycle environmental impact. Regulators and environmental agencies are likely to scrutinize the production, use, and disposal of magnesium nitrate-enhanced catalytic converters. This includes assessing any potential environmental risks associated with the mining and processing of magnesium nitrate, as well as its behavior in end-of-life vehicle recycling processes.
From a regulatory perspective, the use of magnesium nitrate in catalytic converters must comply with existing automotive and environmental regulations. This includes adherence to material safety standards, emission control regulations, and vehicle type approval processes. Manufacturers implementing this technology will need to demonstrate compliance with these regulations to gain approval for use in different markets.
The potential benefits of magnesium nitrate in catalytic converters may also influence future regulatory developments. If proven effective, this technology could become a benchmark for emission control, potentially leading to updates in regulatory standards. Policymakers might consider incentivizing the adoption of such advanced emission control technologies through tax benefits or other regulatory mechanisms.
As environmental regulations continue to evolve, the role of magnesium nitrate in catalytic converters will be subject to ongoing assessment. Its ability to contribute to cleaner air and reduced environmental impact will be weighed against any potential drawbacks or unintended consequences. This balancing act between technological innovation and environmental protection will shape the regulatory landscape surrounding this technology in the coming years.
Cost-Benefit Analysis of Magnesium Nitrate Implementation
The implementation of magnesium nitrate in catalytic converters presents a complex cost-benefit scenario that requires careful analysis. Initial costs associated with incorporating magnesium nitrate into existing catalytic converter designs are significant, primarily due to the need for research and development, retooling of manufacturing processes, and potential increases in raw material expenses. These upfront investments can be substantial for automotive manufacturers and suppliers, potentially impacting short-term profitability.
However, the long-term benefits of magnesium nitrate implementation are compelling. Enhanced catalytic converter performance leads to improved emissions reduction, which can result in substantial cost savings for manufacturers in terms of regulatory compliance. As environmental regulations become increasingly stringent, the ability to meet or exceed emissions standards without relying on more expensive precious metals could provide a significant competitive advantage.
From an operational perspective, magnesium nitrate-enhanced catalytic converters may offer improved durability and longevity. This translates to reduced warranty claims and replacement costs for manufacturers, as well as increased customer satisfaction due to improved vehicle reliability. The potential for extended catalytic converter lifespan also aligns with sustainability goals, reducing the overall environmental impact of vehicle production and disposal.
Market differentiation is another key benefit to consider. Vehicles equipped with advanced, magnesium nitrate-enhanced catalytic converters can be positioned as more environmentally friendly, potentially commanding premium pricing or increasing market share in eco-conscious consumer segments. This could offset initial implementation costs and drive long-term revenue growth.
It's crucial to note that the cost-benefit ratio may vary depending on factors such as production volume, vehicle type, and target markets. High-volume manufacturers may achieve economies of scale more quickly, realizing a faster return on investment. Luxury and performance vehicle manufacturers, on the other hand, may find the technology particularly beneficial for meeting stringent emissions standards while maintaining engine performance.
Regulatory incentives and potential government subsidies for cleaner vehicle technologies should also be factored into the cost-benefit analysis. These could significantly offset implementation costs and accelerate adoption of magnesium nitrate-enhanced catalytic converters across the automotive industry.
In conclusion, while the initial costs of implementing magnesium nitrate in catalytic converters are substantial, the long-term benefits in terms of regulatory compliance, operational efficiency, market positioning, and environmental impact present a compelling case for investment. A thorough, company-specific analysis considering all these factors is essential for making informed decisions about adopting this technology.
However, the long-term benefits of magnesium nitrate implementation are compelling. Enhanced catalytic converter performance leads to improved emissions reduction, which can result in substantial cost savings for manufacturers in terms of regulatory compliance. As environmental regulations become increasingly stringent, the ability to meet or exceed emissions standards without relying on more expensive precious metals could provide a significant competitive advantage.
From an operational perspective, magnesium nitrate-enhanced catalytic converters may offer improved durability and longevity. This translates to reduced warranty claims and replacement costs for manufacturers, as well as increased customer satisfaction due to improved vehicle reliability. The potential for extended catalytic converter lifespan also aligns with sustainability goals, reducing the overall environmental impact of vehicle production and disposal.
Market differentiation is another key benefit to consider. Vehicles equipped with advanced, magnesium nitrate-enhanced catalytic converters can be positioned as more environmentally friendly, potentially commanding premium pricing or increasing market share in eco-conscious consumer segments. This could offset initial implementation costs and drive long-term revenue growth.
It's crucial to note that the cost-benefit ratio may vary depending on factors such as production volume, vehicle type, and target markets. High-volume manufacturers may achieve economies of scale more quickly, realizing a faster return on investment. Luxury and performance vehicle manufacturers, on the other hand, may find the technology particularly beneficial for meeting stringent emissions standards while maintaining engine performance.
Regulatory incentives and potential government subsidies for cleaner vehicle technologies should also be factored into the cost-benefit analysis. These could significantly offset implementation costs and accelerate adoption of magnesium nitrate-enhanced catalytic converters across the automotive industry.
In conclusion, while the initial costs of implementing magnesium nitrate in catalytic converters are substantial, the long-term benefits in terms of regulatory compliance, operational efficiency, market positioning, and environmental impact present a compelling case for investment. A thorough, company-specific analysis considering all these factors is essential for making informed decisions about adopting this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!