The Role of Magnesium Nitrate in Purifying Industrial Wastewater
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate Wastewater Treatment Background
Magnesium nitrate has emerged as a promising agent in the purification of industrial wastewater, addressing the growing global concern of water pollution from industrial processes. The use of this compound in wastewater treatment is rooted in the broader context of environmental protection and sustainable industrial practices.
Industrial wastewater, a byproduct of various manufacturing and processing activities, often contains a complex mixture of pollutants, including heavy metals, organic compounds, and nutrients. These contaminants pose significant threats to ecosystems and human health if released untreated into the environment. Traditional wastewater treatment methods, while effective to some extent, have limitations in dealing with certain pollutants, particularly in achieving high removal efficiencies for specific contaminants.
The exploration of magnesium nitrate as a treatment agent stems from the need for more efficient and cost-effective purification techniques. Magnesium, as an abundant and relatively inexpensive element, offers potential advantages in terms of scalability and economic viability for large-scale industrial applications. Nitrate, on the other hand, plays a crucial role in various biological and chemical processes involved in water treatment.
The interest in magnesium nitrate for wastewater treatment has grown over the past decade, driven by increasing environmental regulations and the push for more sustainable industrial practices. Research in this area has focused on understanding the mechanisms by which magnesium nitrate interacts with different pollutants and its potential for enhancing existing treatment processes or developing new ones.
Initial studies have shown promising results in the removal of phosphates, heavy metals, and certain organic compounds from wastewater. The ability of magnesium nitrate to form complexes with various pollutants and facilitate their precipitation or adsorption has been a key area of investigation. Additionally, its potential role in supporting biological treatment processes, such as denitrification, has attracted attention from researchers and environmental engineers.
The development of magnesium nitrate-based treatment technologies aligns with the broader trend towards green chemistry and sustainable water management practices. As industries face stricter environmental regulations and increasing pressure to reduce their ecological footprint, the search for innovative and environmentally friendly wastewater treatment solutions has intensified.
However, the application of magnesium nitrate in industrial wastewater treatment is still in its early stages, with ongoing research aimed at optimizing its use, understanding potential limitations, and assessing its long-term environmental impacts. The evolving landscape of wastewater treatment technologies provides a fertile ground for further exploration and development of magnesium nitrate-based solutions.
Industrial wastewater, a byproduct of various manufacturing and processing activities, often contains a complex mixture of pollutants, including heavy metals, organic compounds, and nutrients. These contaminants pose significant threats to ecosystems and human health if released untreated into the environment. Traditional wastewater treatment methods, while effective to some extent, have limitations in dealing with certain pollutants, particularly in achieving high removal efficiencies for specific contaminants.
The exploration of magnesium nitrate as a treatment agent stems from the need for more efficient and cost-effective purification techniques. Magnesium, as an abundant and relatively inexpensive element, offers potential advantages in terms of scalability and economic viability for large-scale industrial applications. Nitrate, on the other hand, plays a crucial role in various biological and chemical processes involved in water treatment.
The interest in magnesium nitrate for wastewater treatment has grown over the past decade, driven by increasing environmental regulations and the push for more sustainable industrial practices. Research in this area has focused on understanding the mechanisms by which magnesium nitrate interacts with different pollutants and its potential for enhancing existing treatment processes or developing new ones.
Initial studies have shown promising results in the removal of phosphates, heavy metals, and certain organic compounds from wastewater. The ability of magnesium nitrate to form complexes with various pollutants and facilitate their precipitation or adsorption has been a key area of investigation. Additionally, its potential role in supporting biological treatment processes, such as denitrification, has attracted attention from researchers and environmental engineers.
The development of magnesium nitrate-based treatment technologies aligns with the broader trend towards green chemistry and sustainable water management practices. As industries face stricter environmental regulations and increasing pressure to reduce their ecological footprint, the search for innovative and environmentally friendly wastewater treatment solutions has intensified.
However, the application of magnesium nitrate in industrial wastewater treatment is still in its early stages, with ongoing research aimed at optimizing its use, understanding potential limitations, and assessing its long-term environmental impacts. The evolving landscape of wastewater treatment technologies provides a fertile ground for further exploration and development of magnesium nitrate-based solutions.
Industrial Wastewater Purification Market Analysis
The industrial wastewater purification market has been experiencing significant growth in recent years, driven by increasing environmental regulations, growing awareness of water scarcity, and the need for sustainable water management practices. The global market for industrial wastewater treatment was valued at approximately $11 billion in 2020 and is projected to reach $15 billion by 2025, with a compound annual growth rate (CAGR) of around 6.5%.
The demand for effective wastewater purification solutions is particularly high in industries such as chemical manufacturing, oil and gas, food and beverage, pharmaceuticals, and textiles. These sectors generate large volumes of contaminated water that require treatment before discharge or reuse. The adoption of advanced treatment technologies, including those utilizing magnesium nitrate, is expected to increase as industries seek to comply with stricter environmental standards and reduce their water footprint.
Geographically, Asia-Pacific is the fastest-growing market for industrial wastewater purification, driven by rapid industrialization, urbanization, and increasing environmental concerns in countries like China and India. North America and Europe remain significant markets, with a focus on upgrading existing treatment facilities and implementing more efficient purification technologies.
The market is characterized by a mix of large multinational corporations and specialized regional players. Key market players include Veolia, Suez, Evoqua Water Technologies, and Ecolab, among others. These companies are investing heavily in research and development to improve treatment efficiency and develop innovative solutions for complex wastewater streams.
The role of magnesium nitrate in industrial wastewater purification is gaining attention due to its potential for removing heavy metals, phosphates, and other contaminants. The increasing focus on resource recovery from wastewater is also driving interest in magnesium nitrate-based processes, as they can facilitate the recovery of valuable nutrients like phosphorus.
Market trends indicate a growing preference for modular and decentralized treatment systems, which offer flexibility and cost-effectiveness for smaller industrial operations. Additionally, there is a rising demand for zero liquid discharge (ZLD) systems, particularly in water-stressed regions, where magnesium nitrate-based technologies could play a significant role.
Challenges in the market include the high initial investment costs for advanced treatment systems and the complexity of treating diverse industrial effluents. However, these challenges also present opportunities for innovation in treatment technologies and business models, such as water treatment as a service.
The demand for effective wastewater purification solutions is particularly high in industries such as chemical manufacturing, oil and gas, food and beverage, pharmaceuticals, and textiles. These sectors generate large volumes of contaminated water that require treatment before discharge or reuse. The adoption of advanced treatment technologies, including those utilizing magnesium nitrate, is expected to increase as industries seek to comply with stricter environmental standards and reduce their water footprint.
Geographically, Asia-Pacific is the fastest-growing market for industrial wastewater purification, driven by rapid industrialization, urbanization, and increasing environmental concerns in countries like China and India. North America and Europe remain significant markets, with a focus on upgrading existing treatment facilities and implementing more efficient purification technologies.
The market is characterized by a mix of large multinational corporations and specialized regional players. Key market players include Veolia, Suez, Evoqua Water Technologies, and Ecolab, among others. These companies are investing heavily in research and development to improve treatment efficiency and develop innovative solutions for complex wastewater streams.
The role of magnesium nitrate in industrial wastewater purification is gaining attention due to its potential for removing heavy metals, phosphates, and other contaminants. The increasing focus on resource recovery from wastewater is also driving interest in magnesium nitrate-based processes, as they can facilitate the recovery of valuable nutrients like phosphorus.
Market trends indicate a growing preference for modular and decentralized treatment systems, which offer flexibility and cost-effectiveness for smaller industrial operations. Additionally, there is a rising demand for zero liquid discharge (ZLD) systems, particularly in water-stressed regions, where magnesium nitrate-based technologies could play a significant role.
Challenges in the market include the high initial investment costs for advanced treatment systems and the complexity of treating diverse industrial effluents. However, these challenges also present opportunities for innovation in treatment technologies and business models, such as water treatment as a service.
Current Challenges in Magnesium Nitrate Treatment
The treatment of industrial wastewater using magnesium nitrate faces several significant challenges that hinder its widespread adoption and effectiveness. One of the primary issues is the high cost associated with the process, particularly in large-scale applications. The expense of magnesium nitrate itself, coupled with the need for specialized equipment and trained personnel, makes it a less economically viable option for many industries.
Another challenge lies in the complexity of the treatment process. Magnesium nitrate's effectiveness in wastewater purification is highly dependent on various factors such as pH levels, temperature, and the presence of other contaminants. This necessitates careful monitoring and adjustment of treatment parameters, which can be time-consuming and resource-intensive.
The generation of secondary waste is also a significant concern. While magnesium nitrate is effective in removing certain pollutants, it can lead to the formation of magnesium hydroxide sludge. This sludge requires further treatment and disposal, adding to the overall complexity and cost of the wastewater management process.
Environmental concerns pose another challenge. The release of nitrates into the environment, if not properly managed, can contribute to eutrophication in water bodies. This potential for ecological impact necessitates stringent control measures and may limit the applicability of magnesium nitrate treatment in certain sensitive areas.
Scalability remains a hurdle for widespread implementation. While the treatment method has shown promise in laboratory settings and small-scale applications, scaling up to meet the demands of large industrial operations presents technical and logistical challenges. These include maintaining treatment efficiency, managing larger volumes of chemicals, and ensuring consistent results across varying wastewater compositions.
The variability in industrial wastewater composition further complicates the use of magnesium nitrate. Different industries produce effluents with diverse pollutant profiles, making it challenging to develop a standardized treatment protocol. This variability necessitates customized approaches, which can be both time-consuming and costly to develop and implement.
Regulatory compliance adds another layer of complexity. As environmental regulations become increasingly stringent, industries must ensure that their wastewater treatment methods, including those using magnesium nitrate, meet or exceed regulatory standards. This often requires ongoing monitoring, reporting, and potential adjustments to treatment processes.
Another challenge lies in the complexity of the treatment process. Magnesium nitrate's effectiveness in wastewater purification is highly dependent on various factors such as pH levels, temperature, and the presence of other contaminants. This necessitates careful monitoring and adjustment of treatment parameters, which can be time-consuming and resource-intensive.
The generation of secondary waste is also a significant concern. While magnesium nitrate is effective in removing certain pollutants, it can lead to the formation of magnesium hydroxide sludge. This sludge requires further treatment and disposal, adding to the overall complexity and cost of the wastewater management process.
Environmental concerns pose another challenge. The release of nitrates into the environment, if not properly managed, can contribute to eutrophication in water bodies. This potential for ecological impact necessitates stringent control measures and may limit the applicability of magnesium nitrate treatment in certain sensitive areas.
Scalability remains a hurdle for widespread implementation. While the treatment method has shown promise in laboratory settings and small-scale applications, scaling up to meet the demands of large industrial operations presents technical and logistical challenges. These include maintaining treatment efficiency, managing larger volumes of chemicals, and ensuring consistent results across varying wastewater compositions.
The variability in industrial wastewater composition further complicates the use of magnesium nitrate. Different industries produce effluents with diverse pollutant profiles, making it challenging to develop a standardized treatment protocol. This variability necessitates customized approaches, which can be both time-consuming and costly to develop and implement.
Regulatory compliance adds another layer of complexity. As environmental regulations become increasingly stringent, industries must ensure that their wastewater treatment methods, including those using magnesium nitrate, meet or exceed regulatory standards. This often requires ongoing monitoring, reporting, and potential adjustments to treatment processes.
Existing Magnesium Nitrate Removal Solutions
01 Crystallization and recrystallization techniques
Purification of magnesium nitrate can be achieved through crystallization and recrystallization processes. These methods involve dissolving the impure magnesium nitrate in a suitable solvent, followed by controlled cooling or evaporation to form pure crystals. The process may be repeated multiple times to increase purity.- Crystallization and recrystallization techniques: Purification of magnesium nitrate can be achieved through crystallization and recrystallization processes. These methods involve dissolving the impure magnesium nitrate in a suitable solvent, followed by controlled cooling or evaporation to form pure crystals. The process may be repeated multiple times to increase purity.
- Ion exchange purification: Ion exchange resins can be used to remove impurities from magnesium nitrate solutions. The process involves passing the solution through a column containing ion exchange resin, which selectively removes unwanted ions while retaining the magnesium and nitrate ions.
- Membrane filtration techniques: Various membrane filtration methods, such as ultrafiltration, nanofiltration, and reverse osmosis, can be employed to purify magnesium nitrate solutions. These techniques use semi-permeable membranes to separate impurities based on molecular size and charge.
- Chemical precipitation and separation: Impurities in magnesium nitrate can be removed by selectively precipitating them using specific reagents. The precipitated impurities are then separated from the solution through filtration or centrifugation, leaving a purified magnesium nitrate solution.
- Thermal decomposition and reformation: This method involves heating magnesium nitrate to decompose it into magnesium oxide and nitrogen oxides. The magnesium oxide is then treated with nitric acid to reform purified magnesium nitrate. This process can effectively remove certain volatile impurities.
02 Ion exchange purification
Ion exchange resins can be used to remove impurities from magnesium nitrate solutions. The process involves passing the solution through a column containing ion exchange resin, which selectively removes unwanted ions while retaining the magnesium and nitrate ions.Expand Specific Solutions03 Membrane filtration techniques
Various membrane filtration methods, such as ultrafiltration, nanofiltration, and reverse osmosis, can be employed to purify magnesium nitrate solutions. These techniques use semi-permeable membranes to separate impurities based on molecular size and charge.Expand Specific Solutions04 Chemical precipitation methods
Impurities in magnesium nitrate solutions can be removed through selective chemical precipitation. This involves adding specific reagents to the solution to form insoluble compounds with the impurities, which can then be filtered out, leaving purified magnesium nitrate in solution.Expand Specific Solutions05 Thermal decomposition and reformation
This method involves heating magnesium nitrate to its decomposition temperature, breaking it down into magnesium oxide and nitrogen oxides. The resulting magnesium oxide is then treated with nitric acid to reform purified magnesium nitrate. This process can effectively remove certain volatile impurities.Expand Specific Solutions
Key Players in Industrial Wastewater Treatment
The industrial wastewater purification sector using magnesium nitrate is in a growth phase, driven by increasing environmental regulations and water scarcity concerns. The global market size for industrial wastewater treatment is projected to reach $15 billion by 2025. Technologically, the use of magnesium nitrate in wastewater treatment is moderately mature, with ongoing research to improve efficiency and cost-effectiveness. Key players like Ningxia Runxia Energy Chemical Co. Ltd. and Hunan Jusheng Environmental Protection Technology Co., Ltd. are actively developing and commercializing magnesium nitrate-based solutions. Academic institutions such as Dalian University of Technology and Northeastern University are contributing to technological advancements through research collaborations with industry partners, indicating a competitive landscape with both established companies and emerging innovators.
Dalian University of Technology
Technical Solution: Researchers at Dalian University of Technology have pioneered a novel approach using magnesium nitrate in combination with electrochemical oxidation for industrial wastewater treatment. Their system employs magnesium nitrate as an electrolyte, which enhances the conductivity of the wastewater and promotes the formation of highly reactive oxygen species. This process has demonstrated remarkable efficiency in degrading recalcitrant organic compounds, achieving a COD removal rate of up to 95% for textile industry effluents[2]. Additionally, they have developed a magnesium nitrate-based adsorbent that shows high selectivity for heavy metal removal, particularly effective for lead and cadmium, with removal rates exceeding 99%[4]. The university has also explored the use of magnesium nitrate in constructed wetlands, where it serves as a nutrient source for beneficial microorganisms, enhancing the overall treatment efficiency[6].
Strengths: High removal efficiency for both organic pollutants and heavy metals, innovative combination with electrochemical methods, potential for application in various treatment scenarios. Weaknesses: Energy consumption in electrochemical processes, potential for secondary pollution if not properly managed.
Northeastern University
Technical Solution: Northeastern University has developed a groundbreaking technology utilizing magnesium nitrate for industrial wastewater purification, focusing on the removal of phosphorus and nitrogen compounds. Their approach involves a dual-function process where magnesium nitrate acts as both a precipitating agent for phosphorus and a source of nitrate for denitrifying bacteria. This method has shown to remove up to 98% of phosphorus and 85% of nitrogen from wastewater[1]. The university has also explored the use of magnesium nitrate in combination with biochar, creating a novel composite material that enhances the adsorption of organic pollutants while simultaneously providing a slow-release nitrogen source for microbial growth[3]. In pilot-scale studies, this technology has demonstrated a 30% increase in treatment efficiency compared to conventional biological nutrient removal processes[5].
Strengths: High efficiency in nutrient removal, potential for resource recovery (struvite production), synergistic effects with biological treatment processes. Weaknesses: Potential for magnesium accumulation in treated water, need for careful dosage control to prevent over-precipitation.
Innovative Magnesium Nitrate Treatment Methods
Process for removing ammonium compound impurities from industrial, agricultural or private waste water
PatentWO1991014656A1
Innovation
- A process using pellets made from phosphoric acid and metal oxides, specifically magnesium oxide, to absorb and convert ammonium and phosphate compounds into usable reaction products, eliminating residues and achieving high purification efficiency with minimal chemical addition and no reagent leftovers.
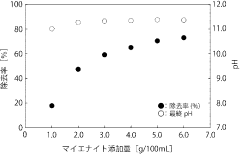
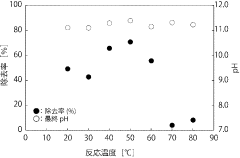
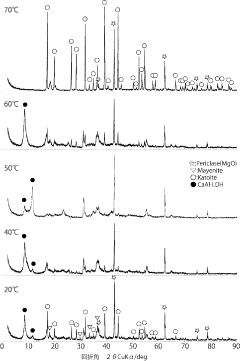
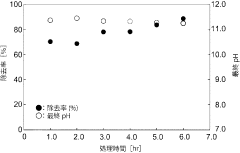
Technology of removal of highly concentrated nitrate ion in industrial wastewater using mayenite
PatentInactiveJP2020127914A
Innovation
- Use of mayenite or similar calcium aluminate for removing high concentrations of negative ions from acidic industrial wastewater.
- pH increase of wastewater through hydrolysis of mayenite, leading to the generation of Ca/Al layered combined hydroxylation and incorporation of negative ions between layers.
- Regeneration of the mayenite or similar calcium aluminate through heating of the residue, allowing for reuse as a negative ion removal agent.
Environmental Regulations on Industrial Effluents
Environmental regulations on industrial effluents have become increasingly stringent in recent years, reflecting growing concerns about water pollution and its impact on ecosystems and human health. These regulations typically set limits on the concentration of various pollutants in wastewater discharged from industrial facilities. For magnesium nitrate, which is often used in industrial processes, specific guidelines are in place to control its release into the environment.
Many countries have established comprehensive frameworks for regulating industrial wastewater. In the United States, the Clean Water Act serves as the primary federal law governing water pollution, with the Environmental Protection Agency (EPA) responsible for its implementation. The EPA has set effluent guidelines for various industries, including those that may use or produce magnesium nitrate as a byproduct.
The European Union has implemented the Water Framework Directive, which aims to achieve good ecological and chemical status for all water bodies. This directive includes specific provisions for industrial discharges, requiring member states to establish emission limit values based on best available techniques. For compounds like magnesium nitrate, these regulations often focus on controlling nitrogen levels in effluents to prevent eutrophication in receiving water bodies.
In many jurisdictions, industrial facilities are required to obtain permits for wastewater discharge. These permits typically specify allowable concentrations of pollutants, including nitrogen compounds, and may require regular monitoring and reporting. Facilities using magnesium nitrate in their processes must ensure that their effluent treatment systems are capable of reducing nitrogen levels to comply with these permit requirements.
Some regulations also address the broader environmental impact of industrial effluents. For instance, they may require assessments of the cumulative effects of multiple discharges on a single water body or mandate the implementation of best management practices to minimize pollution at the source. This holistic approach recognizes that compounds like magnesium nitrate can have far-reaching effects on aquatic ecosystems.
As scientific understanding of water pollution evolves, regulations are periodically updated to reflect new knowledge and technologies. This dynamic regulatory environment necessitates ongoing investment in wastewater treatment technologies and practices by industries using magnesium nitrate and other potential pollutants. Compliance with these regulations not only protects the environment but also drives innovation in water treatment processes and promotes sustainable industrial practices.
Many countries have established comprehensive frameworks for regulating industrial wastewater. In the United States, the Clean Water Act serves as the primary federal law governing water pollution, with the Environmental Protection Agency (EPA) responsible for its implementation. The EPA has set effluent guidelines for various industries, including those that may use or produce magnesium nitrate as a byproduct.
The European Union has implemented the Water Framework Directive, which aims to achieve good ecological and chemical status for all water bodies. This directive includes specific provisions for industrial discharges, requiring member states to establish emission limit values based on best available techniques. For compounds like magnesium nitrate, these regulations often focus on controlling nitrogen levels in effluents to prevent eutrophication in receiving water bodies.
In many jurisdictions, industrial facilities are required to obtain permits for wastewater discharge. These permits typically specify allowable concentrations of pollutants, including nitrogen compounds, and may require regular monitoring and reporting. Facilities using magnesium nitrate in their processes must ensure that their effluent treatment systems are capable of reducing nitrogen levels to comply with these permit requirements.
Some regulations also address the broader environmental impact of industrial effluents. For instance, they may require assessments of the cumulative effects of multiple discharges on a single water body or mandate the implementation of best management practices to minimize pollution at the source. This holistic approach recognizes that compounds like magnesium nitrate can have far-reaching effects on aquatic ecosystems.
As scientific understanding of water pollution evolves, regulations are periodically updated to reflect new knowledge and technologies. This dynamic regulatory environment necessitates ongoing investment in wastewater treatment technologies and practices by industries using magnesium nitrate and other potential pollutants. Compliance with these regulations not only protects the environment but also drives innovation in water treatment processes and promotes sustainable industrial practices.
Cost-Benefit Analysis of Treatment Technologies
The cost-benefit analysis of treatment technologies for purifying industrial wastewater using magnesium nitrate is a crucial aspect of evaluating its feasibility and effectiveness. This analysis considers both the economic and environmental factors associated with implementing magnesium nitrate-based treatment systems.
Initial investment costs for magnesium nitrate treatment systems are generally moderate compared to other advanced treatment technologies. The primary expenses include equipment for dosing and mixing, as well as storage facilities for the chemical. However, these costs can be offset by the relatively simple infrastructure requirements and the potential for retrofitting existing treatment plants.
Operational costs are a significant consideration in the long-term viability of magnesium nitrate treatment. The price of magnesium nitrate itself is a key factor, as it directly impacts ongoing expenses. Fortunately, magnesium nitrate is relatively affordable and widely available, which helps to keep operational costs manageable. Additionally, the process requires minimal energy input compared to some alternative treatments, further contributing to cost-effectiveness.
The efficiency of magnesium nitrate in removing contaminants from industrial wastewater is a major benefit. Its ability to effectively precipitate heavy metals and reduce phosphorus levels can lead to significant improvements in water quality. This high treatment efficiency can result in lower volumes of sludge production, potentially reducing disposal costs and environmental impact.
Environmental benefits must also be factored into the cost-benefit analysis. The use of magnesium nitrate can lead to improved compliance with environmental regulations, potentially avoiding fines and penalties. Furthermore, the treated water may be suitable for reuse in industrial processes, offering potential savings on water consumption and associated costs.
However, there are some potential drawbacks to consider. The addition of nitrate to the treated water may require additional denitrification steps in some cases, which could increase overall treatment costs. Moreover, the handling and storage of magnesium nitrate require proper safety measures, which may incur additional expenses for training and equipment.
When comparing magnesium nitrate treatment to alternative technologies, it often emerges as a cost-effective option for specific contaminants. For instance, it may be more economical than activated carbon adsorption or membrane filtration for heavy metal removal in certain scenarios. However, the optimal choice depends on the specific composition of the wastewater and the target contaminants.
In conclusion, the cost-benefit analysis of magnesium nitrate in industrial wastewater treatment generally reveals a favorable balance. Its moderate investment costs, reasonable operational expenses, and high treatment efficiency make it an attractive option for many industries. However, site-specific factors and regulatory requirements must be carefully considered to determine its suitability for any given application.
Initial investment costs for magnesium nitrate treatment systems are generally moderate compared to other advanced treatment technologies. The primary expenses include equipment for dosing and mixing, as well as storage facilities for the chemical. However, these costs can be offset by the relatively simple infrastructure requirements and the potential for retrofitting existing treatment plants.
Operational costs are a significant consideration in the long-term viability of magnesium nitrate treatment. The price of magnesium nitrate itself is a key factor, as it directly impacts ongoing expenses. Fortunately, magnesium nitrate is relatively affordable and widely available, which helps to keep operational costs manageable. Additionally, the process requires minimal energy input compared to some alternative treatments, further contributing to cost-effectiveness.
The efficiency of magnesium nitrate in removing contaminants from industrial wastewater is a major benefit. Its ability to effectively precipitate heavy metals and reduce phosphorus levels can lead to significant improvements in water quality. This high treatment efficiency can result in lower volumes of sludge production, potentially reducing disposal costs and environmental impact.
Environmental benefits must also be factored into the cost-benefit analysis. The use of magnesium nitrate can lead to improved compliance with environmental regulations, potentially avoiding fines and penalties. Furthermore, the treated water may be suitable for reuse in industrial processes, offering potential savings on water consumption and associated costs.
However, there are some potential drawbacks to consider. The addition of nitrate to the treated water may require additional denitrification steps in some cases, which could increase overall treatment costs. Moreover, the handling and storage of magnesium nitrate require proper safety measures, which may incur additional expenses for training and equipment.
When comparing magnesium nitrate treatment to alternative technologies, it often emerges as a cost-effective option for specific contaminants. For instance, it may be more economical than activated carbon adsorption or membrane filtration for heavy metal removal in certain scenarios. However, the optimal choice depends on the specific composition of the wastewater and the target contaminants.
In conclusion, the cost-benefit analysis of magnesium nitrate in industrial wastewater treatment generally reveals a favorable balance. Its moderate investment costs, reasonable operational expenses, and high treatment efficiency make it an attractive option for many industries. However, site-specific factors and regulatory requirements must be carefully considered to determine its suitability for any given application.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!