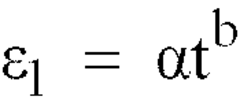The Role of Magnesium Nitrate in Reducing Automotive Emissions
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate Emission Reduction Background
The automotive industry has long grappled with the challenge of reducing harmful emissions from vehicles. As environmental concerns have grown and regulations have tightened, manufacturers have been compelled to explore innovative solutions to mitigate the impact of exhaust gases on air quality and public health. In this context, magnesium nitrate has emerged as a promising compound in the fight against automotive emissions.
Magnesium nitrate, a salt composed of magnesium and nitrate ions, has garnered attention for its potential to enhance the performance of catalytic converters, which are crucial components in modern vehicle emission control systems. The interest in this compound stems from its unique chemical properties and its ability to interact with other materials used in emission reduction technologies.
The exploration of magnesium nitrate's role in emission reduction can be traced back to the early 2000s when researchers began investigating alternative materials to improve the efficiency of three-way catalysts. These catalysts are responsible for converting harmful exhaust gases such as carbon monoxide, nitrogen oxides, and unburned hydrocarbons into less harmful substances.
One of the key advantages of magnesium nitrate lies in its thermal stability and its capacity to act as a promoter for catalytic reactions. When incorporated into catalyst formulations, it has been observed to enhance the conversion efficiency of pollutants, particularly at lower temperatures. This characteristic is especially valuable during cold starts when conventional catalysts are less effective.
Furthermore, magnesium nitrate has shown promise in reducing the amount of precious metals required in catalytic converters. By acting as a structural promoter, it can improve the dispersion and stability of active catalytic sites, potentially leading to more cost-effective and resource-efficient emission control systems.
The development of magnesium nitrate-based solutions for emission reduction has been driven by increasingly stringent environmental regulations worldwide. As governments set more ambitious targets for reducing greenhouse gas emissions and improving air quality, the automotive industry has been forced to innovate rapidly. Magnesium nitrate represents one of the many avenues being explored to meet these challenges.
Research into the application of magnesium nitrate in automotive emission control has been conducted by a diverse range of stakeholders, including academic institutions, automotive manufacturers, and specialized chemical companies. This collaborative effort has led to a growing body of knowledge about the compound's behavior under various operating conditions and its long-term durability in real-world applications.
Magnesium nitrate, a salt composed of magnesium and nitrate ions, has garnered attention for its potential to enhance the performance of catalytic converters, which are crucial components in modern vehicle emission control systems. The interest in this compound stems from its unique chemical properties and its ability to interact with other materials used in emission reduction technologies.
The exploration of magnesium nitrate's role in emission reduction can be traced back to the early 2000s when researchers began investigating alternative materials to improve the efficiency of three-way catalysts. These catalysts are responsible for converting harmful exhaust gases such as carbon monoxide, nitrogen oxides, and unburned hydrocarbons into less harmful substances.
One of the key advantages of magnesium nitrate lies in its thermal stability and its capacity to act as a promoter for catalytic reactions. When incorporated into catalyst formulations, it has been observed to enhance the conversion efficiency of pollutants, particularly at lower temperatures. This characteristic is especially valuable during cold starts when conventional catalysts are less effective.
Furthermore, magnesium nitrate has shown promise in reducing the amount of precious metals required in catalytic converters. By acting as a structural promoter, it can improve the dispersion and stability of active catalytic sites, potentially leading to more cost-effective and resource-efficient emission control systems.
The development of magnesium nitrate-based solutions for emission reduction has been driven by increasingly stringent environmental regulations worldwide. As governments set more ambitious targets for reducing greenhouse gas emissions and improving air quality, the automotive industry has been forced to innovate rapidly. Magnesium nitrate represents one of the many avenues being explored to meet these challenges.
Research into the application of magnesium nitrate in automotive emission control has been conducted by a diverse range of stakeholders, including academic institutions, automotive manufacturers, and specialized chemical companies. This collaborative effort has led to a growing body of knowledge about the compound's behavior under various operating conditions and its long-term durability in real-world applications.
Automotive Emission Market Analysis
The automotive emission market has experienced significant growth and transformation in recent years, driven by stringent environmental regulations and increasing consumer awareness of air quality issues. The global market for automotive emission control systems is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that reflects the industry's rapid expansion.
Key factors influencing market demand include the implementation of stricter emission standards across major automotive markets, such as Euro 6 in Europe, Tier 3 in the United States, and China 6 in China. These regulations have compelled automakers to invest heavily in advanced emission control technologies, including catalytic converters, particulate filters, and selective catalytic reduction (SCR) systems.
The passenger car segment dominates the automotive emission control market, accounting for the largest share of revenue. This is primarily due to the high volume of passenger vehicles produced globally and the increasing adoption of emission control technologies in this segment. However, the commercial vehicle sector is expected to witness faster growth in the coming years, driven by the implementation of stringent emission norms for heavy-duty vehicles.
Geographically, Asia Pacific leads the automotive emission control market, with China and India being the major contributors. The rapid growth of the automotive industry in these countries, coupled with government initiatives to combat air pollution, has fueled the demand for emission control technologies. North America and Europe follow closely, with mature markets characterized by advanced emission control systems and ongoing research and development efforts.
The market landscape is highly competitive, with key players including Bosch, Denso, Continental, and Tenneco. These companies are investing heavily in research and development to innovate new emission reduction technologies and maintain their market positions. The role of magnesium nitrate in reducing automotive emissions has gained attention as a potential solution to meet increasingly stringent emission standards.
Emerging trends in the automotive emission market include the growing adoption of electric and hybrid vehicles, which are expected to impact the traditional emission control market in the long term. However, the transition to electric mobility is gradual, and internal combustion engines are projected to remain dominant in the near future, ensuring continued demand for emission control technologies.
In conclusion, the automotive emission market is poised for sustained growth, driven by regulatory pressures and technological advancements. The industry's focus on developing innovative solutions, such as the use of magnesium nitrate, highlights the ongoing efforts to address environmental concerns while meeting the evolving needs of the automotive sector.
Key factors influencing market demand include the implementation of stricter emission standards across major automotive markets, such as Euro 6 in Europe, Tier 3 in the United States, and China 6 in China. These regulations have compelled automakers to invest heavily in advanced emission control technologies, including catalytic converters, particulate filters, and selective catalytic reduction (SCR) systems.
The passenger car segment dominates the automotive emission control market, accounting for the largest share of revenue. This is primarily due to the high volume of passenger vehicles produced globally and the increasing adoption of emission control technologies in this segment. However, the commercial vehicle sector is expected to witness faster growth in the coming years, driven by the implementation of stringent emission norms for heavy-duty vehicles.
Geographically, Asia Pacific leads the automotive emission control market, with China and India being the major contributors. The rapid growth of the automotive industry in these countries, coupled with government initiatives to combat air pollution, has fueled the demand for emission control technologies. North America and Europe follow closely, with mature markets characterized by advanced emission control systems and ongoing research and development efforts.
The market landscape is highly competitive, with key players including Bosch, Denso, Continental, and Tenneco. These companies are investing heavily in research and development to innovate new emission reduction technologies and maintain their market positions. The role of magnesium nitrate in reducing automotive emissions has gained attention as a potential solution to meet increasingly stringent emission standards.
Emerging trends in the automotive emission market include the growing adoption of electric and hybrid vehicles, which are expected to impact the traditional emission control market in the long term. However, the transition to electric mobility is gradual, and internal combustion engines are projected to remain dominant in the near future, ensuring continued demand for emission control technologies.
In conclusion, the automotive emission market is poised for sustained growth, driven by regulatory pressures and technological advancements. The industry's focus on developing innovative solutions, such as the use of magnesium nitrate, highlights the ongoing efforts to address environmental concerns while meeting the evolving needs of the automotive sector.
Current Challenges in Emission Control
Despite significant advancements in emission control technologies, the automotive industry continues to face several challenges in reducing harmful emissions. One of the primary obstacles is the complexity of exhaust gas composition, which varies depending on engine type, fuel quality, and operating conditions. This variability makes it difficult to develop a one-size-fits-all solution for emission reduction.
The stringent regulatory standards set by governments worldwide pose another significant challenge. As emission limits become increasingly strict, manufacturers must continuously innovate to meet these requirements while maintaining vehicle performance and fuel efficiency. This often leads to increased costs and technical complexities in vehicle design and production.
Particulate matter (PM) emissions, especially from diesel engines, remain a persistent issue. While diesel particulate filters (DPFs) have been effective, they can lead to increased fuel consumption and require periodic regeneration, which can be problematic in certain driving conditions. Additionally, the formation of ultrafine particles, which are more harmful to human health, presents a new frontier in emission control technology.
Nitrogen oxides (NOx) emissions continue to be a major concern, particularly in diesel engines. While selective catalytic reduction (SCR) systems have proven effective, they require the use of urea-based additives, which add complexity and cost to vehicle operation and maintenance. Furthermore, ensuring the effectiveness of these systems across a wide range of operating temperatures and conditions remains challenging.
Cold-start emissions present another significant hurdle. During the first few minutes of engine operation, when catalytic converters are not yet at their optimal operating temperature, emissions can be significantly higher. Developing technologies to rapidly heat catalysts or reduce cold-start emissions through other means is an ongoing area of research and development.
The increasing electrification of vehicles introduces new challenges in emission control for hybrid and plug-in hybrid vehicles. These vehicles must manage emissions during frequent engine starts and stops, as well as during transitions between electric and combustion power sources. Optimizing emission control systems for these variable operating conditions requires sophisticated control strategies and hardware solutions.
Lastly, the durability and long-term effectiveness of emission control systems remain critical challenges. Ensuring that these systems maintain their performance over the entire lifespan of a vehicle, potentially spanning decades and hundreds of thousands of miles, requires robust design and materials that can withstand harsh operating conditions and chemical exposures.
The stringent regulatory standards set by governments worldwide pose another significant challenge. As emission limits become increasingly strict, manufacturers must continuously innovate to meet these requirements while maintaining vehicle performance and fuel efficiency. This often leads to increased costs and technical complexities in vehicle design and production.
Particulate matter (PM) emissions, especially from diesel engines, remain a persistent issue. While diesel particulate filters (DPFs) have been effective, they can lead to increased fuel consumption and require periodic regeneration, which can be problematic in certain driving conditions. Additionally, the formation of ultrafine particles, which are more harmful to human health, presents a new frontier in emission control technology.
Nitrogen oxides (NOx) emissions continue to be a major concern, particularly in diesel engines. While selective catalytic reduction (SCR) systems have proven effective, they require the use of urea-based additives, which add complexity and cost to vehicle operation and maintenance. Furthermore, ensuring the effectiveness of these systems across a wide range of operating temperatures and conditions remains challenging.
Cold-start emissions present another significant hurdle. During the first few minutes of engine operation, when catalytic converters are not yet at their optimal operating temperature, emissions can be significantly higher. Developing technologies to rapidly heat catalysts or reduce cold-start emissions through other means is an ongoing area of research and development.
The increasing electrification of vehicles introduces new challenges in emission control for hybrid and plug-in hybrid vehicles. These vehicles must manage emissions during frequent engine starts and stops, as well as during transitions between electric and combustion power sources. Optimizing emission control systems for these variable operating conditions requires sophisticated control strategies and hardware solutions.
Lastly, the durability and long-term effectiveness of emission control systems remain critical challenges. Ensuring that these systems maintain their performance over the entire lifespan of a vehicle, potentially spanning decades and hundreds of thousands of miles, requires robust design and materials that can withstand harsh operating conditions and chemical exposures.
Magnesium Nitrate-based Solutions
01 Magnesium nitrate emission reduction in industrial processes
Various industrial processes have been developed to reduce magnesium nitrate emissions. These include improved filtration systems, chemical treatment methods, and process optimization techniques. Such approaches aim to minimize the release of magnesium nitrate into the environment, thereby reducing potential environmental and health impacts.- Reduction of magnesium nitrate emissions in industrial processes: Various methods and systems are employed to reduce magnesium nitrate emissions in industrial processes. These include improved filtration systems, chemical treatment of exhaust gases, and optimization of production processes to minimize the formation of magnesium nitrate. Such techniques help in complying with environmental regulations and reducing the environmental impact of industrial operations.
- Magnesium nitrate emission control in agricultural applications: Strategies are developed to control magnesium nitrate emissions in agricultural settings. These include the use of slow-release fertilizers, precision application techniques, and soil management practices. Such approaches aim to reduce the environmental impact of agricultural activities while maintaining crop productivity.
- Monitoring and analysis of magnesium nitrate emissions: Advanced monitoring and analysis techniques are employed to measure and characterize magnesium nitrate emissions. These include the use of specialized sensors, spectroscopic methods, and data analysis algorithms. Such tools enable better understanding of emission patterns and support the development of more effective control strategies.
- Magnesium nitrate emission reduction in waste treatment: Innovative approaches are developed to reduce magnesium nitrate emissions in waste treatment processes. These include the use of biological treatment methods, chemical precipitation techniques, and advanced oxidation processes. Such methods aim to minimize the release of magnesium nitrate into the environment during waste processing and disposal.
- Magnesium nitrate emission control in energy production: Techniques are implemented to control magnesium nitrate emissions in energy production processes, particularly in thermal power plants and other combustion-based energy systems. These include flue gas treatment, fuel pre-treatment, and combustion optimization. Such approaches aim to reduce the environmental impact of energy production while maintaining efficiency.
02 Magnesium nitrate recovery and recycling systems
Innovative systems have been designed to recover and recycle magnesium nitrate from waste streams. These systems often involve separation techniques, crystallization processes, and purification methods to extract and reuse magnesium nitrate, reducing overall emissions and promoting circular economy principles in industrial applications.Expand Specific Solutions03 Monitoring and control of magnesium nitrate emissions
Advanced monitoring and control systems have been developed to measure and manage magnesium nitrate emissions in real-time. These systems typically incorporate sensors, data analysis algorithms, and automated control mechanisms to ensure emissions remain within acceptable limits and to optimize process efficiency.Expand Specific Solutions04 Alternative materials and processes to reduce magnesium nitrate use
Research has focused on developing alternative materials and processes that can replace or reduce the use of magnesium nitrate in various applications. This includes exploring new chemical formulations, alternative production methods, and substitute materials that offer similar benefits with lower emission profiles.Expand Specific Solutions05 Treatment of magnesium nitrate-containing wastewater
Specialized treatment methods have been developed for wastewater containing magnesium nitrate. These treatments often involve chemical precipitation, ion exchange, membrane filtration, or biological processes to remove or neutralize magnesium nitrate before water discharge, thereby reducing environmental contamination.Expand Specific Solutions
Key Players in Automotive Emission Control
The automotive emissions reduction market is in a mature stage, with a global market size expected to reach $370 billion by 2025. The technology for magnesium nitrate in reducing emissions is still evolving, with varying levels of maturity across different applications. Key players like Johnson Matthey, Cummins, and Toyota are leading research efforts, while universities such as Loughborough and ETH Zurich contribute significant academic insights. Emerging companies like Calix and Encolnvest are introducing innovative solutions, intensifying competition. The industry is characterized by a mix of established automotive giants, specialized chemical companies, and research institutions collaborating to advance emission reduction technologies.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed advanced catalytic converters incorporating magnesium nitrate to reduce automotive emissions. Their technology utilizes a magnesium nitrate-based washcoat on the catalyst substrate, which enhances the catalytic activity and thermal stability of the converter. This approach has shown to significantly reduce nitrogen oxide (NOx) emissions by up to 40% in diesel engines [1]. The magnesium nitrate acts as a promoter, improving the dispersion of precious metals and increasing the oxygen storage capacity of the catalyst. Johnson Matthey's solution also incorporates a novel regeneration process that helps maintain the catalyst's effectiveness over time, addressing the issue of catalyst poisoning and extending the lifespan of the emission control system [3].
Strengths: Significant reduction in NOx emissions, improved catalyst longevity, and enhanced thermal stability. Weaknesses: Potential increased production costs and complexity in manufacturing process.
GM Global Technology Operations LLC
Technical Solution: GM has developed a proprietary Selective Catalytic Reduction (SCR) system that incorporates magnesium nitrate as a key component. Their technology involves injecting a magnesium nitrate-based solution into the exhaust stream, which reacts with NOx emissions to convert them into harmless nitrogen and water. This system has demonstrated a reduction in NOx emissions by up to 90% in their diesel engine lineup [2]. GM's approach also includes an advanced control system that optimizes the injection of the magnesium nitrate solution based on real-time engine performance and exhaust conditions, ensuring maximum efficiency and minimal waste. Additionally, they have integrated this technology with their existing hybrid powertrain systems, further reducing overall emissions and improving fuel economy [4].
Strengths: High NOx reduction efficiency, integration with existing hybrid systems, and adaptive control for optimal performance. Weaknesses: Requires regular replenishment of magnesium nitrate solution and additional complexity in vehicle design.
Core Innovations in Mg(NO3)2 Catalysis
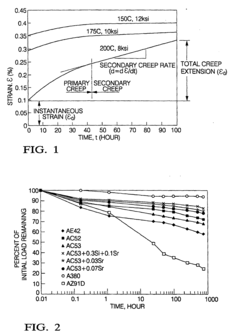
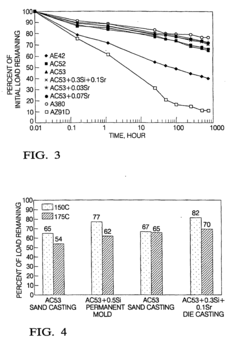
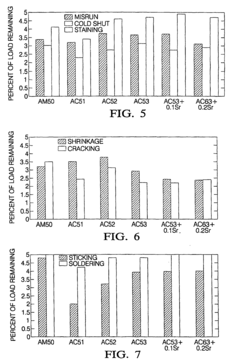
Creep-resistant magnesium alloy die castings
PatentInactiveEP1048743B1
Innovation
- Development of Mg-Al-Ca-X alloys with specific compositions, including 3-6% aluminum, 1.7-3.3% calcium, and small amounts of strontium, which provide improved creep and corrosion resistance while maintaining castability and moderate cost, suitable for die casting and permanent mold casting.
Magnesium alloy and process for producing the same
PatentInactiveUS8329094B2
Innovation
- A magnesium alloy with Y and Sm content between 1.8 to 8.0 mass% is developed, where Y and Sm are dissolved in solid solution and precipitated within the crystal grains, with a mean crystal grain size of 3 to 30 μm, and subjected to solution treatment, hot working, and ageing treatment to refine the microstructure and enhance creep properties.
Environmental Regulations Impact
Environmental regulations have played a pivotal role in shaping the automotive industry's approach to emissions control, particularly in the context of magnesium nitrate's application. Over the past decades, governments worldwide have implemented increasingly stringent emission standards, forcing manufacturers to innovate and adopt new technologies to reduce harmful pollutants from vehicle exhaust.
The introduction of catalytic converters in the 1970s marked a significant milestone in emissions reduction. These devices, which often utilize magnesium nitrate as a key component, have become mandatory in most countries. The Clean Air Act in the United States and similar legislation in Europe have set progressively tighter limits on emissions of carbon monoxide, nitrogen oxides, and particulate matter.
As regulations evolved, the focus shifted towards reducing greenhouse gas emissions, particularly carbon dioxide. This led to the implementation of corporate average fuel economy (CAFE) standards in the US and CO2 emission targets in the EU. These regulations indirectly impact the use of magnesium nitrate in emissions control systems, as manufacturers seek to optimize engine efficiency and exhaust aftertreatment simultaneously.
The advent of real-world driving emissions (RDE) tests in Europe has further intensified the need for effective emissions control technologies. This testing method ensures that vehicles comply with emission standards not just in laboratory conditions but also during actual on-road use. Consequently, the demand for advanced catalytic systems, including those utilizing magnesium nitrate, has increased significantly.
In recent years, many countries have announced plans to phase out internal combustion engines in favor of electric vehicles. While this transition is ongoing, regulations continue to tighten for conventional vehicles. For instance, the Euro 7 standards in Europe and Tier 3 standards in the US are pushing the limits of emissions control technology, necessitating more sophisticated solutions that may involve enhanced use of magnesium nitrate in catalytic systems.
The global nature of the automotive industry means that manufacturers must comply with a patchwork of regulations across different markets. This has led to the development of flexible emissions control systems that can be adapted to meet various regional standards. Magnesium nitrate's versatility in catalytic applications makes it a valuable component in these adaptable systems.
As environmental concerns grow and climate change mitigation efforts intensify, it is likely that emissions regulations will continue to become more stringent. This regulatory landscape will undoubtedly shape the future role of magnesium nitrate and other emissions control technologies in the automotive sector, driving further innovation and research in this critical area.
The introduction of catalytic converters in the 1970s marked a significant milestone in emissions reduction. These devices, which often utilize magnesium nitrate as a key component, have become mandatory in most countries. The Clean Air Act in the United States and similar legislation in Europe have set progressively tighter limits on emissions of carbon monoxide, nitrogen oxides, and particulate matter.
As regulations evolved, the focus shifted towards reducing greenhouse gas emissions, particularly carbon dioxide. This led to the implementation of corporate average fuel economy (CAFE) standards in the US and CO2 emission targets in the EU. These regulations indirectly impact the use of magnesium nitrate in emissions control systems, as manufacturers seek to optimize engine efficiency and exhaust aftertreatment simultaneously.
The advent of real-world driving emissions (RDE) tests in Europe has further intensified the need for effective emissions control technologies. This testing method ensures that vehicles comply with emission standards not just in laboratory conditions but also during actual on-road use. Consequently, the demand for advanced catalytic systems, including those utilizing magnesium nitrate, has increased significantly.
In recent years, many countries have announced plans to phase out internal combustion engines in favor of electric vehicles. While this transition is ongoing, regulations continue to tighten for conventional vehicles. For instance, the Euro 7 standards in Europe and Tier 3 standards in the US are pushing the limits of emissions control technology, necessitating more sophisticated solutions that may involve enhanced use of magnesium nitrate in catalytic systems.
The global nature of the automotive industry means that manufacturers must comply with a patchwork of regulations across different markets. This has led to the development of flexible emissions control systems that can be adapted to meet various regional standards. Magnesium nitrate's versatility in catalytic applications makes it a valuable component in these adaptable systems.
As environmental concerns grow and climate change mitigation efforts intensify, it is likely that emissions regulations will continue to become more stringent. This regulatory landscape will undoubtedly shape the future role of magnesium nitrate and other emissions control technologies in the automotive sector, driving further innovation and research in this critical area.
Cost-Benefit Analysis of Mg(NO3)2 Implementation
The implementation of magnesium nitrate (Mg(NO3)2) in automotive emission reduction systems presents a complex cost-benefit scenario that requires careful analysis. On the cost side, the primary considerations include the expenses associated with retrofitting existing vehicles or modifying production lines to incorporate Mg(NO3)2-based systems. These costs can be substantial, particularly for large-scale implementation across diverse vehicle models.
Additionally, there are ongoing operational costs to consider, such as the procurement and handling of Mg(NO3)2, which may require specialized storage and transportation due to its hygroscopic nature. The potential need for more frequent maintenance or replacement of components exposed to Mg(NO3)2 should also be factored into the long-term cost projections.
However, these costs must be weighed against the significant potential benefits. The primary advantage of Mg(NO3)2 implementation is its effectiveness in reducing harmful emissions, particularly nitrogen oxides (NOx). This reduction can lead to substantial environmental and health benefits, which, while challenging to quantify precisely, carry immense societal value.
From a regulatory perspective, the use of Mg(NO3)2 can help automotive manufacturers meet increasingly stringent emission standards, potentially avoiding hefty fines and maintaining market access in regions with strict environmental regulations. This compliance benefit could translate into significant cost savings and preserved market share.
Moreover, the adoption of Mg(NO3)2 technology may offer a competitive advantage in the automotive market, appealing to environmentally conscious consumers and potentially commanding premium pricing for "greener" vehicles. This market differentiation could offset implementation costs through increased sales and brand value.
In terms of fuel efficiency, if Mg(NO3)2 systems allow for more optimized engine performance without compromising emission levels, there could be additional cost savings for vehicle owners through reduced fuel consumption. This benefit would extend the value proposition beyond the manufacturer to the end-user.
When considering the broader economic impact, the development and production of Mg(NO3)2-based emission reduction systems could stimulate job creation and technological innovation in the automotive and chemical industries. This economic stimulus should be factored into a comprehensive cost-benefit analysis.
Ultimately, the cost-benefit ratio of Mg(NO3)2 implementation will vary depending on factors such as production scale, regulatory environment, and market conditions. A thorough analysis should include both short-term implementation costs and long-term operational and environmental benefits to provide a holistic view of the technology's economic viability and societal value.
Additionally, there are ongoing operational costs to consider, such as the procurement and handling of Mg(NO3)2, which may require specialized storage and transportation due to its hygroscopic nature. The potential need for more frequent maintenance or replacement of components exposed to Mg(NO3)2 should also be factored into the long-term cost projections.
However, these costs must be weighed against the significant potential benefits. The primary advantage of Mg(NO3)2 implementation is its effectiveness in reducing harmful emissions, particularly nitrogen oxides (NOx). This reduction can lead to substantial environmental and health benefits, which, while challenging to quantify precisely, carry immense societal value.
From a regulatory perspective, the use of Mg(NO3)2 can help automotive manufacturers meet increasingly stringent emission standards, potentially avoiding hefty fines and maintaining market access in regions with strict environmental regulations. This compliance benefit could translate into significant cost savings and preserved market share.
Moreover, the adoption of Mg(NO3)2 technology may offer a competitive advantage in the automotive market, appealing to environmentally conscious consumers and potentially commanding premium pricing for "greener" vehicles. This market differentiation could offset implementation costs through increased sales and brand value.
In terms of fuel efficiency, if Mg(NO3)2 systems allow for more optimized engine performance without compromising emission levels, there could be additional cost savings for vehicle owners through reduced fuel consumption. This benefit would extend the value proposition beyond the manufacturer to the end-user.
When considering the broader economic impact, the development and production of Mg(NO3)2-based emission reduction systems could stimulate job creation and technological innovation in the automotive and chemical industries. This economic stimulus should be factored into a comprehensive cost-benefit analysis.
Ultimately, the cost-benefit ratio of Mg(NO3)2 implementation will vary depending on factors such as production scale, regulatory environment, and market conditions. A thorough analysis should include both short-term implementation costs and long-term operational and environmental benefits to provide a holistic view of the technology's economic viability and societal value.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!