The Use of Magnesium Nitrate in Aqueous Phase Reforming Processes
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate APR Background and Objectives
Aqueous Phase Reforming (APR) has emerged as a promising technology for the production of hydrogen and value-added chemicals from biomass-derived oxygenated hydrocarbons. The use of magnesium nitrate in APR processes represents a significant advancement in this field, offering potential improvements in catalyst performance and process efficiency.
The development of APR technology can be traced back to the early 2000s when researchers sought alternative methods for hydrogen production from renewable resources. Traditional steam reforming processes, while effective for fossil fuels, were less suitable for biomass-derived feedstocks due to their high oxygen content and tendency to form coke deposits on catalysts.
Magnesium nitrate's introduction into APR processes stems from the need to enhance catalyst stability and selectivity. Conventional APR catalysts, typically based on noble metals such as platinum or palladium, often suffer from deactivation due to sintering and carbon deposition. The incorporation of magnesium nitrate as a promoter or support modifier aims to address these challenges.
The primary objective of utilizing magnesium nitrate in APR is to improve the overall efficiency and sustainability of the process. Specifically, researchers aim to achieve higher hydrogen yields, increased selectivity towards desired products, and enhanced catalyst longevity. These improvements could potentially reduce the economic barriers to large-scale implementation of APR technology.
Another key goal is to expand the range of feedstocks that can be effectively processed through APR. Magnesium nitrate's role in modifying catalyst properties may enable the efficient conversion of a broader spectrum of biomass-derived compounds, including those traditionally considered recalcitrant or problematic.
Furthermore, the use of magnesium nitrate aligns with the broader trend towards developing more environmentally friendly catalytic processes. As a relatively abundant and non-toxic compound, magnesium nitrate offers a sustainable alternative to some of the more environmentally problematic catalyst additives used in other reforming technologies.
The evolution of APR technology, including the integration of magnesium nitrate, is driven by the global push for renewable energy sources and the circular economy. As governments and industries worldwide seek to reduce dependence on fossil fuels and minimize carbon emissions, APR with magnesium nitrate presents a potential pathway to produce clean hydrogen and valuable chemicals from biomass waste streams.
In conclusion, the background and objectives of magnesium nitrate use in APR processes reflect a confluence of technological innovation, environmental considerations, and economic drivers. The ongoing research in this area aims to unlock the full potential of biomass resources, contributing to the development of more sustainable and efficient energy production methods.
The development of APR technology can be traced back to the early 2000s when researchers sought alternative methods for hydrogen production from renewable resources. Traditional steam reforming processes, while effective for fossil fuels, were less suitable for biomass-derived feedstocks due to their high oxygen content and tendency to form coke deposits on catalysts.
Magnesium nitrate's introduction into APR processes stems from the need to enhance catalyst stability and selectivity. Conventional APR catalysts, typically based on noble metals such as platinum or palladium, often suffer from deactivation due to sintering and carbon deposition. The incorporation of magnesium nitrate as a promoter or support modifier aims to address these challenges.
The primary objective of utilizing magnesium nitrate in APR is to improve the overall efficiency and sustainability of the process. Specifically, researchers aim to achieve higher hydrogen yields, increased selectivity towards desired products, and enhanced catalyst longevity. These improvements could potentially reduce the economic barriers to large-scale implementation of APR technology.
Another key goal is to expand the range of feedstocks that can be effectively processed through APR. Magnesium nitrate's role in modifying catalyst properties may enable the efficient conversion of a broader spectrum of biomass-derived compounds, including those traditionally considered recalcitrant or problematic.
Furthermore, the use of magnesium nitrate aligns with the broader trend towards developing more environmentally friendly catalytic processes. As a relatively abundant and non-toxic compound, magnesium nitrate offers a sustainable alternative to some of the more environmentally problematic catalyst additives used in other reforming technologies.
The evolution of APR technology, including the integration of magnesium nitrate, is driven by the global push for renewable energy sources and the circular economy. As governments and industries worldwide seek to reduce dependence on fossil fuels and minimize carbon emissions, APR with magnesium nitrate presents a potential pathway to produce clean hydrogen and valuable chemicals from biomass waste streams.
In conclusion, the background and objectives of magnesium nitrate use in APR processes reflect a confluence of technological innovation, environmental considerations, and economic drivers. The ongoing research in this area aims to unlock the full potential of biomass resources, contributing to the development of more sustainable and efficient energy production methods.
Market Analysis for APR Technologies
The Aqueous Phase Reforming (APR) technology market is experiencing significant growth and attracting increasing attention from various industries. This innovative process, which converts biomass-derived oxygenated hydrocarbons into hydrogen and alkanes, has shown promising potential in the renewable energy sector. The global market for APR technologies is projected to expand rapidly in the coming years, driven by the growing demand for sustainable energy solutions and the push towards a circular economy.
One of the key factors driving the APR market is the rising interest in hydrogen as a clean energy carrier. As countries and industries worldwide seek to reduce their carbon footprint, hydrogen produced through APR processes offers a viable alternative to fossil fuel-based energy sources. This has led to increased investment in research and development of APR technologies, particularly in regions with strong environmental policies and renewable energy targets.
The market for APR technologies is also benefiting from the abundance of biomass feedstocks. Agricultural residues, food processing waste, and other organic materials can be efficiently converted into valuable products through APR processes. This not only addresses waste management issues but also creates new revenue streams for industries in the bioeconomy sector.
In terms of application areas, the APR market is diversifying. While initially focused on hydrogen production, APR technologies are now finding applications in the production of value-added chemicals and biofuels. This expansion of potential end-uses is attracting interest from chemical manufacturers, fuel producers, and other industrial sectors, further driving market growth.
Geographically, North America and Europe are currently leading the APR technology market, with significant research activities and pilot projects underway. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing energy demands and government initiatives to promote clean energy technologies.
The competitive landscape of the APR market is characterized by a mix of established chemical and energy companies, as well as innovative startups. These players are actively engaged in developing and optimizing APR processes, with a focus on improving catalyst performance, enhancing process efficiency, and reducing overall costs.
Despite the promising outlook, the APR technology market faces certain challenges. The high capital costs associated with setting up APR facilities and the need for further technological advancements to improve process efficiency are potential barriers to widespread adoption. Additionally, the market's growth is influenced by factors such as government regulations, energy prices, and competition from other renewable energy technologies.
One of the key factors driving the APR market is the rising interest in hydrogen as a clean energy carrier. As countries and industries worldwide seek to reduce their carbon footprint, hydrogen produced through APR processes offers a viable alternative to fossil fuel-based energy sources. This has led to increased investment in research and development of APR technologies, particularly in regions with strong environmental policies and renewable energy targets.
The market for APR technologies is also benefiting from the abundance of biomass feedstocks. Agricultural residues, food processing waste, and other organic materials can be efficiently converted into valuable products through APR processes. This not only addresses waste management issues but also creates new revenue streams for industries in the bioeconomy sector.
In terms of application areas, the APR market is diversifying. While initially focused on hydrogen production, APR technologies are now finding applications in the production of value-added chemicals and biofuels. This expansion of potential end-uses is attracting interest from chemical manufacturers, fuel producers, and other industrial sectors, further driving market growth.
Geographically, North America and Europe are currently leading the APR technology market, with significant research activities and pilot projects underway. However, Asia-Pacific is emerging as a rapidly growing market, driven by increasing energy demands and government initiatives to promote clean energy technologies.
The competitive landscape of the APR market is characterized by a mix of established chemical and energy companies, as well as innovative startups. These players are actively engaged in developing and optimizing APR processes, with a focus on improving catalyst performance, enhancing process efficiency, and reducing overall costs.
Despite the promising outlook, the APR technology market faces certain challenges. The high capital costs associated with setting up APR facilities and the need for further technological advancements to improve process efficiency are potential barriers to widespread adoption. Additionally, the market's growth is influenced by factors such as government regulations, energy prices, and competition from other renewable energy technologies.
Current Challenges in Magnesium Nitrate APR
The aqueous phase reforming (APR) of magnesium nitrate presents several significant challenges that hinder its widespread adoption and efficiency. One of the primary obstacles is the high energy requirement for the process. The APR of magnesium nitrate typically requires elevated temperatures and pressures, which translates to substantial energy input and increased operational costs. This energy-intensive nature of the process poses a significant barrier to its economic viability and sustainability.
Another critical challenge is the catalyst deactivation and stability issues. The harsh reaction conditions in APR processes can lead to rapid degradation of catalysts, reducing their effectiveness and lifespan. This necessitates frequent catalyst replacement or regeneration, adding to the overall process costs and complexity. Moreover, finding catalysts that can withstand the corrosive environment created by magnesium nitrate while maintaining high activity and selectivity remains a significant research focus.
The formation of unwanted by-products during the APR of magnesium nitrate is another substantial challenge. Side reactions can lead to the production of undesired compounds, reducing the overall yield of the target products and complicating downstream separation processes. Controlling reaction selectivity to maximize the production of desired compounds while minimizing by-product formation is a complex task that requires precise control of reaction conditions and catalyst design.
Water management in the APR process presents another set of challenges. The presence of water as both a reactant and a product complicates the reaction kinetics and thermodynamics. Maintaining the optimal water concentration throughout the process is crucial for efficiency but can be difficult to achieve consistently. Additionally, the high water content can lead to corrosion issues in process equipment, necessitating the use of specialized materials and increasing maintenance costs.
The scale-up of magnesium nitrate APR processes from laboratory to industrial scale introduces further challenges. Issues such as heat and mass transfer limitations, which may not be significant at smaller scales, can become critical factors in larger reactors. Ensuring uniform temperature and concentration profiles throughout the reactor volume is essential for maintaining process efficiency and product quality but becomes increasingly difficult as the scale increases.
Lastly, the environmental impact of magnesium nitrate APR processes remains a concern. While APR is generally considered a more environmentally friendly approach compared to traditional reforming methods, the potential release of nitrogen-containing compounds and the energy-intensive nature of the process still pose environmental challenges. Developing strategies to mitigate these impacts, such as improving process efficiency and implementing effective waste treatment methods, is crucial for the sustainable implementation of magnesium nitrate APR technology.
Another critical challenge is the catalyst deactivation and stability issues. The harsh reaction conditions in APR processes can lead to rapid degradation of catalysts, reducing their effectiveness and lifespan. This necessitates frequent catalyst replacement or regeneration, adding to the overall process costs and complexity. Moreover, finding catalysts that can withstand the corrosive environment created by magnesium nitrate while maintaining high activity and selectivity remains a significant research focus.
The formation of unwanted by-products during the APR of magnesium nitrate is another substantial challenge. Side reactions can lead to the production of undesired compounds, reducing the overall yield of the target products and complicating downstream separation processes. Controlling reaction selectivity to maximize the production of desired compounds while minimizing by-product formation is a complex task that requires precise control of reaction conditions and catalyst design.
Water management in the APR process presents another set of challenges. The presence of water as both a reactant and a product complicates the reaction kinetics and thermodynamics. Maintaining the optimal water concentration throughout the process is crucial for efficiency but can be difficult to achieve consistently. Additionally, the high water content can lead to corrosion issues in process equipment, necessitating the use of specialized materials and increasing maintenance costs.
The scale-up of magnesium nitrate APR processes from laboratory to industrial scale introduces further challenges. Issues such as heat and mass transfer limitations, which may not be significant at smaller scales, can become critical factors in larger reactors. Ensuring uniform temperature and concentration profiles throughout the reactor volume is essential for maintaining process efficiency and product quality but becomes increasingly difficult as the scale increases.
Lastly, the environmental impact of magnesium nitrate APR processes remains a concern. While APR is generally considered a more environmentally friendly approach compared to traditional reforming methods, the potential release of nitrogen-containing compounds and the energy-intensive nature of the process still pose environmental challenges. Developing strategies to mitigate these impacts, such as improving process efficiency and implementing effective waste treatment methods, is crucial for the sustainable implementation of magnesium nitrate APR technology.
Existing Magnesium Nitrate APR Solutions
01 Use of magnesium nitrate in fertilizers
Magnesium nitrate is commonly used in fertilizer compositions due to its high solubility and ability to provide both magnesium and nitrogen to plants. It can be combined with other nutrients to create balanced fertilizer formulations for various crops and soil types.- Magnesium nitrate in fertilizer compositions: Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer compositions can be tailored for specific crops or soil conditions.
- Magnesium nitrate in energy storage applications: Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its heat absorption and release properties during melting and solidification. This makes it valuable for solar energy storage and temperature regulation in buildings.
- Magnesium nitrate in water treatment processes: Magnesium nitrate is employed in water treatment processes for various purposes. It can be used to remove contaminants, adjust water hardness, or as a coagulant aid in wastewater treatment. Its application helps improve water quality and meets environmental standards.
- Magnesium nitrate in flame retardant formulations: Magnesium nitrate is incorporated into flame retardant formulations for various materials. It acts as an effective fire suppressant by releasing non-flammable gases when exposed to high temperatures. This property makes it useful in improving the fire resistance of textiles, plastics, and other combustible materials.
- Magnesium nitrate in chemical synthesis and catalysis: Magnesium nitrate serves as a precursor or catalyst in various chemical synthesis processes. It is used in the production of other magnesium compounds, as a catalyst support, or as a reagent in organic synthesis reactions. Its applications span across pharmaceutical, petrochemical, and fine chemical industries.
02 Application in energy storage systems
Magnesium nitrate is utilized in thermal energy storage systems, particularly in phase change materials (PCMs). Its properties allow for efficient heat absorption and release, making it suitable for applications in solar energy storage and building temperature regulation.Expand Specific Solutions03 Use in flame retardant compositions
Magnesium nitrate is incorporated into flame retardant formulations for various materials, including textiles, plastics, and wood products. It enhances fire resistance by releasing non-flammable gases and forming a protective char layer when exposed to high temperatures.Expand Specific Solutions04 Application in wastewater treatment
Magnesium nitrate is employed in wastewater treatment processes, particularly for the removal of phosphates and heavy metals. It can be used in precipitation reactions to form insoluble compounds, facilitating the separation of contaminants from water.Expand Specific Solutions05 Use in chemical synthesis and catalysis
Magnesium nitrate serves as a precursor in the synthesis of various magnesium-containing compounds and materials. It is also used as a catalyst or catalyst support in certain chemical reactions, particularly in the production of fine chemicals and pharmaceuticals.Expand Specific Solutions
Key Players in APR Industry
The use of magnesium nitrate in aqueous phase reforming processes is an emerging field with significant potential for growth. The market is in its early stages, characterized by ongoing research and development efforts. Key players like Korea Research Institute of Chemical Technology, Virent, Inc., and Fraunhofer-Gesellschaft are driving innovation in this area. The technology is still evolving, with varying levels of maturity across different applications. Companies such as Indian Oil Corp. Ltd. and Chemetall GmbH are exploring industrial applications, while academic institutions like Tianjin University and the Institute of Chemical Technology are contributing to fundamental research. As the technology matures, we can expect increased market adoption and expansion of commercial applications in the coming years.
Korea Research Institute of Chemical Technology
Technical Solution: The Korea Research Institute of Chemical Technology (KRICT) has developed an innovative approach to aqueous phase reforming (APR) using magnesium nitrate as a catalyst promoter. Their method involves incorporating magnesium nitrate into traditional noble metal catalysts, such as platinum or palladium, supported on carbon or alumina. This modification enhances the catalytic activity and selectivity towards hydrogen production from biomass-derived oxygenates[1]. The process operates at relatively low temperatures (200-250°C) and moderate pressures (15-50 bar), which is advantageous for energy efficiency[2]. KRICT's research has shown that the addition of magnesium nitrate can increase hydrogen yield by up to 30% compared to unpromoted catalysts, while also improving catalyst stability over extended reaction times[3].
Strengths: Enhanced catalytic activity, improved hydrogen yield, and better catalyst stability. The use of magnesium nitrate is cost-effective and environmentally friendly. Weaknesses: The process may still require noble metal catalysts, which can be expensive, and the long-term effects of magnesium nitrate on catalyst life cycle need further investigation.
Virent, Inc.
Technical Solution: Virent, Inc. has pioneered the BioForming® process, which utilizes aqueous phase reforming (APR) technology to convert plant-based sugars into a range of hydrocarbon products. While their primary focus is not specifically on magnesium nitrate, they have explored various catalyst promoters, including alkaline earth metal salts like magnesium nitrate, to enhance their APR process[4]. Virent's approach involves a multi-step process where biomass-derived oxygenates are first converted to intermediate compounds through APR, followed by catalytic condensation to produce drop-in fuels and chemicals[5]. The company has reported that the use of promoters such as magnesium nitrate can improve catalyst performance by increasing active site density and modifying the electronic properties of the catalyst surface[6]. This results in higher conversion rates and improved selectivity towards desired products.
Strengths: Established commercial-scale technology, versatile product range including fuels and chemicals, and continuous process improvement. Weaknesses: The specific impact of magnesium nitrate on their process may be proprietary, limiting public information, and the technology may be more focused on fuel production rather than pure hydrogen generation.
Core Innovations in Magnesium Nitrate APR
Systems and methods for processing ammonia
PatentWO2024077179A1
Innovation
- A method involving heating a reformer to a target temperature range, directing ammonia to produce a reformate stream comprising hydrogen and nitrogen, and subsequently using this stream to heat another reformer, while processing additional ammonia to optimize ammonia conversion and reduce storage complexities.
Production of high purity nickel and cobalt compounds
PatentWO2022236381A1
Innovation
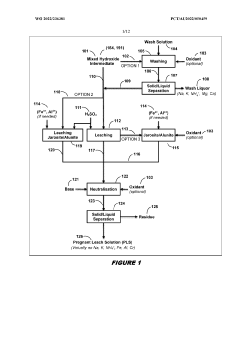
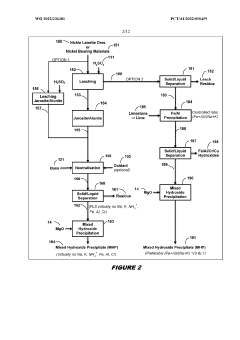
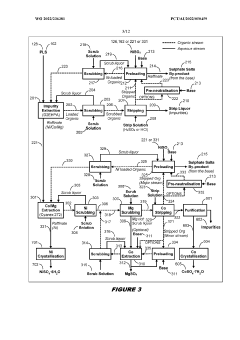
- A two-phase process involving the treatment of nickel laterite ores to create a feed solution free of alkali metals and monovalent cations, followed by novel solvent extraction methods that separate impurities from nickel and cobalt without the need for expensive nickel extraction, using organophosphoric and organophosphinic acid extractants to produce high purity sulphates.
Environmental Impact of APR Processes
The environmental impact of Aqueous Phase Reforming (APR) processes, particularly those involving magnesium nitrate, is a critical consideration in the development and implementation of this technology. APR processes offer several environmental advantages over traditional reforming methods, primarily due to their ability to operate at lower temperatures and pressures, reducing overall energy consumption and greenhouse gas emissions.
One of the key environmental benefits of APR processes is their potential to produce hydrogen from renewable biomass feedstocks. This approach can significantly reduce the carbon footprint associated with hydrogen production compared to conventional methods that rely on fossil fuels. The use of magnesium nitrate as a catalyst or additive in APR processes may further enhance this environmental benefit by improving reaction efficiency and selectivity.
Water consumption is another important environmental factor to consider in APR processes. While these processes inherently require water as a reaction medium, the use of magnesium nitrate may influence water usage and treatment requirements. Proper water management strategies, including recycling and purification techniques, are essential to minimize the environmental impact of water consumption in APR processes.
The potential for wastewater generation and treatment is a significant environmental concern in APR processes. The presence of magnesium nitrate in the reaction system may affect the composition of wastewater streams, potentially requiring specialized treatment methods to remove dissolved salts and other contaminants before discharge. Implementing effective wastewater treatment technologies is crucial to mitigate any negative environmental impacts associated with APR processes.
Air emissions from APR processes are generally lower compared to high-temperature reforming methods. However, the use of magnesium nitrate may influence the composition of gaseous byproducts. Careful monitoring and control of air emissions, particularly nitrogen oxides, is necessary to ensure compliance with environmental regulations and minimize atmospheric pollution.
The life cycle impact of magnesium nitrate production and use in APR processes should also be considered. This includes the environmental effects of mining and processing magnesium and nitrogen compounds, as well as the energy requirements for synthesizing magnesium nitrate. A comprehensive life cycle assessment can help identify opportunities for improving the overall environmental performance of APR processes utilizing magnesium nitrate.
Land use and biodiversity impacts of APR processes are generally minimal compared to other energy production methods. However, the scaling up of these processes and the potential increased demand for biomass feedstocks may have indirect land use implications. Sustainable sourcing of biomass and efficient use of land resources are important considerations in minimizing the environmental footprint of APR technologies.
One of the key environmental benefits of APR processes is their potential to produce hydrogen from renewable biomass feedstocks. This approach can significantly reduce the carbon footprint associated with hydrogen production compared to conventional methods that rely on fossil fuels. The use of magnesium nitrate as a catalyst or additive in APR processes may further enhance this environmental benefit by improving reaction efficiency and selectivity.
Water consumption is another important environmental factor to consider in APR processes. While these processes inherently require water as a reaction medium, the use of magnesium nitrate may influence water usage and treatment requirements. Proper water management strategies, including recycling and purification techniques, are essential to minimize the environmental impact of water consumption in APR processes.
The potential for wastewater generation and treatment is a significant environmental concern in APR processes. The presence of magnesium nitrate in the reaction system may affect the composition of wastewater streams, potentially requiring specialized treatment methods to remove dissolved salts and other contaminants before discharge. Implementing effective wastewater treatment technologies is crucial to mitigate any negative environmental impacts associated with APR processes.
Air emissions from APR processes are generally lower compared to high-temperature reforming methods. However, the use of magnesium nitrate may influence the composition of gaseous byproducts. Careful monitoring and control of air emissions, particularly nitrogen oxides, is necessary to ensure compliance with environmental regulations and minimize atmospheric pollution.
The life cycle impact of magnesium nitrate production and use in APR processes should also be considered. This includes the environmental effects of mining and processing magnesium and nitrogen compounds, as well as the energy requirements for synthesizing magnesium nitrate. A comprehensive life cycle assessment can help identify opportunities for improving the overall environmental performance of APR processes utilizing magnesium nitrate.
Land use and biodiversity impacts of APR processes are generally minimal compared to other energy production methods. However, the scaling up of these processes and the potential increased demand for biomass feedstocks may have indirect land use implications. Sustainable sourcing of biomass and efficient use of land resources are important considerations in minimizing the environmental footprint of APR technologies.
Economic Feasibility of Magnesium Nitrate APR
The economic feasibility of using magnesium nitrate in Aqueous Phase Reforming (APR) processes is a critical consideration for industrial implementation. Initial cost-benefit analyses indicate potential advantages in terms of catalyst efficiency and process optimization. Magnesium nitrate, when used as a promoter or additive in APR catalysts, has shown promising results in enhancing hydrogen production and improving the selectivity of desired products.
From a raw material perspective, magnesium nitrate is relatively inexpensive and widely available, which contributes positively to the economic viability of its use in APR. The cost of incorporating magnesium nitrate into existing APR processes is generally offset by the improved catalytic performance and increased product yields. However, it is essential to consider the long-term economic implications, including potential savings in energy consumption and reduced catalyst degradation rates.
One of the key economic benefits of using magnesium nitrate in APR is the potential for process intensification. By enhancing catalyst activity and stability, it may be possible to achieve higher throughput or operate at lower temperatures, leading to reduced energy costs and improved overall process economics. Additionally, the extended catalyst lifetime resulting from magnesium nitrate incorporation could significantly reduce the frequency of catalyst replacement, further contributing to cost savings.
Market analysis suggests that the demand for APR-derived products, particularly hydrogen and value-added chemicals, is expected to grow in the coming years. This trend could further improve the economic attractiveness of magnesium nitrate-enhanced APR processes. However, it is crucial to consider potential fluctuations in feedstock prices and product market values when assessing long-term economic feasibility.
While the initial capital investment for retrofitting existing APR units or designing new processes to incorporate magnesium nitrate may be substantial, preliminary calculations indicate favorable payback periods. The exact return on investment will vary depending on factors such as plant capacity, operating conditions, and local energy costs. It is recommended that detailed techno-economic assessments be conducted for specific applications to accurately determine the economic viability.
Environmental regulations and potential carbon pricing mechanisms should also be factored into the economic analysis. The improved efficiency and potentially lower carbon footprint of magnesium nitrate-enhanced APR processes could translate into economic benefits in regions with strict emissions controls or carbon taxation systems. This aspect may become increasingly important as global efforts to combat climate change intensify.
In conclusion, the economic feasibility of using magnesium nitrate in APR processes appears promising, with potential benefits outweighing the costs in many scenarios. However, site-specific evaluations and pilot-scale demonstrations are necessary to validate these economic projections and optimize process parameters for maximum financial return.
From a raw material perspective, magnesium nitrate is relatively inexpensive and widely available, which contributes positively to the economic viability of its use in APR. The cost of incorporating magnesium nitrate into existing APR processes is generally offset by the improved catalytic performance and increased product yields. However, it is essential to consider the long-term economic implications, including potential savings in energy consumption and reduced catalyst degradation rates.
One of the key economic benefits of using magnesium nitrate in APR is the potential for process intensification. By enhancing catalyst activity and stability, it may be possible to achieve higher throughput or operate at lower temperatures, leading to reduced energy costs and improved overall process economics. Additionally, the extended catalyst lifetime resulting from magnesium nitrate incorporation could significantly reduce the frequency of catalyst replacement, further contributing to cost savings.
Market analysis suggests that the demand for APR-derived products, particularly hydrogen and value-added chemicals, is expected to grow in the coming years. This trend could further improve the economic attractiveness of magnesium nitrate-enhanced APR processes. However, it is crucial to consider potential fluctuations in feedstock prices and product market values when assessing long-term economic feasibility.
While the initial capital investment for retrofitting existing APR units or designing new processes to incorporate magnesium nitrate may be substantial, preliminary calculations indicate favorable payback periods. The exact return on investment will vary depending on factors such as plant capacity, operating conditions, and local energy costs. It is recommended that detailed techno-economic assessments be conducted for specific applications to accurately determine the economic viability.
Environmental regulations and potential carbon pricing mechanisms should also be factored into the economic analysis. The improved efficiency and potentially lower carbon footprint of magnesium nitrate-enhanced APR processes could translate into economic benefits in regions with strict emissions controls or carbon taxation systems. This aspect may become increasingly important as global efforts to combat climate change intensify.
In conclusion, the economic feasibility of using magnesium nitrate in APR processes appears promising, with potential benefits outweighing the costs in many scenarios. However, site-specific evaluations and pilot-scale demonstrations are necessary to validate these economic projections and optimize process parameters for maximum financial return.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!