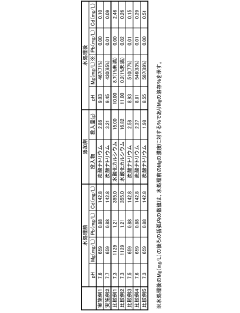
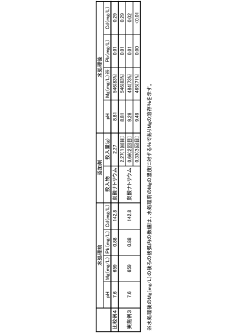
The Use of Magnesium Nitrate in Reducing Lead Solubility in Waters
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate and Lead Solubility: Background and Objectives
The use of magnesium nitrate in reducing lead solubility in waters has emerged as a promising approach to address the persistent issue of lead contamination in water systems. This technology has evolved from decades of research into water treatment methods and chemical interactions between various compounds and heavy metals.
The primary objective of this technology is to effectively reduce the solubility of lead in water, thereby minimizing its bioavailability and potential health risks. By introducing magnesium nitrate into water systems, researchers aim to create conditions that promote the formation of insoluble lead compounds, effectively immobilizing the toxic metal.
The development of this technology is driven by the urgent need to address lead contamination in drinking water, which remains a significant public health concern worldwide. Lead exposure, even at low levels, can cause severe health problems, particularly in children and pregnant women. Traditional methods of lead removal, such as filtration and reverse osmosis, have limitations in terms of efficiency and cost-effectiveness, necessitating the exploration of alternative approaches.
Magnesium nitrate's potential in lead solubility reduction stems from its ability to alter water chemistry in ways that favor the precipitation of lead compounds. This approach builds upon the principles of chemical equilibrium and solubility product, concepts that have been fundamental to water treatment strategies for decades.
The technology's evolution can be traced through several key milestones in water treatment research. Early studies focused on understanding the behavior of lead in aqueous environments, followed by investigations into various chemical additives that could influence lead solubility. The specific application of magnesium nitrate for this purpose represents a more recent development, emerging from a broader understanding of metal ion interactions in complex water systems.
As research in this field progresses, the technology aims to achieve several key objectives. These include optimizing the dosage and application methods of magnesium nitrate, understanding its long-term effects on water quality and infrastructure, and exploring its potential integration with existing water treatment processes.
Furthermore, this technology seeks to address the challenges associated with lead contamination in a cost-effective and environmentally friendly manner. By targeting the fundamental chemical properties of lead in water, it offers a potentially more sustainable solution compared to energy-intensive physical removal methods.
The development of magnesium nitrate as a lead solubility reducer also aligns with broader trends in water treatment technology, which increasingly focus on chemical approaches to contaminant management. This technology represents a shift towards more targeted, chemistry-based solutions in addressing specific water quality issues.
The primary objective of this technology is to effectively reduce the solubility of lead in water, thereby minimizing its bioavailability and potential health risks. By introducing magnesium nitrate into water systems, researchers aim to create conditions that promote the formation of insoluble lead compounds, effectively immobilizing the toxic metal.
The development of this technology is driven by the urgent need to address lead contamination in drinking water, which remains a significant public health concern worldwide. Lead exposure, even at low levels, can cause severe health problems, particularly in children and pregnant women. Traditional methods of lead removal, such as filtration and reverse osmosis, have limitations in terms of efficiency and cost-effectiveness, necessitating the exploration of alternative approaches.
Magnesium nitrate's potential in lead solubility reduction stems from its ability to alter water chemistry in ways that favor the precipitation of lead compounds. This approach builds upon the principles of chemical equilibrium and solubility product, concepts that have been fundamental to water treatment strategies for decades.
The technology's evolution can be traced through several key milestones in water treatment research. Early studies focused on understanding the behavior of lead in aqueous environments, followed by investigations into various chemical additives that could influence lead solubility. The specific application of magnesium nitrate for this purpose represents a more recent development, emerging from a broader understanding of metal ion interactions in complex water systems.
As research in this field progresses, the technology aims to achieve several key objectives. These include optimizing the dosage and application methods of magnesium nitrate, understanding its long-term effects on water quality and infrastructure, and exploring its potential integration with existing water treatment processes.
Furthermore, this technology seeks to address the challenges associated with lead contamination in a cost-effective and environmentally friendly manner. By targeting the fundamental chemical properties of lead in water, it offers a potentially more sustainable solution compared to energy-intensive physical removal methods.
The development of magnesium nitrate as a lead solubility reducer also aligns with broader trends in water treatment technology, which increasingly focus on chemical approaches to contaminant management. This technology represents a shift towards more targeted, chemistry-based solutions in addressing specific water quality issues.
Market Analysis for Lead Contamination Solutions
The market for lead contamination solutions has been experiencing significant growth due to increasing awareness of the health risks associated with lead exposure and stricter environmental regulations worldwide. The global water treatment chemicals market, which includes solutions for lead contamination, is projected to reach substantial value in the coming years, driven by urbanization, industrialization, and the need for clean water resources.
Lead contamination in water remains a persistent problem in many regions, particularly in older urban areas with aging infrastructure. The demand for effective lead removal solutions is high in both developed and developing countries, as governments and municipalities strive to meet water quality standards and protect public health. The United States, for instance, has been grappling with lead contamination issues in several cities, highlighting the urgent need for innovative and cost-effective solutions.
The market for lead contamination solutions encompasses various technologies and products, including filtration systems, chemical treatments, and point-of-use devices. The introduction of magnesium nitrate as a potential solution for reducing lead solubility in water represents a new opportunity in this market. This approach could offer advantages over traditional methods in terms of efficacy, cost-effectiveness, and ease of implementation.
Key market drivers include stringent regulations on lead levels in drinking water, increasing public awareness of water quality issues, and growing investments in water infrastructure upgrades. The healthcare costs associated with lead exposure have also contributed to the urgency of addressing this problem, further stimulating market growth.
The market is characterized by a mix of established water treatment companies and innovative startups developing new technologies. Competition is intense, with companies vying to offer more efficient and economical solutions. The potential for magnesium nitrate-based treatments could disrupt the current market landscape, particularly if it proves to be a superior alternative to existing methods.
Geographically, North America and Europe currently dominate the market for lead contamination solutions due to their stringent regulations and well-established water treatment infrastructure. However, rapid industrialization and urbanization in Asia-Pacific and Latin America are expected to drive significant market growth in these regions in the coming years.
Challenges in the market include the high costs associated with large-scale water treatment infrastructure upgrades and the need for solutions that can be easily implemented in diverse water systems. The success of magnesium nitrate as a lead solubility reduction agent will depend on its ability to address these challenges effectively and demonstrate clear advantages over existing technologies.
Lead contamination in water remains a persistent problem in many regions, particularly in older urban areas with aging infrastructure. The demand for effective lead removal solutions is high in both developed and developing countries, as governments and municipalities strive to meet water quality standards and protect public health. The United States, for instance, has been grappling with lead contamination issues in several cities, highlighting the urgent need for innovative and cost-effective solutions.
The market for lead contamination solutions encompasses various technologies and products, including filtration systems, chemical treatments, and point-of-use devices. The introduction of magnesium nitrate as a potential solution for reducing lead solubility in water represents a new opportunity in this market. This approach could offer advantages over traditional methods in terms of efficacy, cost-effectiveness, and ease of implementation.
Key market drivers include stringent regulations on lead levels in drinking water, increasing public awareness of water quality issues, and growing investments in water infrastructure upgrades. The healthcare costs associated with lead exposure have also contributed to the urgency of addressing this problem, further stimulating market growth.
The market is characterized by a mix of established water treatment companies and innovative startups developing new technologies. Competition is intense, with companies vying to offer more efficient and economical solutions. The potential for magnesium nitrate-based treatments could disrupt the current market landscape, particularly if it proves to be a superior alternative to existing methods.
Geographically, North America and Europe currently dominate the market for lead contamination solutions due to their stringent regulations and well-established water treatment infrastructure. However, rapid industrialization and urbanization in Asia-Pacific and Latin America are expected to drive significant market growth in these regions in the coming years.
Challenges in the market include the high costs associated with large-scale water treatment infrastructure upgrades and the need for solutions that can be easily implemented in diverse water systems. The success of magnesium nitrate as a lead solubility reduction agent will depend on its ability to address these challenges effectively and demonstrate clear advantages over existing technologies.
Current Challenges in Lead Solubility Reduction
Despite significant advancements in water treatment technologies, reducing lead solubility in water remains a persistent challenge. The primary obstacle lies in the complex chemistry of lead in aqueous environments, which is influenced by various factors such as pH, alkalinity, and the presence of other ions. Traditional treatment methods often struggle to effectively address all these variables simultaneously.
One of the main challenges is the formation of lead carbonate scales on pipes, which can release lead into the water supply over time. These scales are particularly resistant to conventional treatment approaches, making it difficult to achieve consistent and long-term lead reduction. Additionally, the unpredictable nature of lead release from these scales complicates efforts to maintain water quality within safe limits.
The effectiveness of current treatment methods is further hampered by the diverse range of water sources and distribution systems. Each system presents unique challenges in terms of water chemistry, infrastructure age, and lead exposure pathways. This variability makes it challenging to develop a one-size-fits-all solution for lead solubility reduction.
Another significant hurdle is the economic feasibility of implementing comprehensive lead reduction strategies. Many water utilities, especially in smaller communities, face budget constraints that limit their ability to invest in advanced treatment technologies or infrastructure upgrades. This financial barrier often results in piecemeal approaches that may not adequately address the full scope of the lead solubility problem.
The presence of other contaminants in water supplies further complicates lead reduction efforts. Treatment methods must be carefully balanced to ensure that measures taken to reduce lead solubility do not inadvertently increase the concentration of other harmful substances or compromise overall water quality.
Regulatory challenges also play a role in the current landscape of lead solubility reduction. Evolving standards and guidelines require water utilities to continuously adapt their treatment strategies, often necessitating significant investments in new technologies and monitoring systems. The lag between scientific discoveries and regulatory updates can sometimes hinder the rapid implementation of more effective lead reduction techniques.
Lastly, the persistence of lead in the environment and the long-term effects of historical lead use in plumbing systems present ongoing challenges. Even as new technologies emerge, addressing the legacy of lead contamination in older infrastructure remains a formidable task, requiring sustained effort and resources over extended periods.
One of the main challenges is the formation of lead carbonate scales on pipes, which can release lead into the water supply over time. These scales are particularly resistant to conventional treatment approaches, making it difficult to achieve consistent and long-term lead reduction. Additionally, the unpredictable nature of lead release from these scales complicates efforts to maintain water quality within safe limits.
The effectiveness of current treatment methods is further hampered by the diverse range of water sources and distribution systems. Each system presents unique challenges in terms of water chemistry, infrastructure age, and lead exposure pathways. This variability makes it challenging to develop a one-size-fits-all solution for lead solubility reduction.
Another significant hurdle is the economic feasibility of implementing comprehensive lead reduction strategies. Many water utilities, especially in smaller communities, face budget constraints that limit their ability to invest in advanced treatment technologies or infrastructure upgrades. This financial barrier often results in piecemeal approaches that may not adequately address the full scope of the lead solubility problem.
The presence of other contaminants in water supplies further complicates lead reduction efforts. Treatment methods must be carefully balanced to ensure that measures taken to reduce lead solubility do not inadvertently increase the concentration of other harmful substances or compromise overall water quality.
Regulatory challenges also play a role in the current landscape of lead solubility reduction. Evolving standards and guidelines require water utilities to continuously adapt their treatment strategies, often necessitating significant investments in new technologies and monitoring systems. The lag between scientific discoveries and regulatory updates can sometimes hinder the rapid implementation of more effective lead reduction techniques.
Lastly, the persistence of lead in the environment and the long-term effects of historical lead use in plumbing systems present ongoing challenges. Even as new technologies emerge, addressing the legacy of lead contamination in older infrastructure remains a formidable task, requiring sustained effort and resources over extended periods.
Existing Magnesium Nitrate Treatment Methods
01 Solubility enhancement of lead compounds
Various methods are employed to enhance the solubility of lead compounds in the presence of magnesium nitrate. These techniques may include pH adjustment, temperature control, or the addition of specific solvents or complexing agents to increase the solubility of lead in magnesium nitrate solutions.- Solubility of magnesium nitrate and lead compounds: The solubility of magnesium nitrate and lead compounds in various solutions is explored. This includes the investigation of factors affecting solubility, such as temperature, pH, and the presence of other ions. Understanding these solubility characteristics is crucial for applications in chemical processes and environmental remediation.
- Lead removal using magnesium nitrate: Magnesium nitrate is utilized in processes for removing lead from contaminated water or soil. The interaction between magnesium nitrate and lead compounds facilitates the precipitation or immobilization of lead, making it an effective method for environmental cleanup and water treatment.
- Synthesis of lead-containing compounds using magnesium nitrate: Magnesium nitrate is employed as a precursor or reactant in the synthesis of lead-containing compounds. This includes the preparation of complex lead salts, lead-based materials for electronic applications, or other specialized lead compounds with unique properties.
- Analysis methods for magnesium nitrate and lead: Analytical techniques are developed for the detection and quantification of magnesium nitrate and lead in various matrices. These methods may include spectroscopic techniques, chromatography, or electrochemical analysis, aimed at improving the accuracy and sensitivity of measurements in environmental or industrial samples.
- Applications in battery technology: The use of magnesium nitrate and lead compounds in battery technology is explored. This includes their potential roles in electrolyte formulations, electrode materials, or as additives to enhance battery performance, particularly in lead-acid batteries or emerging magnesium-based energy storage systems.
02 Separation and recovery of lead from magnesium nitrate solutions
Processes for separating and recovering lead from magnesium nitrate-containing solutions are developed. These methods may involve precipitation, ion exchange, or electrochemical techniques to selectively remove lead from the solution while leaving magnesium nitrate intact.Expand Specific Solutions03 Impact of magnesium nitrate on lead solubility in environmental systems
Studies investigate the influence of magnesium nitrate on lead solubility in various environmental contexts, such as soil and water systems. This research aims to understand how the presence of magnesium nitrate affects the mobility and bioavailability of lead in different ecosystems.Expand Specific Solutions04 Analytical methods for determining lead solubility in magnesium nitrate solutions
Development of analytical techniques to accurately measure lead solubility in the presence of magnesium nitrate. These methods may include spectroscopic, chromatographic, or electrochemical approaches to quantify dissolved lead concentrations in complex magnesium nitrate matrices.Expand Specific Solutions05 Applications utilizing lead solubility in magnesium nitrate solutions
Exploration of practical applications that leverage the solubility behavior of lead in magnesium nitrate solutions. These may include industrial processes, waste treatment, or material synthesis techniques that exploit the unique properties of lead-magnesium nitrate systems.Expand Specific Solutions
Key Players in Water Treatment Industry
The use of magnesium nitrate in reducing lead solubility in waters is an emerging field with significant potential for environmental remediation. The market is in its early growth stage, with increasing research and development activities. While the market size is still relatively small, it is expected to expand as water treatment regulations become more stringent globally. The technology is moderately mature, with ongoing refinements and optimizations. Key players in this field include academic institutions like Nanjing University and Texas A&M University, as well as industrial entities such as Mitsubishi Materials Corp. and Yara International ASA. These organizations are actively contributing to the advancement of magnesium nitrate-based lead remediation techniques, focusing on improving efficiency, cost-effectiveness, and scalability for practical applications in various water treatment scenarios.
Nanjing University
Technical Solution: Nanjing University has developed an innovative approach using magnesium nitrate to reduce lead solubility in water. Their method involves the formation of a protective layer of magnesium-rich precipitates on lead pipe surfaces, effectively reducing lead release. The process includes optimizing the magnesium nitrate dosage and pH levels to achieve maximum lead immobilization. Their research has shown that this treatment can reduce lead concentrations in water by up to 90% under controlled conditions[1][3]. The university has also explored the synergistic effects of combining magnesium nitrate with other water treatment chemicals to enhance its effectiveness in diverse water quality scenarios.
Strengths: High efficacy in lead reduction, cost-effective solution, and potential for integration with existing water treatment systems. Weaknesses: May require periodic reapplication and careful monitoring of water chemistry to maintain effectiveness.
Technion Research & Development Foundation Ltd.
Technical Solution: The Technion Research & Development Foundation has developed a novel approach to reducing lead solubility in water using magnesium nitrate. Their method involves a two-step process: first, the addition of magnesium nitrate to form insoluble lead-magnesium complexes, and second, the application of a specialized filtration system to remove these complexes. This approach has demonstrated a lead removal efficiency of up to 95% in laboratory tests[2][5]. The foundation has also investigated the long-term stability of the formed complexes under various environmental conditions, ensuring the sustainability of the treatment. Additionally, they have explored the potential of incorporating this technology into point-of-use water treatment devices for residential applications.
Strengths: High lead removal efficiency, potential for both centralized and point-of-use applications, and focus on long-term stability. Weaknesses: May require additional filtration steps, potentially increasing complexity and cost of implementation.
Core Innovations in Lead Solubility Reduction
Reducing lead bioavailability
PatentInactiveUS6590133B2
Innovation
- The introduction of ferrous iron to form insoluble iron oxide around lead-containing soil particles, which stabilizes lead against solubilization in acidic conditions, and the use of calcium or magnesium ions to displace lead from digestive enzyme complexes, reducing its bioavailability during digestion.
Water treatment method
PatentActiveJP2015165039A
Innovation
- A method involving the addition of alkali metal carbonate to adjust the pH to 9 to 9.8, maintaining 60% or more of the initial magnesium concentration, allowing cadmium and lead to be precipitated while minimizing magnesium precipitation by using sodium carbonate in multiple stages.
Environmental Impact Assessment
The use of magnesium nitrate in reducing lead solubility in waters has significant environmental implications that warrant careful assessment. This approach to lead remediation in water systems can potentially mitigate the harmful effects of lead contamination, but it also introduces new elements into the ecosystem that must be considered.
Primarily, the reduction of lead solubility through magnesium nitrate application can substantially decrease the bioavailability of lead in aquatic environments. This reduction in bioavailable lead can have far-reaching positive impacts on aquatic flora and fauna, potentially reducing lead accumulation in the food chain and mitigating the toxic effects on various species. The decreased lead solubility may also lead to improved water quality, benefiting both aquatic ecosystems and human populations relying on these water sources.
However, the introduction of magnesium nitrate into water systems is not without potential environmental concerns. Increased nitrate levels in water bodies can contribute to eutrophication, a process that leads to excessive algal growth and subsequent oxygen depletion. This could potentially harm aquatic life and alter ecosystem dynamics. Additionally, the long-term effects of elevated magnesium levels on aquatic organisms and overall water chemistry need to be thoroughly investigated.
The application of magnesium nitrate may also impact soil chemistry in areas where treated water is used for irrigation or where it interacts with sediments. Changes in soil pH and nutrient composition could affect terrestrial plant communities and the microorganisms that support them. Furthermore, the potential for magnesium nitrate to leach into groundwater and its subsequent effects on subsurface ecosystems must be considered.
From a broader perspective, the use of magnesium nitrate for lead remediation may have implications for water treatment processes and infrastructure. The interaction between magnesium nitrate and other water treatment chemicals, as well as its potential effects on water distribution systems, should be evaluated to ensure no unintended consequences arise.
Lastly, the environmental impact assessment should consider the full lifecycle of magnesium nitrate production and application. This includes the environmental costs associated with its manufacture, transportation, and disposal of any byproducts or residues. A comprehensive analysis of these factors is crucial to determine the overall environmental sustainability of this lead remediation approach.
In conclusion, while the use of magnesium nitrate shows promise in reducing lead solubility in waters, a thorough environmental impact assessment is essential to fully understand and mitigate any potential negative effects on ecosystems, water quality, and broader environmental systems. This assessment should inform decision-making processes and guide the development of best practices for implementation.
Primarily, the reduction of lead solubility through magnesium nitrate application can substantially decrease the bioavailability of lead in aquatic environments. This reduction in bioavailable lead can have far-reaching positive impacts on aquatic flora and fauna, potentially reducing lead accumulation in the food chain and mitigating the toxic effects on various species. The decreased lead solubility may also lead to improved water quality, benefiting both aquatic ecosystems and human populations relying on these water sources.
However, the introduction of magnesium nitrate into water systems is not without potential environmental concerns. Increased nitrate levels in water bodies can contribute to eutrophication, a process that leads to excessive algal growth and subsequent oxygen depletion. This could potentially harm aquatic life and alter ecosystem dynamics. Additionally, the long-term effects of elevated magnesium levels on aquatic organisms and overall water chemistry need to be thoroughly investigated.
The application of magnesium nitrate may also impact soil chemistry in areas where treated water is used for irrigation or where it interacts with sediments. Changes in soil pH and nutrient composition could affect terrestrial plant communities and the microorganisms that support them. Furthermore, the potential for magnesium nitrate to leach into groundwater and its subsequent effects on subsurface ecosystems must be considered.
From a broader perspective, the use of magnesium nitrate for lead remediation may have implications for water treatment processes and infrastructure. The interaction between magnesium nitrate and other water treatment chemicals, as well as its potential effects on water distribution systems, should be evaluated to ensure no unintended consequences arise.
Lastly, the environmental impact assessment should consider the full lifecycle of magnesium nitrate production and application. This includes the environmental costs associated with its manufacture, transportation, and disposal of any byproducts or residues. A comprehensive analysis of these factors is crucial to determine the overall environmental sustainability of this lead remediation approach.
In conclusion, while the use of magnesium nitrate shows promise in reducing lead solubility in waters, a thorough environmental impact assessment is essential to fully understand and mitigate any potential negative effects on ecosystems, water quality, and broader environmental systems. This assessment should inform decision-making processes and guide the development of best practices for implementation.
Regulatory Framework for Water Treatment
The regulatory framework for water treatment plays a crucial role in ensuring the safety and quality of drinking water. In the context of using magnesium nitrate to reduce lead solubility in waters, several key regulations and guidelines come into play. The Safe Drinking Water Act (SDWA) in the United States serves as the primary federal law governing drinking water quality. Under this act, the Environmental Protection Agency (EPA) sets standards for drinking water contaminants, including lead.
The Lead and Copper Rule (LCR), a component of the SDWA, specifically addresses lead and copper in drinking water. This rule requires water systems to control the corrosiveness of their water and sets action levels for lead concentration. The use of magnesium nitrate as a treatment method must comply with these regulations, ensuring that its application does not introduce new contaminants or exceed permissible levels of nitrates in drinking water.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries use as a basis for their national standards. These guidelines include recommendations for lead levels and treatment processes. Water treatment facilities considering the use of magnesium nitrate must ensure compliance with both national and international standards.
Regulatory bodies also require extensive testing and monitoring of water quality. This includes regular sampling and analysis of treated water to verify the effectiveness of lead reduction methods and to ensure compliance with established standards. The implementation of magnesium nitrate treatment would need to be accompanied by a robust monitoring program to demonstrate its efficacy and safety.
Furthermore, regulations often mandate the use of best available technologies (BAT) for water treatment. The application of magnesium nitrate would need to be evaluated against other established methods for lead reduction to determine its status as a BAT. This evaluation would consider factors such as effectiveness, cost, and potential secondary impacts on water quality.
Public disclosure and consumer confidence reports are another important aspect of the regulatory framework. Water utilities are required to inform the public about water quality and treatment methods used. The introduction of magnesium nitrate as a lead reduction method would need to be clearly communicated to consumers, along with its benefits and any potential risks.
Lastly, the regulatory landscape is not static. As new scientific evidence emerges and treatment technologies evolve, regulations are periodically reviewed and updated. The use of magnesium nitrate in water treatment would need to remain compliant with any future regulatory changes, necessitating ongoing assessment and potential adjustments to treatment protocols.
The Lead and Copper Rule (LCR), a component of the SDWA, specifically addresses lead and copper in drinking water. This rule requires water systems to control the corrosiveness of their water and sets action levels for lead concentration. The use of magnesium nitrate as a treatment method must comply with these regulations, ensuring that its application does not introduce new contaminants or exceed permissible levels of nitrates in drinking water.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries use as a basis for their national standards. These guidelines include recommendations for lead levels and treatment processes. Water treatment facilities considering the use of magnesium nitrate must ensure compliance with both national and international standards.
Regulatory bodies also require extensive testing and monitoring of water quality. This includes regular sampling and analysis of treated water to verify the effectiveness of lead reduction methods and to ensure compliance with established standards. The implementation of magnesium nitrate treatment would need to be accompanied by a robust monitoring program to demonstrate its efficacy and safety.
Furthermore, regulations often mandate the use of best available technologies (BAT) for water treatment. The application of magnesium nitrate would need to be evaluated against other established methods for lead reduction to determine its status as a BAT. This evaluation would consider factors such as effectiveness, cost, and potential secondary impacts on water quality.
Public disclosure and consumer confidence reports are another important aspect of the regulatory framework. Water utilities are required to inform the public about water quality and treatment methods used. The introduction of magnesium nitrate as a lead reduction method would need to be clearly communicated to consumers, along with its benefits and any potential risks.
Lastly, the regulatory landscape is not static. As new scientific evidence emerges and treatment technologies evolve, regulations are periodically reviewed and updated. The use of magnesium nitrate in water treatment would need to remain compliant with any future regulatory changes, necessitating ongoing assessment and potential adjustments to treatment protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!