Hydrochloric Acid: Advances in Waste Processing
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Waste Processing Evolution and Objectives
The evolution of waste processing using hydrochloric acid (HCl) has been a significant area of technological advancement in recent decades. This field has seen remarkable progress, driven by the increasing need for efficient and environmentally friendly waste management solutions. The primary objective of these advancements is to develop innovative methods for treating and recycling waste materials while minimizing environmental impact and maximizing resource recovery.
Historically, HCl has been widely used in various industrial processes, often resulting in the generation of acidic waste streams. The challenge of managing these waste streams has led to the development of novel treatment technologies. Early approaches focused on neutralization and disposal, but as environmental regulations became more stringent, the focus shifted towards recovery and reuse of both the acid and valuable materials contained in the waste.
One of the key milestones in this field was the development of membrane-based separation techniques. These technologies allowed for the selective recovery of HCl from waste streams, enabling its reuse in industrial processes. This not only reduced the environmental burden but also provided economic benefits by reclaiming a valuable resource.
Another significant advancement has been the integration of HCl waste processing with other waste treatment technologies. For instance, the combination of acid leaching with bioleaching processes has shown promising results in the treatment of metal-containing wastes. This synergistic approach enhances metal recovery rates while reducing the overall acid consumption.
The objectives of current research in HCl waste processing are multifaceted. Firstly, there is a strong focus on improving the efficiency and selectivity of acid recovery processes. This includes the development of advanced membrane materials and the optimization of separation techniques. Secondly, researchers are exploring ways to valorize the residual waste after acid recovery, aiming to transform it into useful products or energy sources.
Furthermore, there is an increasing emphasis on developing closed-loop systems that minimize waste generation and maximize resource utilization. This aligns with the principles of circular economy and sustainable industrial practices. The ultimate goal is to create processes that not only treat HCl waste effectively but also generate value-added products, thereby turning waste management into a profitable and environmentally beneficial activity.
As we look towards the future, the field of HCl waste processing is expected to continue evolving. Emerging technologies such as advanced oxidation processes, electrochemical treatment methods, and nanotechnology-based solutions are likely to play a significant role in shaping the next generation of waste treatment systems. These advancements promise to further enhance the efficiency, sustainability, and economic viability of HCl waste processing.
Historically, HCl has been widely used in various industrial processes, often resulting in the generation of acidic waste streams. The challenge of managing these waste streams has led to the development of novel treatment technologies. Early approaches focused on neutralization and disposal, but as environmental regulations became more stringent, the focus shifted towards recovery and reuse of both the acid and valuable materials contained in the waste.
One of the key milestones in this field was the development of membrane-based separation techniques. These technologies allowed for the selective recovery of HCl from waste streams, enabling its reuse in industrial processes. This not only reduced the environmental burden but also provided economic benefits by reclaiming a valuable resource.
Another significant advancement has been the integration of HCl waste processing with other waste treatment technologies. For instance, the combination of acid leaching with bioleaching processes has shown promising results in the treatment of metal-containing wastes. This synergistic approach enhances metal recovery rates while reducing the overall acid consumption.
The objectives of current research in HCl waste processing are multifaceted. Firstly, there is a strong focus on improving the efficiency and selectivity of acid recovery processes. This includes the development of advanced membrane materials and the optimization of separation techniques. Secondly, researchers are exploring ways to valorize the residual waste after acid recovery, aiming to transform it into useful products or energy sources.
Furthermore, there is an increasing emphasis on developing closed-loop systems that minimize waste generation and maximize resource utilization. This aligns with the principles of circular economy and sustainable industrial practices. The ultimate goal is to create processes that not only treat HCl waste effectively but also generate value-added products, thereby turning waste management into a profitable and environmentally beneficial activity.
As we look towards the future, the field of HCl waste processing is expected to continue evolving. Emerging technologies such as advanced oxidation processes, electrochemical treatment methods, and nanotechnology-based solutions are likely to play a significant role in shaping the next generation of waste treatment systems. These advancements promise to further enhance the efficiency, sustainability, and economic viability of HCl waste processing.
Market Demand for HCl Waste Treatment Solutions
The market demand for hydrochloric acid (HCl) waste treatment solutions has been steadily increasing due to growing environmental concerns and stringent regulations across various industries. As industrial processes continue to generate significant amounts of HCl waste, the need for efficient and cost-effective treatment methods has become paramount.
In the chemical manufacturing sector, which is one of the largest producers of HCl waste, there is a strong demand for advanced treatment technologies that can neutralize and safely dispose of acidic byproducts. This demand is driven by both regulatory compliance requirements and the industry's commitment to sustainable practices. The petrochemical industry, another major source of HCl waste, is also seeking innovative solutions to manage and recycle acid waste streams.
The semiconductor industry, known for its use of HCl in wafer cleaning and etching processes, has shown a growing interest in waste treatment solutions that can recover and reuse HCl. This trend is fueled by the increasing cost of raw materials and the need to reduce environmental impact. Similarly, the metal processing industry, which uses HCl for pickling and surface treatment, is actively seeking methods to treat and recycle acid waste to improve operational efficiency and reduce disposal costs.
Environmental regulations, such as the Resource Conservation and Recovery Act (RCRA) in the United States and similar legislation worldwide, have significantly influenced the market demand for HCl waste treatment solutions. These regulations mandate proper handling, treatment, and disposal of hazardous wastes, including HCl, driving industries to invest in advanced treatment technologies.
The market for HCl waste treatment solutions is also being shaped by the circular economy concept, with a growing emphasis on resource recovery and waste minimization. This has led to increased demand for technologies that can not only treat HCl waste but also recover valuable components for reuse or sale as byproducts.
Geographically, the demand for HCl waste treatment solutions is particularly strong in regions with high industrial activity and strict environmental regulations, such as North America, Europe, and parts of Asia. Emerging economies are also showing increased interest in these technologies as they strive to balance industrial growth with environmental protection.
The water treatment sector represents another significant market for HCl waste treatment solutions, as municipalities and industrial facilities seek to remove chlorides and other contaminants from wastewater streams. This demand is driven by the need to meet discharge standards and protect aquatic ecosystems.
As industries continue to seek more sustainable and economical ways to manage HCl waste, the market for innovative treatment solutions is expected to expand. This growth is likely to be fueled by advancements in membrane technologies, ion exchange systems, and electrochemical processes that offer improved efficiency and reduced environmental impact in HCl waste treatment.
In the chemical manufacturing sector, which is one of the largest producers of HCl waste, there is a strong demand for advanced treatment technologies that can neutralize and safely dispose of acidic byproducts. This demand is driven by both regulatory compliance requirements and the industry's commitment to sustainable practices. The petrochemical industry, another major source of HCl waste, is also seeking innovative solutions to manage and recycle acid waste streams.
The semiconductor industry, known for its use of HCl in wafer cleaning and etching processes, has shown a growing interest in waste treatment solutions that can recover and reuse HCl. This trend is fueled by the increasing cost of raw materials and the need to reduce environmental impact. Similarly, the metal processing industry, which uses HCl for pickling and surface treatment, is actively seeking methods to treat and recycle acid waste to improve operational efficiency and reduce disposal costs.
Environmental regulations, such as the Resource Conservation and Recovery Act (RCRA) in the United States and similar legislation worldwide, have significantly influenced the market demand for HCl waste treatment solutions. These regulations mandate proper handling, treatment, and disposal of hazardous wastes, including HCl, driving industries to invest in advanced treatment technologies.
The market for HCl waste treatment solutions is also being shaped by the circular economy concept, with a growing emphasis on resource recovery and waste minimization. This has led to increased demand for technologies that can not only treat HCl waste but also recover valuable components for reuse or sale as byproducts.
Geographically, the demand for HCl waste treatment solutions is particularly strong in regions with high industrial activity and strict environmental regulations, such as North America, Europe, and parts of Asia. Emerging economies are also showing increased interest in these technologies as they strive to balance industrial growth with environmental protection.
The water treatment sector represents another significant market for HCl waste treatment solutions, as municipalities and industrial facilities seek to remove chlorides and other contaminants from wastewater streams. This demand is driven by the need to meet discharge standards and protect aquatic ecosystems.
As industries continue to seek more sustainable and economical ways to manage HCl waste, the market for innovative treatment solutions is expected to expand. This growth is likely to be fueled by advancements in membrane technologies, ion exchange systems, and electrochemical processes that offer improved efficiency and reduced environmental impact in HCl waste treatment.
Current Challenges in HCl Waste Processing
The processing of hydrochloric acid (HCl) waste presents several significant challenges in today's industrial landscape. One of the primary issues is the corrosive nature of HCl, which poses risks to both equipment and personnel. This corrosivity necessitates the use of specialized materials and protective measures, increasing the overall cost and complexity of waste management systems.
Another major challenge is the environmental impact of HCl waste. Improper disposal can lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. Regulatory bodies worldwide have implemented strict guidelines for HCl waste handling and disposal, adding another layer of complexity to waste processing operations.
The variability in HCl waste composition across different industries further complicates processing efforts. Waste streams from chemical manufacturing, metal processing, and semiconductor production, for example, may contain different concentrations of HCl and various contaminants. This heterogeneity requires flexible and adaptable treatment solutions, which can be technically challenging and economically demanding.
Scale formation in processing equipment is another significant issue. The reaction of HCl with metal ions often leads to the formation of scale deposits, reducing equipment efficiency and necessitating frequent maintenance. This not only increases operational costs but also results in downtime, affecting overall productivity.
The recovery and reuse of HCl from waste streams present both an opportunity and a challenge. While recycling HCl can reduce waste and raw material costs, the process of separating and purifying the acid from complex waste mixtures is often energy-intensive and technologically demanding.
Safety concerns in HCl waste processing extend beyond corrosion risks. The potential for harmful gas emissions, particularly when HCl reacts with other chemicals, requires sophisticated ventilation systems and rigorous safety protocols. This adds to the overall complexity and cost of waste management facilities.
The energy intensity of current HCl waste processing methods is another significant challenge. Many treatment processes, such as neutralization and thermal decomposition, require substantial energy inputs, contributing to high operational costs and environmental footprints.
Lastly, the disposal of byproducts from HCl waste treatment poses its own set of challenges. Neutralization processes, for instance, generate large volumes of salt solutions that require further management. Finding sustainable and economical ways to handle these secondary waste streams remains an ongoing challenge in the field of HCl waste processing.
Another major challenge is the environmental impact of HCl waste. Improper disposal can lead to soil and water contamination, affecting ecosystems and potentially entering the food chain. Regulatory bodies worldwide have implemented strict guidelines for HCl waste handling and disposal, adding another layer of complexity to waste processing operations.
The variability in HCl waste composition across different industries further complicates processing efforts. Waste streams from chemical manufacturing, metal processing, and semiconductor production, for example, may contain different concentrations of HCl and various contaminants. This heterogeneity requires flexible and adaptable treatment solutions, which can be technically challenging and economically demanding.
Scale formation in processing equipment is another significant issue. The reaction of HCl with metal ions often leads to the formation of scale deposits, reducing equipment efficiency and necessitating frequent maintenance. This not only increases operational costs but also results in downtime, affecting overall productivity.
The recovery and reuse of HCl from waste streams present both an opportunity and a challenge. While recycling HCl can reduce waste and raw material costs, the process of separating and purifying the acid from complex waste mixtures is often energy-intensive and technologically demanding.
Safety concerns in HCl waste processing extend beyond corrosion risks. The potential for harmful gas emissions, particularly when HCl reacts with other chemicals, requires sophisticated ventilation systems and rigorous safety protocols. This adds to the overall complexity and cost of waste management facilities.
The energy intensity of current HCl waste processing methods is another significant challenge. Many treatment processes, such as neutralization and thermal decomposition, require substantial energy inputs, contributing to high operational costs and environmental footprints.
Lastly, the disposal of byproducts from HCl waste treatment poses its own set of challenges. Neutralization processes, for instance, generate large volumes of salt solutions that require further management. Finding sustainable and economical ways to handle these secondary waste streams remains an ongoing challenge in the field of HCl waste processing.
Existing HCl Waste Processing Techniques
01 Production methods of hydrochloric acid
Various methods are employed for the production of hydrochloric acid, including chemical reactions and industrial processes. These methods may involve the use of specific catalysts, reactants, or equipment to efficiently produce hydrochloric acid at different concentrations and purities.- Production methods of hydrochloric acid: Various methods are employed to produce hydrochloric acid, including the reaction of chlorine with hydrogen, the chlorination of organic compounds, and as a byproduct in chemical processes. These production methods aim to optimize yield and purity while minimizing environmental impact.
- Purification and concentration techniques: Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for industrial applications.
- Industrial applications of hydrochloric acid: Hydrochloric acid finds wide-ranging industrial applications, including metal processing, chemical synthesis, water treatment, and oil well acidizing. Its strong acidic properties make it valuable in various manufacturing processes and as a cleaning agent.
- Safety measures and handling procedures: Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes the use of appropriate personal protective equipment, storage containers, and neutralization techniques in case of spills.
- Environmental considerations and waste management: Environmental considerations in the production and use of hydrochloric acid include proper waste management, recycling techniques, and emission control. Efforts are made to minimize the environmental impact and comply with regulations regarding its disposal and handling.
02 Applications of hydrochloric acid in chemical processes
Hydrochloric acid is widely used in various chemical processes and industries. It serves as a key reagent in reactions, pH adjustment, and as a cleaning agent. Its applications range from metal treatment to food processing and pharmaceutical manufacturing.Expand Specific Solutions03 Handling and storage of hydrochloric acid
Proper handling and storage of hydrochloric acid are crucial for safety and efficiency. Specialized equipment, containers, and procedures are used to manage this corrosive substance. This includes the use of resistant materials, safety measures, and storage systems designed to prevent leaks and accidents.Expand Specific Solutions04 Purification and concentration of hydrochloric acid
Techniques for purifying and concentrating hydrochloric acid are essential for obtaining high-quality products. These processes may involve distillation, membrane separation, or other advanced methods to remove impurities and achieve desired concentrations for specific applications.Expand Specific Solutions05 Environmental and safety considerations in hydrochloric acid use
The use of hydrochloric acid requires careful consideration of environmental impact and safety measures. This includes developing methods for neutralization, waste treatment, and emission control. Additionally, safety protocols and protective equipment are essential for handling this corrosive substance in industrial and laboratory settings.Expand Specific Solutions
Key Players in HCl Waste Management Industry
The waste processing industry using hydrochloric acid is in a mature stage, with a growing market driven by increasing environmental regulations and the need for sustainable waste management solutions. The global market size for this technology is estimated to be in the billions, with steady growth projected. Technologically, the field is well-established but continues to evolve, with companies like LG Chem, Indaver NV, and Dow Chemical leading innovation. These firms, along with others such as Wacker Chemie and Air Liquide, are investing in research and development to improve efficiency and reduce environmental impact. Universities like Queensland and Florida are contributing to advancements through academic research, while specialized companies like Australian BioRefining and Big Fish Environmental are developing niche solutions for specific waste processing challenges.
The Dow Chemical Co.
Technical Solution: Dow Chemical has developed an advanced waste processing technique using hydrochloric acid for the treatment of industrial waste streams. Their process involves a multi-stage acid leaching system that efficiently extracts valuable metals from complex waste materials[1]. The technology utilizes a series of reactors with controlled pH levels to selectively dissolve and recover metals such as copper, nickel, and zinc. Additionally, Dow has implemented a closed-loop acid regeneration system, which recycles up to 98% of the hydrochloric acid used in the process, significantly reducing chemical consumption and environmental impact[3].
Strengths: High metal recovery rates, efficient acid recycling, reduced environmental footprint. Weaknesses: High initial capital investment, potential for acid-resistant contaminants to remain in waste streams.
Arkema France SA
Technical Solution: Arkema has pioneered a novel hydrochloric acid-based waste processing technology focused on the treatment of fluoropolymer waste. Their process utilizes a high-temperature, pressurized reactor system where hydrochloric acid is used to break down fluoropolymer chains into recoverable monomers[2]. The technology achieves a recovery rate of up to 95% for valuable fluorinated compounds, which can be reused in the production of new fluoropolymers. Arkema's system also incorporates advanced off-gas treatment to capture and neutralize any potentially harmful emissions, ensuring environmental compliance[4].
Strengths: High recovery rate of valuable fluorinated compounds, closed-loop system for monomer reuse. Weaknesses: Limited to specific types of fluoropolymer waste, high energy requirements for the high-temperature process.
Innovative HCl Waste Treatment Patents
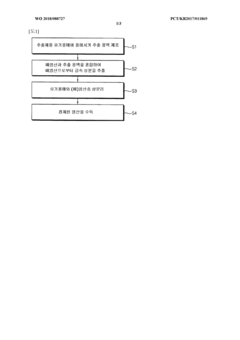
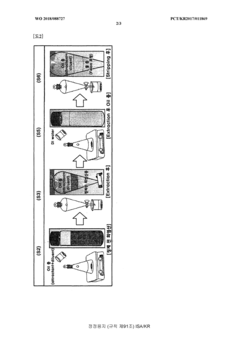
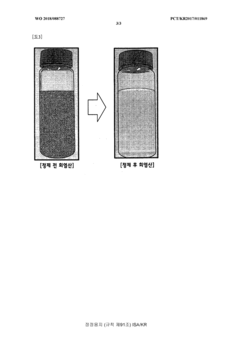
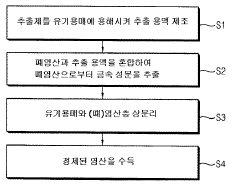
Method for purifying waste hydrochloric acid
PatentWO2018088727A1
Innovation
- A solvent extraction method using an organic solvent and an extractant like trioctylamine to selectively remove metal components, including iron ions, directly from waste hydrochloric acid, allowing for phase separation and regeneration of the solvent, thereby simplifying the process and reducing energy and cost consumption.
Method for absorbing chlorine from gas streams
PatentWO2009138401A1
Innovation
- A continuous process using controlled addition of hydrogen peroxide in water to suppress hypochlorous acid formation, allowing chlorine to react and form hydrochloric acid, which is then bound in the absorption medium, eliminating salt formation and enabling the production of usable hydrochloric acid.
Environmental Impact Assessment
The environmental impact assessment of hydrochloric acid (HCl) in waste processing reveals both potential benefits and concerns. HCl's use in waste treatment can significantly enhance the efficiency of metal recovery from electronic waste and industrial sludges, reducing the volume of waste destined for landfills. This process contributes to the circular economy by reclaiming valuable resources that would otherwise be lost.
However, the use of HCl in waste processing also presents several environmental challenges. The production and transportation of HCl itself have associated carbon footprints and energy costs. During the waste treatment process, there is a risk of acid vapors being released into the atmosphere, which can contribute to air pollution and potentially harm local ecosystems if not properly controlled.
Water pollution is another significant concern. The acidic wastewater generated from HCl-based waste processing requires careful management and neutralization before discharge. If not adequately treated, this effluent can alter the pH of receiving water bodies, negatively impacting aquatic life and water quality.
Soil contamination is also a potential risk, particularly if there are accidental spills or improper disposal of acid-treated waste. The low pH of HCl can mobilize heavy metals in soil, potentially leading to groundwater contamination and long-term ecological damage.
On the positive side, advanced HCl recovery and recycling systems can mitigate many of these environmental risks. Closed-loop processes that capture and reuse HCl minimize waste and reduce the need for fresh acid production. Additionally, the use of HCl in waste processing can indirectly benefit the environment by reducing the need for mining raw materials, thus decreasing the environmental impact associated with extractive industries.
The overall environmental impact of HCl use in waste processing depends heavily on the implementation of best practices and technologies. Proper emission control systems, wastewater treatment facilities, and safety protocols are essential to minimize negative environmental effects. Furthermore, life cycle assessments should be conducted to compare the environmental footprint of HCl-based waste processing with alternative methods, ensuring that the chosen approach offers the best balance of resource recovery and environmental protection.
However, the use of HCl in waste processing also presents several environmental challenges. The production and transportation of HCl itself have associated carbon footprints and energy costs. During the waste treatment process, there is a risk of acid vapors being released into the atmosphere, which can contribute to air pollution and potentially harm local ecosystems if not properly controlled.
Water pollution is another significant concern. The acidic wastewater generated from HCl-based waste processing requires careful management and neutralization before discharge. If not adequately treated, this effluent can alter the pH of receiving water bodies, negatively impacting aquatic life and water quality.
Soil contamination is also a potential risk, particularly if there are accidental spills or improper disposal of acid-treated waste. The low pH of HCl can mobilize heavy metals in soil, potentially leading to groundwater contamination and long-term ecological damage.
On the positive side, advanced HCl recovery and recycling systems can mitigate many of these environmental risks. Closed-loop processes that capture and reuse HCl minimize waste and reduce the need for fresh acid production. Additionally, the use of HCl in waste processing can indirectly benefit the environment by reducing the need for mining raw materials, thus decreasing the environmental impact associated with extractive industries.
The overall environmental impact of HCl use in waste processing depends heavily on the implementation of best practices and technologies. Proper emission control systems, wastewater treatment facilities, and safety protocols are essential to minimize negative environmental effects. Furthermore, life cycle assessments should be conducted to compare the environmental footprint of HCl-based waste processing with alternative methods, ensuring that the chosen approach offers the best balance of resource recovery and environmental protection.
Regulatory Framework for HCl Waste Management
The regulatory framework for hydrochloric acid (HCl) waste management has become increasingly stringent in recent years, reflecting growing concerns about environmental protection and public health. Governments and international organizations have implemented comprehensive regulations to govern the handling, storage, transportation, and disposal of HCl waste.
At the national level, many countries have established specific guidelines for HCl waste management. In the United States, the Environmental Protection Agency (EPA) regulates HCl waste under the Resource Conservation and Recovery Act (RCRA). The EPA classifies HCl as a hazardous waste when its concentration exceeds certain thresholds, requiring strict compliance with handling and disposal procedures.
Similarly, the European Union has implemented the Waste Framework Directive, which sets out a hierarchy for waste management prioritizing prevention, reuse, and recycling over disposal. For HCl waste specifically, the EU's Classification, Labelling, and Packaging (CLP) Regulation provides guidelines for proper identification and handling of hazardous substances.
International agreements also play a crucial role in shaping the regulatory landscape. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal regulates the international transport of hazardous wastes, including HCl waste. This convention ensures that countries take responsibility for their waste and prevents the dumping of hazardous materials in developing nations.
Industry-specific regulations further complement these broader frameworks. For instance, the semiconductor industry, which uses large quantities of HCl in manufacturing processes, must adhere to additional guidelines set by organizations such as the Semiconductor Equipment and Materials International (SEMI).
Regulatory bodies are increasingly focusing on promoting circular economy principles in waste management. This approach encourages the recovery and reuse of HCl from waste streams, aligning with broader sustainability goals. As a result, regulations are evolving to incentivize the development and adoption of advanced waste processing technologies that can effectively recover and purify HCl from waste materials.
Compliance with these regulations often requires significant investment in infrastructure and technology. Companies must implement robust waste management systems, including proper storage facilities, treatment technologies, and monitoring equipment. Regular audits and reporting are typically mandated to ensure ongoing compliance with regulatory standards.
As environmental concerns continue to grow, it is anticipated that regulations governing HCl waste management will become even more stringent in the future. This trend is likely to drive further innovation in waste processing technologies, particularly those that can efficiently recover and reuse HCl, thereby reducing environmental impact and improving resource efficiency.
At the national level, many countries have established specific guidelines for HCl waste management. In the United States, the Environmental Protection Agency (EPA) regulates HCl waste under the Resource Conservation and Recovery Act (RCRA). The EPA classifies HCl as a hazardous waste when its concentration exceeds certain thresholds, requiring strict compliance with handling and disposal procedures.
Similarly, the European Union has implemented the Waste Framework Directive, which sets out a hierarchy for waste management prioritizing prevention, reuse, and recycling over disposal. For HCl waste specifically, the EU's Classification, Labelling, and Packaging (CLP) Regulation provides guidelines for proper identification and handling of hazardous substances.
International agreements also play a crucial role in shaping the regulatory landscape. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal regulates the international transport of hazardous wastes, including HCl waste. This convention ensures that countries take responsibility for their waste and prevents the dumping of hazardous materials in developing nations.
Industry-specific regulations further complement these broader frameworks. For instance, the semiconductor industry, which uses large quantities of HCl in manufacturing processes, must adhere to additional guidelines set by organizations such as the Semiconductor Equipment and Materials International (SEMI).
Regulatory bodies are increasingly focusing on promoting circular economy principles in waste management. This approach encourages the recovery and reuse of HCl from waste streams, aligning with broader sustainability goals. As a result, regulations are evolving to incentivize the development and adoption of advanced waste processing technologies that can effectively recover and purify HCl from waste materials.
Compliance with these regulations often requires significant investment in infrastructure and technology. Companies must implement robust waste management systems, including proper storage facilities, treatment technologies, and monitoring equipment. Regular audits and reporting are typically mandated to ensure ongoing compliance with regulatory standards.
As environmental concerns continue to grow, it is anticipated that regulations governing HCl waste management will become even more stringent in the future. This trend is likely to drive further innovation in waste processing technologies, particularly those that can efficiently recover and reuse HCl, thereby reducing environmental impact and improving resource efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!