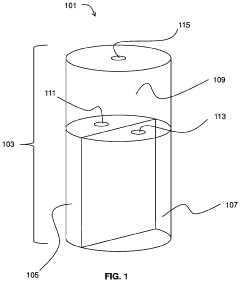
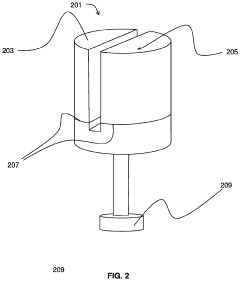
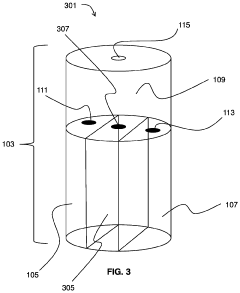
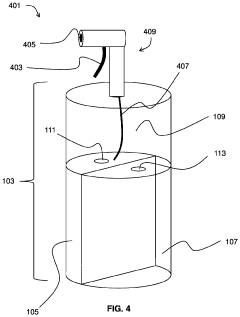
Hydrochloric Acid: Enhancements in Industrial Hygiene
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Safety Evolution
The evolution of safety practices in hydrochloric acid (HCl) handling has been a critical aspect of industrial hygiene over the past century. In the early 1900s, the dangers of HCl exposure were not fully understood, leading to numerous workplace accidents and health issues. As scientific knowledge advanced, the corrosive and toxic nature of HCl became apparent, prompting the development of initial safety measures.
By the 1950s, basic personal protective equipment (PPE) such as rubber gloves and safety goggles became standard in industries handling HCl. However, these measures were often inadequate, and incidents of chemical burns and respiratory problems remained common. The 1970s marked a significant turning point with the establishment of regulatory bodies like the Occupational Safety and Health Administration (OSHA) in the United States, which set strict guidelines for HCl handling and exposure limits.
The 1980s and 1990s saw rapid advancements in safety technology. Improved ventilation systems and fume hoods became mandatory in laboratories and industrial settings. More sophisticated PPE, including chemical-resistant suits and respirators with specific HCl filters, was introduced. Safety showers and eyewash stations became ubiquitous in facilities dealing with HCl.
The turn of the millennium brought a shift towards preventive measures and engineering controls. Closed-loop systems for HCl transfer and storage significantly reduced the risk of exposure. Advanced monitoring technologies, such as real-time HCl vapor detectors, allowed for immediate detection of leaks or spills. Training programs for workers became more comprehensive, focusing on proper handling techniques, emergency response, and the importance of regular equipment maintenance.
In recent years, the focus has expanded to include environmental concerns alongside worker safety. Stricter regulations on HCl emissions and waste disposal have led to the development of more efficient scrubbing systems and neutralization techniques. The integration of digital technologies has further enhanced safety measures, with automated safety systems and predictive maintenance algorithms helping to prevent accidents before they occur.
Today, the approach to HCl safety is holistic, combining advanced engineering controls, stringent administrative procedures, and state-of-the-art PPE. Ongoing research continues to refine our understanding of long-term health effects, driving the development of even safer handling practices and alternative processes that minimize HCl use where possible. The evolution of HCl safety practices serves as a model for continuous improvement in industrial hygiene, demonstrating the importance of adapting to new knowledge and technologies to protect worker health and the environment.
By the 1950s, basic personal protective equipment (PPE) such as rubber gloves and safety goggles became standard in industries handling HCl. However, these measures were often inadequate, and incidents of chemical burns and respiratory problems remained common. The 1970s marked a significant turning point with the establishment of regulatory bodies like the Occupational Safety and Health Administration (OSHA) in the United States, which set strict guidelines for HCl handling and exposure limits.
The 1980s and 1990s saw rapid advancements in safety technology. Improved ventilation systems and fume hoods became mandatory in laboratories and industrial settings. More sophisticated PPE, including chemical-resistant suits and respirators with specific HCl filters, was introduced. Safety showers and eyewash stations became ubiquitous in facilities dealing with HCl.
The turn of the millennium brought a shift towards preventive measures and engineering controls. Closed-loop systems for HCl transfer and storage significantly reduced the risk of exposure. Advanced monitoring technologies, such as real-time HCl vapor detectors, allowed for immediate detection of leaks or spills. Training programs for workers became more comprehensive, focusing on proper handling techniques, emergency response, and the importance of regular equipment maintenance.
In recent years, the focus has expanded to include environmental concerns alongside worker safety. Stricter regulations on HCl emissions and waste disposal have led to the development of more efficient scrubbing systems and neutralization techniques. The integration of digital technologies has further enhanced safety measures, with automated safety systems and predictive maintenance algorithms helping to prevent accidents before they occur.
Today, the approach to HCl safety is holistic, combining advanced engineering controls, stringent administrative procedures, and state-of-the-art PPE. Ongoing research continues to refine our understanding of long-term health effects, driving the development of even safer handling practices and alternative processes that minimize HCl use where possible. The evolution of HCl safety practices serves as a model for continuous improvement in industrial hygiene, demonstrating the importance of adapting to new knowledge and technologies to protect worker health and the environment.
Industrial HCl Demand
The global demand for hydrochloric acid (HCl) in industrial applications continues to grow steadily, driven by its versatile use across various sectors. The chemical industry remains the largest consumer of HCl, utilizing it as a key raw material in the production of various chemicals, including PVC, water treatment chemicals, and pharmaceuticals. The increasing demand for these end-products directly correlates with the rising need for HCl in industrial processes.
In the metal processing industry, HCl plays a crucial role in steel pickling and metal surface treatment. As the global steel production and metal manufacturing sectors expand, particularly in emerging economies, the demand for HCl in these applications is expected to rise significantly. The automotive and construction industries, major consumers of processed metals, indirectly contribute to the increased demand for HCl.
The oil and gas sector represents another significant market for HCl, where it is used in well acidizing and hydraulic fracturing processes. As exploration and production activities continue to grow, especially in unconventional oil and gas reserves, the demand for HCl in this sector is projected to increase.
Water treatment and purification processes also contribute substantially to the industrial demand for HCl. With growing urbanization and industrialization, coupled with increasing environmental regulations, the need for effective water treatment solutions is escalating. HCl's role in pH adjustment and as a precursor for water treatment chemicals positions it as a critical component in this expanding market.
The electronics industry, particularly in the production of semiconductors and printed circuit boards, represents a growing market for high-purity HCl. As the demand for electronic devices continues to surge globally, driven by technological advancements and digitalization trends, the need for HCl in this sector is expected to rise proportionally.
Geographically, Asia-Pacific remains the largest consumer and producer of HCl, with China leading the market. The region's rapid industrialization, expanding manufacturing base, and increasing investments in infrastructure development are key factors driving the demand. North America and Europe follow, with steady consumption in established industrial sectors and growing demand in specialized applications.
The market dynamics are further influenced by the increasing focus on sustainable practices and circular economy principles. This trend is driving research into more efficient HCl production methods and recycling techniques, potentially impacting the supply-demand balance in the coming years. Additionally, the development of alternative technologies in some application areas may pose challenges to HCl demand growth in specific sectors.
In the metal processing industry, HCl plays a crucial role in steel pickling and metal surface treatment. As the global steel production and metal manufacturing sectors expand, particularly in emerging economies, the demand for HCl in these applications is expected to rise significantly. The automotive and construction industries, major consumers of processed metals, indirectly contribute to the increased demand for HCl.
The oil and gas sector represents another significant market for HCl, where it is used in well acidizing and hydraulic fracturing processes. As exploration and production activities continue to grow, especially in unconventional oil and gas reserves, the demand for HCl in this sector is projected to increase.
Water treatment and purification processes also contribute substantially to the industrial demand for HCl. With growing urbanization and industrialization, coupled with increasing environmental regulations, the need for effective water treatment solutions is escalating. HCl's role in pH adjustment and as a precursor for water treatment chemicals positions it as a critical component in this expanding market.
The electronics industry, particularly in the production of semiconductors and printed circuit boards, represents a growing market for high-purity HCl. As the demand for electronic devices continues to surge globally, driven by technological advancements and digitalization trends, the need for HCl in this sector is expected to rise proportionally.
Geographically, Asia-Pacific remains the largest consumer and producer of HCl, with China leading the market. The region's rapid industrialization, expanding manufacturing base, and increasing investments in infrastructure development are key factors driving the demand. North America and Europe follow, with steady consumption in established industrial sectors and growing demand in specialized applications.
The market dynamics are further influenced by the increasing focus on sustainable practices and circular economy principles. This trend is driving research into more efficient HCl production methods and recycling techniques, potentially impacting the supply-demand balance in the coming years. Additionally, the development of alternative technologies in some application areas may pose challenges to HCl demand growth in specific sectors.
HCl Handling Challenges
Handling hydrochloric acid (HCl) in industrial settings presents significant challenges due to its corrosive nature and potential health hazards. The primary concerns revolve around worker safety, equipment integrity, and environmental protection. One of the most pressing issues is the risk of chemical burns and respiratory problems caused by HCl exposure. Even at low concentrations, HCl vapors can irritate the eyes, nose, and throat, while higher concentrations can lead to severe respiratory distress and tissue damage.
Equipment corrosion is another major challenge in HCl handling. The acid's aggressive nature can rapidly degrade storage tanks, pipelines, and processing equipment, leading to leaks, spills, and potential catastrophic failures. This necessitates the use of specialized materials such as high-grade stainless steel, fiber-reinforced plastics, or rubber-lined vessels, which significantly increases infrastructure costs and maintenance requirements.
Proper containment and storage of HCl pose additional challenges. The acid must be stored in tightly sealed containers to prevent vapor emissions and potential reactions with other chemicals. Temperature control is crucial, as HCl can become more volatile at higher temperatures, increasing the risk of pressure build-up in storage vessels. Furthermore, the hygroscopic nature of HCl means it readily absorbs moisture from the air, potentially leading to concentration changes and increased corrosivity.
Transportation of HCl presents its own set of challenges. Strict regulations govern the movement of hazardous materials, requiring specialized tankers, proper labeling, and trained personnel. Accidental spills during transport can have severe environmental consequences, necessitating comprehensive emergency response plans and specialized clean-up procedures.
Waste management is another significant concern in HCl handling. Neutralization and disposal of HCl waste must be carefully managed to comply with environmental regulations and prevent ecological damage. This often requires sophisticated treatment systems and ongoing monitoring to ensure compliance with discharge limits.
The challenges extend to personal protective equipment (PPE) for workers. Standard PPE may be insufficient for handling concentrated HCl, requiring specialized acid-resistant suits, gloves, and respiratory protection. Ensuring proper fit, maintenance, and training for this equipment is crucial for worker safety but can be complex and resource-intensive.
Lastly, the evolving regulatory landscape poses ongoing challenges for industries using HCl. Stricter environmental and safety regulations require continuous adaptation of handling procedures, equipment, and training protocols. This necessitates significant investment in compliance measures and regular audits to ensure adherence to the latest standards.
Equipment corrosion is another major challenge in HCl handling. The acid's aggressive nature can rapidly degrade storage tanks, pipelines, and processing equipment, leading to leaks, spills, and potential catastrophic failures. This necessitates the use of specialized materials such as high-grade stainless steel, fiber-reinforced plastics, or rubber-lined vessels, which significantly increases infrastructure costs and maintenance requirements.
Proper containment and storage of HCl pose additional challenges. The acid must be stored in tightly sealed containers to prevent vapor emissions and potential reactions with other chemicals. Temperature control is crucial, as HCl can become more volatile at higher temperatures, increasing the risk of pressure build-up in storage vessels. Furthermore, the hygroscopic nature of HCl means it readily absorbs moisture from the air, potentially leading to concentration changes and increased corrosivity.
Transportation of HCl presents its own set of challenges. Strict regulations govern the movement of hazardous materials, requiring specialized tankers, proper labeling, and trained personnel. Accidental spills during transport can have severe environmental consequences, necessitating comprehensive emergency response plans and specialized clean-up procedures.
Waste management is another significant concern in HCl handling. Neutralization and disposal of HCl waste must be carefully managed to comply with environmental regulations and prevent ecological damage. This often requires sophisticated treatment systems and ongoing monitoring to ensure compliance with discharge limits.
The challenges extend to personal protective equipment (PPE) for workers. Standard PPE may be insufficient for handling concentrated HCl, requiring specialized acid-resistant suits, gloves, and respiratory protection. Ensuring proper fit, maintenance, and training for this equipment is crucial for worker safety but can be complex and resource-intensive.
Lastly, the evolving regulatory landscape poses ongoing challenges for industries using HCl. Stricter environmental and safety regulations require continuous adaptation of handling procedures, equipment, and training protocols. This necessitates significant investment in compliance measures and regular audits to ensure adherence to the latest standards.
Current HCl Safety Methods
01 Safety measures for handling hydrochloric acid
Implementing proper safety measures is crucial when handling hydrochloric acid in industrial settings. This includes using appropriate personal protective equipment (PPE), such as chemical-resistant gloves, goggles, and protective clothing. Adequate ventilation systems should be in place to prevent the accumulation of harmful vapors. Emergency eyewash stations and safety showers should be readily accessible in case of accidental exposure.- Personal protective equipment for handling hydrochloric acid: Proper personal protective equipment (PPE) is essential for safe handling of hydrochloric acid in industrial settings. This includes acid-resistant gloves, goggles, face shields, and protective clothing to prevent skin and eye contact. Respiratory protection may also be necessary in certain situations to prevent inhalation of acid vapors.
- Ventilation and exhaust systems for acid handling areas: Adequate ventilation and exhaust systems are crucial for maintaining safe air quality in areas where hydrochloric acid is used or stored. These systems help to remove acid vapors and prevent the buildup of potentially harmful concentrations in the workplace, reducing the risk of respiratory issues for workers.
- Spill containment and neutralization procedures: Proper spill containment and neutralization procedures are essential for managing accidental releases of hydrochloric acid. This includes the use of appropriate absorbent materials, neutralizing agents, and disposal methods to minimize environmental impact and protect worker safety during cleanup operations.
- Storage and handling guidelines for hydrochloric acid: Specific storage and handling guidelines must be followed to ensure the safe management of hydrochloric acid in industrial settings. This includes using corrosion-resistant containers, proper labeling, segregation from incompatible materials, and implementing appropriate transfer procedures to minimize the risk of spills or accidents.
- Emergency response and first aid procedures: Comprehensive emergency response and first aid procedures are crucial for addressing incidents involving hydrochloric acid exposure. This includes having readily available eyewash stations and safety showers, training personnel in proper response techniques, and establishing clear protocols for seeking medical attention in case of acid contact or inhalation.
02 Storage and containment of hydrochloric acid
Proper storage and containment of hydrochloric acid are essential for industrial hygiene. This involves using corrosion-resistant containers and storage tanks, implementing secondary containment measures to prevent spills, and ensuring proper labeling and signage. Regular inspections and maintenance of storage facilities are necessary to prevent leaks and maintain the integrity of containment systems.Expand Specific Solutions03 Monitoring and detection systems
Implementing monitoring and detection systems is crucial for maintaining industrial hygiene when working with hydrochloric acid. This includes the use of gas detectors, pH sensors, and other monitoring equipment to detect leaks or releases. Regular air quality monitoring and workplace exposure assessments should be conducted to ensure that exposure levels remain within safe limits.Expand Specific Solutions04 Emergency response and spill management
Developing and implementing emergency response and spill management procedures is essential for hydrochloric acid industrial hygiene. This includes training employees on proper response techniques, having appropriate spill containment and neutralization materials readily available, and establishing clear communication protocols for reporting and addressing incidents.Expand Specific Solutions05 Waste management and disposal
Proper waste management and disposal of hydrochloric acid and related materials are crucial for maintaining industrial hygiene. This involves implementing appropriate neutralization and treatment processes, adhering to local and national regulations for hazardous waste disposal, and maintaining accurate records of waste generation and disposal activities.Expand Specific Solutions
Key HCl Industry Players
The industrial hygiene enhancements for hydrochloric acid are in a mature stage, with a growing market driven by increasing safety regulations and industrial applications. The technology's maturity is evident from the diverse range of established players, including major chemical corporations like China Petroleum & Chemical Corp. and Henkel AG & Co. KGaA, as well as specialized firms such as Fluid Energy Group Ltd. and Ecolab USA, Inc. These companies are focusing on developing safer handling methods, improved containment systems, and more efficient neutralization techniques. The market is characterized by ongoing innovation in personal protective equipment, monitoring systems, and waste management solutions, reflecting the industry's commitment to worker safety and environmental protection.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group has pioneered the development of safer alternatives to traditional hydrochloric acid for industrial applications. Their ENVIRO-SYN® HCR series products are designed to match or exceed the performance of conventional HCl while significantly reducing health and environmental risks. These products utilize a proprietary blend of organic acids and surfactants that provide similar pH and reactivity to HCl but with lower corrosivity and vapor pressure. This results in reduced equipment wear and improved worker safety. Fluid Energy Group's solutions also incorporate advanced buffering systems that maintain acidity over extended periods, reducing the frequency of acid replenishment and associated handling risks[6]. The company has further developed specialized delivery systems and training programs to ensure safe handling and application of their products in various industrial settings.
Strengths: Innovative, safer alternatives to traditional HCl with comparable performance. Comprehensive approach including product formulation, delivery systems, and training. Weaknesses: May require process modifications for adoption in some industries accustomed to traditional HCl use.
Ecolab USA, Inc.
Technical Solution: Ecolab has developed advanced hydrochloric acid (HCl) handling and safety solutions for industrial applications. Their approach includes innovative closed-loop systems that minimize worker exposure to HCl vapors. The company's Acid-A-Vator™ system automates acid delivery, significantly reducing the risk of spills and exposure[1]. Ecolab also offers specialized personal protective equipment (PPE) designed specifically for HCl handling, including chemical-resistant suits and respiratory protection. Their comprehensive approach extends to environmental controls, with advanced scrubber systems that capture and neutralize HCl emissions, ensuring compliance with stringent air quality regulations[2].
Strengths: Comprehensive approach combining engineering controls, PPE, and environmental solutions. Weaknesses: May require significant initial investment for implementation.
HCl Safety Innovations
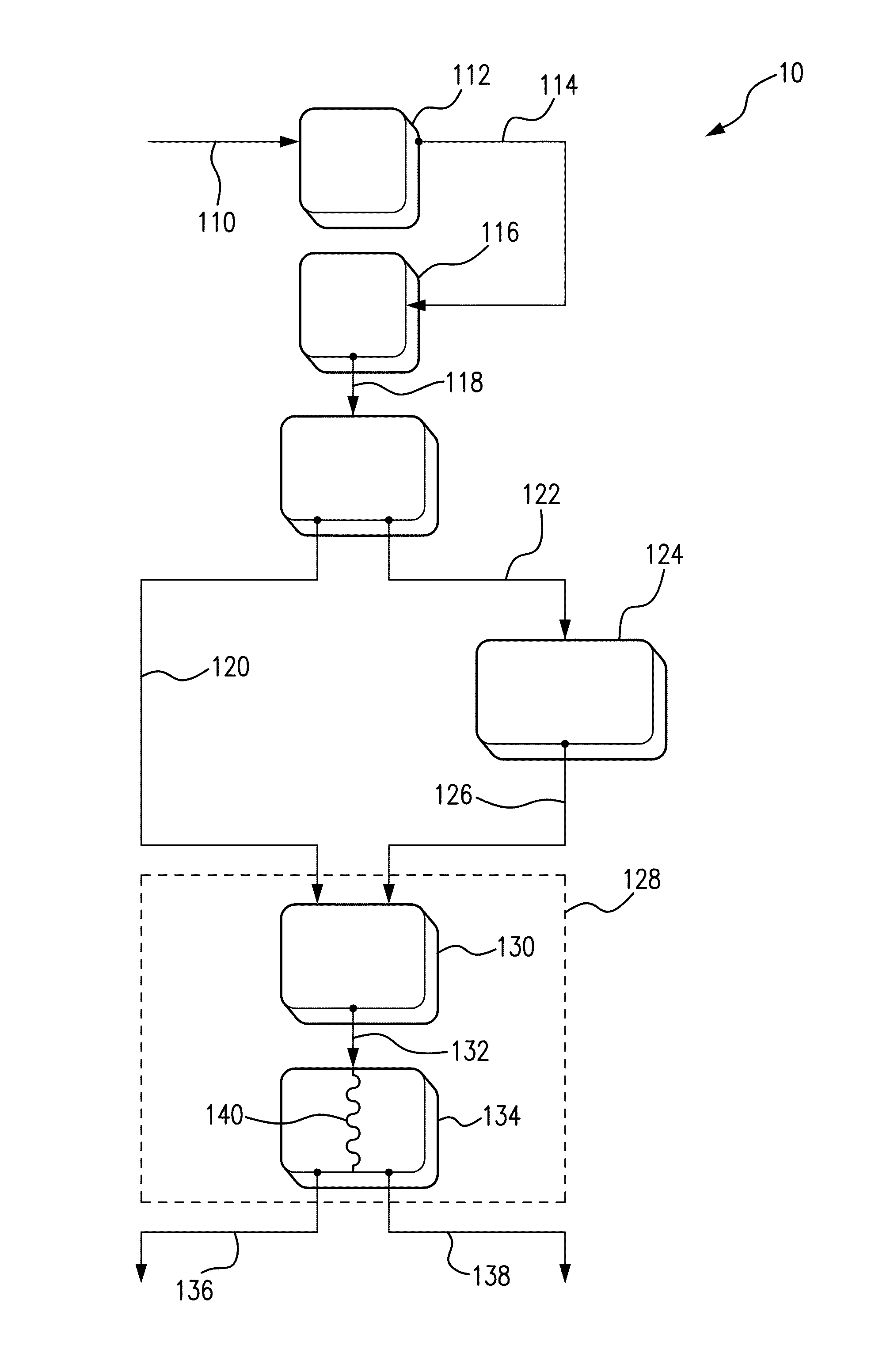
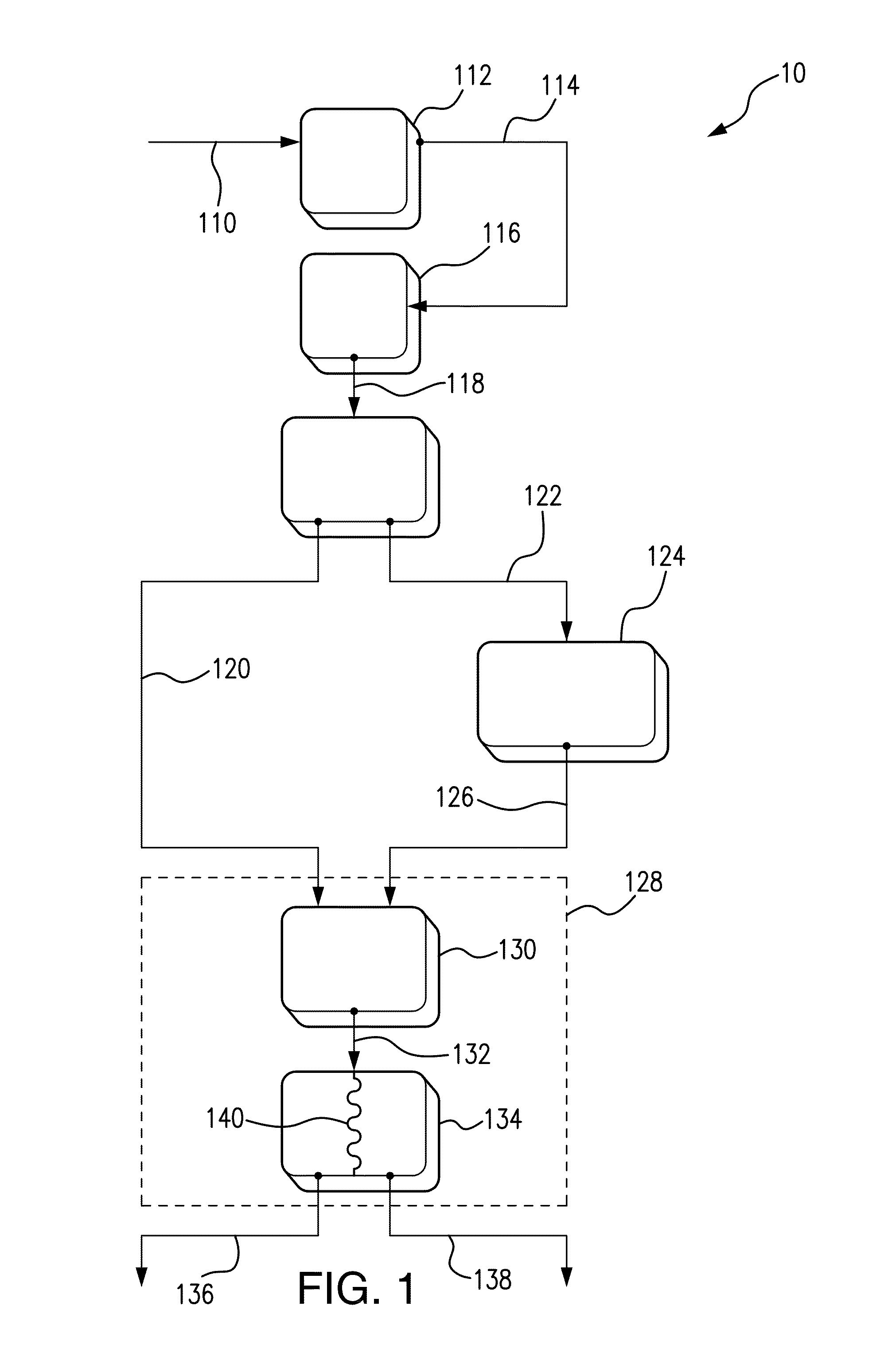
Multi-chamber hypochlorous acid dispenser
PatentActiveUS20230399228A1
Innovation
- A multi-chambered container system that separates and stabilizes the components for producing hypochlorous acid, using one-way valves and buffering agents to maintain a stable pH range, allowing for on-site preparation and long-term storage by controlling pH, ion concentration, and air exposure, thereby preventing degradation.
Method for Producing Shelf Stable Hypochlorous Acid Solutions
PatentInactiveUS20150119245A1
Innovation
- A method involving the production of hypochlorous acid using high purity water from a combination of softening and reverse osmosis steps, followed by electrolysis with a bipolar membrane, and storage in opaque PET bottles or Nylon/PE bags, which significantly extends the shelf life to 24 months.
HCl Regulations Overview
Hydrochloric acid (HCl) is a widely used industrial chemical with significant applications across various sectors. Due to its corrosive nature and potential health hazards, stringent regulations have been established to govern its handling, storage, and use. These regulations aim to protect workers, the environment, and the general public from the risks associated with HCl exposure.
At the federal level in the United States, the Occupational Safety and Health Administration (OSHA) has set permissible exposure limits (PELs) for HCl. The current PEL is 5 parts per million (ppm) as a ceiling limit, meaning that exposure should never exceed this concentration during any part of the workday. OSHA also requires employers to provide appropriate personal protective equipment (PPE) and implement engineering controls to minimize worker exposure.
The Environmental Protection Agency (EPA) regulates HCl under the Clean Air Act as a hazardous air pollutant. Facilities that emit HCl above certain thresholds must comply with Maximum Achievable Control Technology (MACT) standards, which mandate the use of specific control technologies and work practices to reduce emissions.
In addition to federal regulations, many states have implemented their own rules regarding HCl use and handling. These state-level regulations often mirror or exceed federal standards, addressing specific local concerns or industry practices. For instance, California's Proposition 65 requires businesses to provide warnings about significant exposures to chemicals that can cause cancer, birth defects, or other reproductive harm, including HCl.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of HCl and other chemicals. REACH requires manufacturers and importers to register substances and provide safety information, including exposure scenarios and risk management measures.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted, providing a standardized approach to communicating chemical hazards through labels and safety data sheets. This system ensures consistent hazard communication for HCl across different countries and regions.
Industry-specific regulations also play a crucial role in HCl management. For example, in the semiconductor industry, where ultra-pure HCl is used, additional guidelines are in place to ensure product quality and worker safety. Similarly, the food industry has specific regulations for the use of HCl in food processing applications.
As environmental and health concerns continue to evolve, regulations surrounding HCl are subject to periodic review and updates. Regulatory bodies often collaborate with industry stakeholders, research institutions, and public health organizations to refine standards based on the latest scientific evidence and technological advancements in industrial hygiene.
At the federal level in the United States, the Occupational Safety and Health Administration (OSHA) has set permissible exposure limits (PELs) for HCl. The current PEL is 5 parts per million (ppm) as a ceiling limit, meaning that exposure should never exceed this concentration during any part of the workday. OSHA also requires employers to provide appropriate personal protective equipment (PPE) and implement engineering controls to minimize worker exposure.
The Environmental Protection Agency (EPA) regulates HCl under the Clean Air Act as a hazardous air pollutant. Facilities that emit HCl above certain thresholds must comply with Maximum Achievable Control Technology (MACT) standards, which mandate the use of specific control technologies and work practices to reduce emissions.
In addition to federal regulations, many states have implemented their own rules regarding HCl use and handling. These state-level regulations often mirror or exceed federal standards, addressing specific local concerns or industry practices. For instance, California's Proposition 65 requires businesses to provide warnings about significant exposures to chemicals that can cause cancer, birth defects, or other reproductive harm, including HCl.
Internationally, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the use of HCl and other chemicals. REACH requires manufacturers and importers to register substances and provide safety information, including exposure scenarios and risk management measures.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) has been widely adopted, providing a standardized approach to communicating chemical hazards through labels and safety data sheets. This system ensures consistent hazard communication for HCl across different countries and regions.
Industry-specific regulations also play a crucial role in HCl management. For example, in the semiconductor industry, where ultra-pure HCl is used, additional guidelines are in place to ensure product quality and worker safety. Similarly, the food industry has specific regulations for the use of HCl in food processing applications.
As environmental and health concerns continue to evolve, regulations surrounding HCl are subject to periodic review and updates. Regulatory bodies often collaborate with industry stakeholders, research institutions, and public health organizations to refine standards based on the latest scientific evidence and technological advancements in industrial hygiene.
Environmental Impact HCl
The environmental impact of hydrochloric acid (HCl) is a critical consideration in industrial hygiene and safety practices. HCl, a strong and corrosive mineral acid, can have significant effects on both the natural environment and human health if not properly managed.
In aquatic ecosystems, the release of HCl can lead to severe acidification of water bodies. This pH reduction can disrupt the delicate balance of aquatic life, affecting fish populations, algae growth, and the overall biodiversity of affected areas. The acid can also mobilize heavy metals from sediments, potentially increasing their bioavailability and toxicity to aquatic organisms.
Soil contamination is another concern associated with HCl spills or improper disposal. The acid can alter soil chemistry, affecting nutrient availability and microbial activity. This can lead to reduced soil fertility and impaired plant growth in affected areas. In severe cases, it may result in long-term changes to local ecosystems and agricultural productivity.
Atmospheric emissions of HCl, often from industrial processes or accidental releases, contribute to air pollution and can form acid rain. When HCl vapor combines with moisture in the air, it can create acidic precipitation that damages vegetation, corrodes buildings and infrastructure, and further impacts water and soil quality over large areas.
The impact on human health is equally significant. Exposure to HCl vapors can cause respiratory irritation, coughing, and in severe cases, pulmonary edema. Skin contact with the acid can result in burns and tissue damage. Long-term occupational exposure may lead to dental erosion and chronic respiratory issues.
To mitigate these environmental and health risks, stringent regulations and best practices have been developed for the handling, storage, and disposal of HCl in industrial settings. These include the use of corrosion-resistant containment systems, proper ventilation in work areas, and the implementation of spill response protocols.
Advancements in industrial hygiene practices focus on minimizing HCl exposure and environmental release. This includes the development of closed-loop systems that reduce emissions, improved personal protective equipment for workers, and more efficient scrubbing technologies to neutralize HCl vapors before they are released into the atmosphere.
Monitoring and remediation strategies are crucial in areas where HCl contamination has occurred. Environmental scientists employ various techniques to assess and mitigate the impact, including soil and water testing, pH adjustment treatments, and long-term ecosystem restoration projects.
As industries continue to use HCl for various applications, ongoing research and development efforts are directed towards finding less hazardous alternatives or developing more environmentally friendly production and handling methods. This includes exploring bio-based acids and green chemistry approaches that could potentially reduce the overall environmental footprint of industrial processes relying on strong acids like HCl.
In aquatic ecosystems, the release of HCl can lead to severe acidification of water bodies. This pH reduction can disrupt the delicate balance of aquatic life, affecting fish populations, algae growth, and the overall biodiversity of affected areas. The acid can also mobilize heavy metals from sediments, potentially increasing their bioavailability and toxicity to aquatic organisms.
Soil contamination is another concern associated with HCl spills or improper disposal. The acid can alter soil chemistry, affecting nutrient availability and microbial activity. This can lead to reduced soil fertility and impaired plant growth in affected areas. In severe cases, it may result in long-term changes to local ecosystems and agricultural productivity.
Atmospheric emissions of HCl, often from industrial processes or accidental releases, contribute to air pollution and can form acid rain. When HCl vapor combines with moisture in the air, it can create acidic precipitation that damages vegetation, corrodes buildings and infrastructure, and further impacts water and soil quality over large areas.
The impact on human health is equally significant. Exposure to HCl vapors can cause respiratory irritation, coughing, and in severe cases, pulmonary edema. Skin contact with the acid can result in burns and tissue damage. Long-term occupational exposure may lead to dental erosion and chronic respiratory issues.
To mitigate these environmental and health risks, stringent regulations and best practices have been developed for the handling, storage, and disposal of HCl in industrial settings. These include the use of corrosion-resistant containment systems, proper ventilation in work areas, and the implementation of spill response protocols.
Advancements in industrial hygiene practices focus on minimizing HCl exposure and environmental release. This includes the development of closed-loop systems that reduce emissions, improved personal protective equipment for workers, and more efficient scrubbing technologies to neutralize HCl vapors before they are released into the atmosphere.
Monitoring and remediation strategies are crucial in areas where HCl contamination has occurred. Environmental scientists employ various techniques to assess and mitigate the impact, including soil and water testing, pH adjustment treatments, and long-term ecosystem restoration projects.
As industries continue to use HCl for various applications, ongoing research and development efforts are directed towards finding less hazardous alternatives or developing more environmentally friendly production and handling methods. This includes exploring bio-based acids and green chemistry approaches that could potentially reduce the overall environmental footprint of industrial processes relying on strong acids like HCl.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!