Hydrochloric Acid Across Technological Platforms
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Tech Background
Hydrochloric acid (HCl) has been a cornerstone in various industrial processes for over a century. Its discovery dates back to the early alchemists, but it wasn't until the Industrial Revolution that its full potential began to be realized. The development of the Leblanc process in the late 18th century marked a significant milestone, as it allowed for large-scale production of sodium carbonate, with HCl as a byproduct.
The evolution of HCl technology has been closely tied to advancements in chemical engineering and materials science. In the early 20th century, the introduction of the Chlor-alkali process revolutionized HCl production, making it more efficient and economically viable. This process, which involves the electrolysis of brine, remains the primary method for industrial-scale HCl production today.
Over the years, the applications of HCl have expanded dramatically. Initially used primarily in the production of fertilizers and dyes, it now plays a crucial role in diverse industries such as pharmaceuticals, food processing, and semiconductor manufacturing. The oil and gas industry, in particular, has seen a surge in HCl usage for hydraulic fracturing and well acidizing operations.
Recent technological trends in HCl research focus on improving production efficiency, reducing environmental impact, and exploring novel applications. Membrane-based technologies are being developed to enhance the purity of HCl and minimize waste. Additionally, there is growing interest in the use of HCl in hydrogen production, aligning with the global push towards clean energy solutions.
The current technological landscape surrounding HCl is characterized by a drive towards sustainability and circular economy principles. Researchers are exploring methods to recover and recycle HCl from industrial waste streams, aiming to reduce the environmental footprint of its production and use. Simultaneously, efforts are being made to develop greener alternatives for processes traditionally reliant on HCl.
Looking ahead, the technological goals for HCl research include developing more energy-efficient production methods, improving handling and storage safety, and expanding its role in emerging fields such as nanotechnology and advanced materials processing. There is also a growing emphasis on understanding and mitigating the long-term environmental impacts of HCl use, particularly in sensitive ecosystems.
The evolution of HCl technology has been closely tied to advancements in chemical engineering and materials science. In the early 20th century, the introduction of the Chlor-alkali process revolutionized HCl production, making it more efficient and economically viable. This process, which involves the electrolysis of brine, remains the primary method for industrial-scale HCl production today.
Over the years, the applications of HCl have expanded dramatically. Initially used primarily in the production of fertilizers and dyes, it now plays a crucial role in diverse industries such as pharmaceuticals, food processing, and semiconductor manufacturing. The oil and gas industry, in particular, has seen a surge in HCl usage for hydraulic fracturing and well acidizing operations.
Recent technological trends in HCl research focus on improving production efficiency, reducing environmental impact, and exploring novel applications. Membrane-based technologies are being developed to enhance the purity of HCl and minimize waste. Additionally, there is growing interest in the use of HCl in hydrogen production, aligning with the global push towards clean energy solutions.
The current technological landscape surrounding HCl is characterized by a drive towards sustainability and circular economy principles. Researchers are exploring methods to recover and recycle HCl from industrial waste streams, aiming to reduce the environmental footprint of its production and use. Simultaneously, efforts are being made to develop greener alternatives for processes traditionally reliant on HCl.
Looking ahead, the technological goals for HCl research include developing more energy-efficient production methods, improving handling and storage safety, and expanding its role in emerging fields such as nanotechnology and advanced materials processing. There is also a growing emphasis on understanding and mitigating the long-term environmental impacts of HCl use, particularly in sensitive ecosystems.
Market Demand Analysis
The global market for hydrochloric acid has shown steady growth in recent years, driven by its widespread applications across various industries. The chemical sector remains the largest consumer of hydrochloric acid, utilizing it in the production of various chemicals, including PVC, polyurethane, and other chlorinated compounds. The steel industry also contributes significantly to the demand, using hydrochloric acid for pickling and descaling processes.
In the oil and gas sector, hydrochloric acid plays a crucial role in well acidizing and hydraulic fracturing operations, enhancing oil recovery rates. This application has seen increased demand, particularly in regions with expanding shale gas exploration activities. The food industry utilizes hydrochloric acid in the production of various food additives and in the processing of corn syrup, contributing to the overall market growth.
The water treatment industry has emerged as a key growth driver for hydrochloric acid demand. As global concerns over water scarcity and quality intensify, the use of hydrochloric acid in water purification and pH adjustment processes has increased significantly. This trend is expected to continue, especially in developing regions facing water infrastructure challenges.
Geographically, Asia-Pacific dominates the hydrochloric acid market, with China being the largest producer and consumer. The region's rapid industrialization, particularly in countries like India and Southeast Asian nations, has fueled the demand for hydrochloric acid across various applications. North America and Europe follow, with stable demand from established industrial sectors.
The market for high-purity hydrochloric acid, used in semiconductor manufacturing and pharmaceutical production, has shown particularly strong growth. This segment is driven by the expanding electronics industry and the increasing demand for advanced medical treatments and drugs.
Environmental regulations have influenced market dynamics, with a growing emphasis on sustainable production methods and recycling of hydrochloric acid. This has led to innovations in production technologies and increased focus on closed-loop systems in industrial applications.
Looking ahead, the global hydrochloric acid market is projected to continue its growth trajectory. Emerging applications in renewable energy technologies, such as the production of solar panels and batteries, are expected to create new demand streams. Additionally, the ongoing research into novel uses of hydrochloric acid in materials science and nanotechnology could potentially open up new market opportunities in the coming years.
In the oil and gas sector, hydrochloric acid plays a crucial role in well acidizing and hydraulic fracturing operations, enhancing oil recovery rates. This application has seen increased demand, particularly in regions with expanding shale gas exploration activities. The food industry utilizes hydrochloric acid in the production of various food additives and in the processing of corn syrup, contributing to the overall market growth.
The water treatment industry has emerged as a key growth driver for hydrochloric acid demand. As global concerns over water scarcity and quality intensify, the use of hydrochloric acid in water purification and pH adjustment processes has increased significantly. This trend is expected to continue, especially in developing regions facing water infrastructure challenges.
Geographically, Asia-Pacific dominates the hydrochloric acid market, with China being the largest producer and consumer. The region's rapid industrialization, particularly in countries like India and Southeast Asian nations, has fueled the demand for hydrochloric acid across various applications. North America and Europe follow, with stable demand from established industrial sectors.
The market for high-purity hydrochloric acid, used in semiconductor manufacturing and pharmaceutical production, has shown particularly strong growth. This segment is driven by the expanding electronics industry and the increasing demand for advanced medical treatments and drugs.
Environmental regulations have influenced market dynamics, with a growing emphasis on sustainable production methods and recycling of hydrochloric acid. This has led to innovations in production technologies and increased focus on closed-loop systems in industrial applications.
Looking ahead, the global hydrochloric acid market is projected to continue its growth trajectory. Emerging applications in renewable energy technologies, such as the production of solar panels and batteries, are expected to create new demand streams. Additionally, the ongoing research into novel uses of hydrochloric acid in materials science and nanotechnology could potentially open up new market opportunities in the coming years.
HCl Tech Challenges
The development of hydrochloric acid (HCl) technology faces several significant challenges across various technological platforms. One of the primary obstacles is the corrosive nature of HCl, which poses substantial risks to equipment and infrastructure. This corrosivity necessitates the use of specialized materials and coatings, driving up costs and limiting the scalability of HCl-related processes.
Environmental concerns present another major challenge in HCl technology. The production and handling of HCl often result in emissions of chlorine and other potentially harmful substances. Stringent regulations and increasing public awareness of environmental issues have put pressure on industries to develop cleaner, more sustainable methods for HCl production and utilization.
Safety considerations remain a critical challenge in HCl technology. The hazardous nature of HCl requires robust safety protocols, specialized training for personnel, and advanced containment systems. These safety requirements add complexity and cost to HCl-related operations, particularly in large-scale industrial applications.
The transportation and storage of HCl pose significant logistical challenges. Due to its corrosive properties, specialized containers and handling equipment are necessary, which increases transportation costs and limits the feasibility of long-distance supply chains. This challenge is particularly acute in regions with limited infrastructure or harsh environmental conditions.
In the realm of HCl recycling and recovery, technological limitations persist. While recycling HCl is crucial for sustainability and cost-effectiveness, current methods often struggle with efficiency and purity issues. Developing more effective recycling technologies that can handle diverse waste streams remains an ongoing challenge.
The integration of HCl technology with emerging fields such as nanotechnology and advanced materials science presents both opportunities and challenges. While these fields offer potential for innovative HCl applications, they also require overcoming compatibility issues and developing new methodologies for handling HCl at microscopic scales.
Lastly, the challenge of finding alternatives to HCl in various applications continues to drive research and development efforts. In some industries, there is a push to reduce or eliminate HCl usage due to its associated risks and environmental impact. However, finding suitable replacements that match HCl's effectiveness and cost-efficiency remains a significant technological hurdle.
Environmental concerns present another major challenge in HCl technology. The production and handling of HCl often result in emissions of chlorine and other potentially harmful substances. Stringent regulations and increasing public awareness of environmental issues have put pressure on industries to develop cleaner, more sustainable methods for HCl production and utilization.
Safety considerations remain a critical challenge in HCl technology. The hazardous nature of HCl requires robust safety protocols, specialized training for personnel, and advanced containment systems. These safety requirements add complexity and cost to HCl-related operations, particularly in large-scale industrial applications.
The transportation and storage of HCl pose significant logistical challenges. Due to its corrosive properties, specialized containers and handling equipment are necessary, which increases transportation costs and limits the feasibility of long-distance supply chains. This challenge is particularly acute in regions with limited infrastructure or harsh environmental conditions.
In the realm of HCl recycling and recovery, technological limitations persist. While recycling HCl is crucial for sustainability and cost-effectiveness, current methods often struggle with efficiency and purity issues. Developing more effective recycling technologies that can handle diverse waste streams remains an ongoing challenge.
The integration of HCl technology with emerging fields such as nanotechnology and advanced materials science presents both opportunities and challenges. While these fields offer potential for innovative HCl applications, they also require overcoming compatibility issues and developing new methodologies for handling HCl at microscopic scales.
Lastly, the challenge of finding alternatives to HCl in various applications continues to drive research and development efforts. In some industries, there is a push to reduce or eliminate HCl usage due to its associated risks and environmental impact. However, finding suitable replacements that match HCl's effectiveness and cost-efficiency remains a significant technological hurdle.
Current HCl Solutions
01 Production and purification of hydrochloric acid
Various methods and processes for producing and purifying hydrochloric acid, including techniques for improving yield and quality. This may involve specific reaction conditions, catalysts, or separation methods to obtain high-purity hydrochloric acid efficiently.- Production and purification of hydrochloric acid: Various methods and processes for producing and purifying hydrochloric acid, including techniques for improving yield and quality. This may involve specific reaction conditions, catalysts, or separation methods to obtain high-purity hydrochloric acid for industrial applications.
- Applications of hydrochloric acid in chemical processes: Hydrochloric acid is used in numerous chemical processes across different industries. This includes its role as a reagent, catalyst, or pH adjuster in various reactions and manufacturing processes, such as in the production of other chemicals, pharmaceuticals, or materials.
- Handling and storage of hydrochloric acid: Specialized equipment and methods for safely handling, storing, and transporting hydrochloric acid. This includes corrosion-resistant materials, safety measures, and containment systems designed to manage the hazardous nature of the acid in industrial settings.
- Environmental and waste management related to hydrochloric acid: Techniques and systems for managing hydrochloric acid waste, reducing environmental impact, and recovering or recycling the acid from industrial processes. This may include treatment methods, neutralization processes, or closed-loop systems to minimize acid discharge.
- Analytical methods involving hydrochloric acid: Use of hydrochloric acid in various analytical and testing procedures, including its role in sample preparation, extraction processes, or as a reagent in chemical analysis. This covers applications in fields such as environmental monitoring, quality control, and scientific research.
02 Applications of hydrochloric acid in chemical processes
Hydrochloric acid is used in numerous chemical processes and industrial applications. This includes its role as a reagent in various reactions, as a pH adjuster, and in the production of other chemicals. The acid's properties make it valuable in diverse fields such as metallurgy, water treatment, and pharmaceuticals.Expand Specific Solutions03 Handling and storage of hydrochloric acid
Specialized equipment and methods for safely handling, storing, and transporting hydrochloric acid. This includes corrosion-resistant materials, safety measures, and containment systems designed to manage the acid's corrosive nature and potential hazards.Expand Specific Solutions04 Recovery and recycling of hydrochloric acid
Techniques for recovering and recycling hydrochloric acid from industrial processes or waste streams. These methods aim to reduce environmental impact and improve resource efficiency by reusing the acid in various applications.Expand Specific Solutions05 Environmental and safety considerations
Approaches to mitigate the environmental impact and safety risks associated with hydrochloric acid use. This includes emission control technologies, neutralization methods, and safety protocols for handling and disposal of the acid in industrial settings.Expand Specific Solutions
Key Industry Players
The research on hydrochloric acid across technological platforms is in a mature stage, with a diverse competitive landscape. The market size is substantial, driven by widespread industrial applications. Established players like Covestro, AkzoNobel, and Dorf Ketal Chemicals dominate the industry, while academic institutions such as MIT, Harvard, and Tsinghua University contribute to technological advancements. The field also sees participation from specialized companies like Jiangyin Jianghua Micro-electronics Materials and KriSan Biotech, focusing on high-purity electronic-grade hydrochloric acid. This mix of industry giants, academic research, and niche players indicates a well-developed ecosystem with ongoing innovation potential.
Covestro Deutschland AG
Technical Solution: Covestro has developed innovative solutions for hydrochloric acid management in their production processes. They have implemented a closed-loop recycling system that captures and purifies HCl byproducts for reuse in other chemical processes[1]. This approach not only reduces waste but also improves resource efficiency. Additionally, Covestro has invested in advanced membrane technology for HCl recovery, which allows for the separation of HCl from other process streams with high purity[2]. The company has also developed corrosion-resistant materials and coatings that can withstand exposure to hydrochloric acid, extending the lifespan of equipment used in HCl-related processes[3].
Strengths: Efficient resource utilization, reduced environmental impact, and improved process economics. Weaknesses: High initial investment costs for implementing recycling systems and potential complexity in integrating new technologies into existing processes.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has focused on developing sustainable solutions for hydrochloric acid production and utilization. They have pioneered a membrane electrolysis process that produces high-purity hydrochloric acid from industrial waste streams containing chloride ions[4]. This technology not only reduces waste but also creates a valuable product from what would otherwise be a disposal challenge. Akzo Nobel has also developed advanced catalysts for HCl oxidation, enabling the production of chlorine gas from HCl, which can then be used in various chemical processes[5]. Furthermore, the company has invested in safety technologies for HCl handling, including advanced containment systems and real-time monitoring solutions to prevent leaks and ensure worker safety[6].
Strengths: Innovative waste-to-product conversion, improved safety measures, and circular economy approach. Weaknesses: Dependence on specific waste streams for HCl production and potential regulatory challenges in implementing new chemical processes.
HCl Tech Innovations
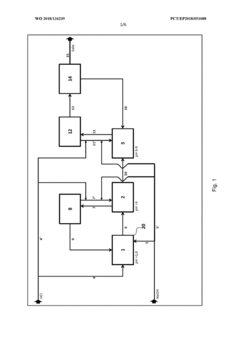
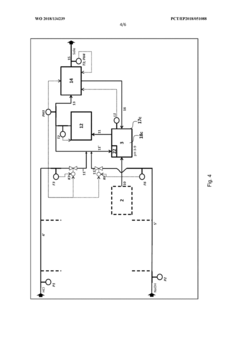
Method for flexibly controlling the use of hydrochloric acid from chemical production
PatentWO2018134239A1
Innovation
- A flexible control process for hydrochloric acid management involves neutralizing hydrochloric acid with concentrated alkali, specifically sodium hydroxide, in a multi-stage continuous process that adjusts pH values and compensates for flow and concentration variations, allowing for efficient handling and recycling of hydrochloric acid even when traditional acceptance points are unavailable.
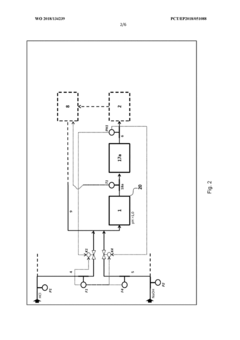
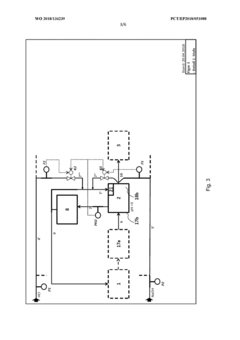
Method for flexibly controlling the use of hydrochloric acid from chemical production
PatentInactiveEP3571157A1
Innovation
- A multi-stage neutralization process using concentrated alkali, specifically sodium hydroxide, to control the pH value of hydrochloric acid in a continuously automated mode, allowing for flexible handling and recycling of hydrochloric acid, even when traditional acceptance points are unavailable, by employing a three-stage neutralization system with cooled recirculated partial streams to manage reaction heat and maintain target pH values.
Environmental Impact
The environmental impact of hydrochloric acid (HCl) across technological platforms is a critical consideration in various industries. HCl, a strong and corrosive acid, poses significant risks to ecosystems and human health if not properly managed. Its production, transportation, and use can lead to air, water, and soil pollution if appropriate safeguards are not in place.
In industrial processes, HCl emissions can contribute to acid rain formation, affecting vegetation, aquatic life, and infrastructure. The acid's corrosive nature can damage buildings, monuments, and metal structures, leading to increased maintenance costs and potential safety hazards. Moreover, accidental releases or spills can have severe consequences on local ecosystems, causing immediate harm to flora and fauna.
Water pollution is another major concern associated with HCl. Improper disposal or accidental discharge into water bodies can drastically alter pH levels, devastating aquatic ecosystems. This can lead to fish kills, disruption of food chains, and long-term ecological imbalances. Additionally, contaminated water sources may become unsuitable for human consumption or agricultural use, necessitating costly treatment processes.
The production of HCl often involves energy-intensive processes, contributing to greenhouse gas emissions and climate change. Efforts to reduce the carbon footprint of HCl production have led to the development of more efficient manufacturing techniques and the exploration of alternative production methods.
Occupational health and safety is a crucial aspect of HCl's environmental impact. Workers in industries using HCl face risks of exposure, which can cause severe respiratory issues, skin burns, and eye damage. Implementing robust safety protocols, proper handling procedures, and personal protective equipment is essential to mitigate these risks.
Regulatory frameworks play a vital role in managing HCl's environmental impact. Many countries have implemented strict regulations governing its production, transportation, use, and disposal. These regulations often require industries to adopt best practices, install emission control technologies, and implement comprehensive waste management systems.
Technological advancements have led to improved containment, handling, and recycling methods for HCl. Closed-loop systems, which recycle and reuse the acid within industrial processes, have gained popularity as they minimize environmental release and reduce raw material consumption. Additionally, innovative neutralization techniques and waste treatment processes have been developed to render HCl less harmful before disposal.
As industries continue to rely on HCl for various applications, ongoing research focuses on developing more environmentally friendly alternatives or modifying processes to reduce its usage. This includes exploring bio-based acids, implementing green chemistry principles, and optimizing industrial processes to minimize HCl consumption and waste generation.
In industrial processes, HCl emissions can contribute to acid rain formation, affecting vegetation, aquatic life, and infrastructure. The acid's corrosive nature can damage buildings, monuments, and metal structures, leading to increased maintenance costs and potential safety hazards. Moreover, accidental releases or spills can have severe consequences on local ecosystems, causing immediate harm to flora and fauna.
Water pollution is another major concern associated with HCl. Improper disposal or accidental discharge into water bodies can drastically alter pH levels, devastating aquatic ecosystems. This can lead to fish kills, disruption of food chains, and long-term ecological imbalances. Additionally, contaminated water sources may become unsuitable for human consumption or agricultural use, necessitating costly treatment processes.
The production of HCl often involves energy-intensive processes, contributing to greenhouse gas emissions and climate change. Efforts to reduce the carbon footprint of HCl production have led to the development of more efficient manufacturing techniques and the exploration of alternative production methods.
Occupational health and safety is a crucial aspect of HCl's environmental impact. Workers in industries using HCl face risks of exposure, which can cause severe respiratory issues, skin burns, and eye damage. Implementing robust safety protocols, proper handling procedures, and personal protective equipment is essential to mitigate these risks.
Regulatory frameworks play a vital role in managing HCl's environmental impact. Many countries have implemented strict regulations governing its production, transportation, use, and disposal. These regulations often require industries to adopt best practices, install emission control technologies, and implement comprehensive waste management systems.
Technological advancements have led to improved containment, handling, and recycling methods for HCl. Closed-loop systems, which recycle and reuse the acid within industrial processes, have gained popularity as they minimize environmental release and reduce raw material consumption. Additionally, innovative neutralization techniques and waste treatment processes have been developed to render HCl less harmful before disposal.
As industries continue to rely on HCl for various applications, ongoing research focuses on developing more environmentally friendly alternatives or modifying processes to reduce its usage. This includes exploring bio-based acids, implementing green chemistry principles, and optimizing industrial processes to minimize HCl consumption and waste generation.
Safety Regulations
Safety regulations surrounding the use and handling of hydrochloric acid are critical across various technological platforms due to its corrosive and hazardous nature. These regulations are designed to protect workers, the environment, and the general public from potential harm associated with this widely used industrial chemical.
In the United States, the Occupational Safety and Health Administration (OSHA) has established comprehensive guidelines for the safe handling of hydrochloric acid in workplace settings. These regulations mandate the use of personal protective equipment (PPE), including chemical-resistant gloves, goggles, and face shields when working with hydrochloric acid. Additionally, OSHA requires proper labeling of containers, adequate ventilation in work areas, and the availability of emergency eyewash stations and safety showers.
The Environmental Protection Agency (EPA) regulates the storage, transportation, and disposal of hydrochloric acid to prevent environmental contamination. Strict protocols are in place for the containment and cleanup of spills, with specific requirements for reporting incidents to relevant authorities. The EPA also sets limits on the release of hydrochloric acid into the air and water, necessitating the implementation of emission control technologies in industrial processes.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. This system ensures consistent labeling and safety data sheets for hydrochloric acid across different countries, facilitating safer international trade and use.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of hydrochloric acid. REACH requires manufacturers and importers to register chemical substances and provide detailed safety information, including exposure scenarios and risk management measures.
For transportation, the International Maritime Dangerous Goods (IMDG) Code and the International Air Transport Association (IATA) Dangerous Goods Regulations set forth specific requirements for shipping hydrochloric acid by sea and air, respectively. These regulations cover packaging, labeling, documentation, and handling procedures to ensure safe transport.
In research and development settings, additional safety protocols are often implemented. These may include specialized training for laboratory personnel, strict inventory control measures, and the use of fume hoods or other containment systems when working with hydrochloric acid.
As technology advances, safety regulations continue to evolve. Emerging areas of focus include the development of safer alternatives to hydrochloric acid in certain applications, improved risk assessment methodologies, and the integration of digital technologies for real-time monitoring of chemical handling and storage conditions.
In the United States, the Occupational Safety and Health Administration (OSHA) has established comprehensive guidelines for the safe handling of hydrochloric acid in workplace settings. These regulations mandate the use of personal protective equipment (PPE), including chemical-resistant gloves, goggles, and face shields when working with hydrochloric acid. Additionally, OSHA requires proper labeling of containers, adequate ventilation in work areas, and the availability of emergency eyewash stations and safety showers.
The Environmental Protection Agency (EPA) regulates the storage, transportation, and disposal of hydrochloric acid to prevent environmental contamination. Strict protocols are in place for the containment and cleanup of spills, with specific requirements for reporting incidents to relevant authorities. The EPA also sets limits on the release of hydrochloric acid into the air and water, necessitating the implementation of emission control technologies in industrial processes.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. This system ensures consistent labeling and safety data sheets for hydrochloric acid across different countries, facilitating safer international trade and use.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of hydrochloric acid. REACH requires manufacturers and importers to register chemical substances and provide detailed safety information, including exposure scenarios and risk management measures.
For transportation, the International Maritime Dangerous Goods (IMDG) Code and the International Air Transport Association (IATA) Dangerous Goods Regulations set forth specific requirements for shipping hydrochloric acid by sea and air, respectively. These regulations cover packaging, labeling, documentation, and handling procedures to ensure safe transport.
In research and development settings, additional safety protocols are often implemented. These may include specialized training for laboratory personnel, strict inventory control measures, and the use of fume hoods or other containment systems when working with hydrochloric acid.
As technology advances, safety regulations continue to evolve. Emerging areas of focus include the development of safer alternatives to hydrochloric acid in certain applications, improved risk assessment methodologies, and the integration of digital technologies for real-time monitoring of chemical handling and storage conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!