How to Ensure Compliance When Using Hydrochloric Acid?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Compliance Background and Objectives
Hydrochloric acid (HCl) is a widely used chemical compound in various industries, including manufacturing, pharmaceuticals, and water treatment. Its corrosive nature and potential health hazards necessitate strict compliance measures to ensure safe handling and usage. The evolution of HCl compliance regulations has been driven by increasing awareness of workplace safety and environmental protection.
The primary objective of HCl compliance is to minimize risks associated with its use while maximizing its benefits in industrial processes. This involves implementing comprehensive safety protocols, proper storage and handling procedures, and effective waste management strategies. Compliance measures have evolved from basic personal protective equipment requirements to sophisticated containment systems and real-time monitoring technologies.
Over the years, regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) have played crucial roles in shaping HCl compliance standards. These agencies have established guidelines for permissible exposure limits, emergency response procedures, and environmental release prevention. The development of these regulations has been influenced by scientific research on the long-term effects of HCl exposure and advancements in safety technologies.
The technological trend in HCl compliance has shifted towards automation and digital monitoring systems. Smart sensors and IoT-enabled devices are increasingly being employed to detect HCl leaks, monitor air quality, and alert personnel to potential hazards in real-time. This trend is expected to continue, with artificial intelligence and machine learning algorithms being integrated to predict and prevent compliance issues before they occur.
Another significant trend is the focus on sustainable practices in HCl usage. Industries are exploring ways to reduce HCl consumption, recycle waste products, and develop less hazardous alternatives. This aligns with broader environmental goals and reflects the growing emphasis on corporate social responsibility in chemical management.
The global nature of supply chains and manufacturing processes has led to the harmonization of HCl compliance standards across different countries and regions. International organizations such as the United Nations and the World Health Organization have contributed to establishing universal guidelines for the safe transport and handling of hydrochloric acid.
As we look towards the future, the expected technological advancements in HCl compliance include the development of more resistant materials for containment, improved neutralization techniques, and enhanced personal protective equipment. These innovations aim to further reduce the risks associated with HCl use and improve overall safety standards in industrial settings.
The primary objective of HCl compliance is to minimize risks associated with its use while maximizing its benefits in industrial processes. This involves implementing comprehensive safety protocols, proper storage and handling procedures, and effective waste management strategies. Compliance measures have evolved from basic personal protective equipment requirements to sophisticated containment systems and real-time monitoring technologies.
Over the years, regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) have played crucial roles in shaping HCl compliance standards. These agencies have established guidelines for permissible exposure limits, emergency response procedures, and environmental release prevention. The development of these regulations has been influenced by scientific research on the long-term effects of HCl exposure and advancements in safety technologies.
The technological trend in HCl compliance has shifted towards automation and digital monitoring systems. Smart sensors and IoT-enabled devices are increasingly being employed to detect HCl leaks, monitor air quality, and alert personnel to potential hazards in real-time. This trend is expected to continue, with artificial intelligence and machine learning algorithms being integrated to predict and prevent compliance issues before they occur.
Another significant trend is the focus on sustainable practices in HCl usage. Industries are exploring ways to reduce HCl consumption, recycle waste products, and develop less hazardous alternatives. This aligns with broader environmental goals and reflects the growing emphasis on corporate social responsibility in chemical management.
The global nature of supply chains and manufacturing processes has led to the harmonization of HCl compliance standards across different countries and regions. International organizations such as the United Nations and the World Health Organization have contributed to establishing universal guidelines for the safe transport and handling of hydrochloric acid.
As we look towards the future, the expected technological advancements in HCl compliance include the development of more resistant materials for containment, improved neutralization techniques, and enhanced personal protective equipment. These innovations aim to further reduce the risks associated with HCl use and improve overall safety standards in industrial settings.
Market Analysis for HCl Applications
The global market for hydrochloric acid (HCl) applications continues to expand, driven by diverse industrial sectors and technological advancements. The chemical industry remains the largest consumer of HCl, utilizing it in various processes such as the production of vinyl chloride monomer (VCM) and other chlorinated compounds. Steel pickling and oil well acidizing represent significant segments, with the former benefiting from the growth in construction and automotive industries.
In recent years, the electronics sector has emerged as a notable consumer of high-purity HCl, particularly in the manufacturing of semiconductors and printed circuit boards. This trend aligns with the increasing demand for electronic devices and the expansion of 5G networks worldwide. The water treatment industry also contributes substantially to HCl demand, employing it for pH adjustment and chlorine dioxide generation.
Geographically, Asia-Pacific dominates the HCl market, with China leading in both production and consumption. The region's rapid industrialization and urbanization continue to fuel demand across various applications. North America and Europe maintain stable markets, primarily driven by established chemical and manufacturing sectors.
Environmental regulations and sustainability concerns are shaping market dynamics. There is a growing emphasis on recycling and recovering HCl from industrial processes, particularly in developed economies. This trend is expected to influence production methods and supply chains in the coming years.
The food industry represents a niche but growing market for HCl, where it is used in food processing and as a food additive. Pharmaceutical applications, including drug synthesis and formulation, also contribute to market growth, albeit on a smaller scale compared to industrial uses.
Market analysts project a compound annual growth rate (CAGR) for the global HCl market in the range of 3-5% over the next five years. This growth is attributed to the expanding industrial base in developing countries and the continuous innovation in high-purity applications. However, fluctuations in raw material prices and stringent environmental regulations pose challenges to market expansion.
Emerging applications in renewable energy sectors, such as the production of polysilicon for solar panels, are expected to create new opportunities for HCl suppliers. Additionally, the development of more efficient and environmentally friendly production methods is likely to reshape the competitive landscape in the medium to long term.
In recent years, the electronics sector has emerged as a notable consumer of high-purity HCl, particularly in the manufacturing of semiconductors and printed circuit boards. This trend aligns with the increasing demand for electronic devices and the expansion of 5G networks worldwide. The water treatment industry also contributes substantially to HCl demand, employing it for pH adjustment and chlorine dioxide generation.
Geographically, Asia-Pacific dominates the HCl market, with China leading in both production and consumption. The region's rapid industrialization and urbanization continue to fuel demand across various applications. North America and Europe maintain stable markets, primarily driven by established chemical and manufacturing sectors.
Environmental regulations and sustainability concerns are shaping market dynamics. There is a growing emphasis on recycling and recovering HCl from industrial processes, particularly in developed economies. This trend is expected to influence production methods and supply chains in the coming years.
The food industry represents a niche but growing market for HCl, where it is used in food processing and as a food additive. Pharmaceutical applications, including drug synthesis and formulation, also contribute to market growth, albeit on a smaller scale compared to industrial uses.
Market analysts project a compound annual growth rate (CAGR) for the global HCl market in the range of 3-5% over the next five years. This growth is attributed to the expanding industrial base in developing countries and the continuous innovation in high-purity applications. However, fluctuations in raw material prices and stringent environmental regulations pose challenges to market expansion.
Emerging applications in renewable energy sectors, such as the production of polysilicon for solar panels, are expected to create new opportunities for HCl suppliers. Additionally, the development of more efficient and environmentally friendly production methods is likely to reshape the competitive landscape in the medium to long term.
Current Challenges in HCl Handling
The handling of hydrochloric acid (HCl) presents several significant challenges in ensuring compliance with safety regulations and environmental standards. One of the primary concerns is the corrosive nature of HCl, which poses risks to both personnel and equipment. Proper storage and containment systems are crucial to prevent leaks and spills, but maintaining these systems can be complex and costly. Corrosion-resistant materials must be used throughout the handling process, from storage tanks to transfer pipelines, requiring regular inspections and maintenance to ensure integrity.
Worker safety is another major challenge in HCl handling. Exposure to HCl vapors or liquid can cause severe respiratory issues, skin burns, and eye damage. Implementing comprehensive personal protective equipment (PPE) protocols and ensuring strict adherence to safety procedures are ongoing challenges for organizations. Training programs must be regularly updated to reflect the latest safety standards and best practices, which can be resource-intensive.
Environmental compliance presents additional hurdles in HCl handling. Strict regulations govern the disposal of HCl and its byproducts, necessitating sophisticated waste management systems. Neutralization processes must be carefully controlled to prevent the release of harmful substances into the environment. Monitoring and reporting emissions and effluents in accordance with local and national regulations require advanced tracking systems and expertise.
The transportation of HCl is another area fraught with compliance challenges. Stringent regulations govern the packaging, labeling, and shipping of HCl, with requirements varying across different jurisdictions. Ensuring that all transportation activities meet these diverse standards demands meticulous planning and documentation.
Emergency response preparedness is a critical aspect of HCl handling compliance. Organizations must develop and maintain comprehensive emergency plans, including spill response procedures and evacuation protocols. Regular drills and training exercises are necessary to ensure readiness, but coordinating these activities across large facilities or multiple sites can be logistically challenging.
Keeping up with evolving regulations and industry standards poses an ongoing challenge for HCl handlers. Regulatory frameworks are subject to frequent updates, requiring constant vigilance and adaptability. Implementing changes to comply with new standards often involves significant investments in equipment upgrades, process modifications, and staff training.
Lastly, the documentation and record-keeping requirements associated with HCl handling compliance are extensive. Maintaining accurate and up-to-date records of safety protocols, incident reports, training logs, and environmental monitoring data is crucial for demonstrating compliance during audits and inspections. Developing robust systems to manage this information effectively while ensuring data integrity and accessibility remains a persistent challenge for many organizations.
Worker safety is another major challenge in HCl handling. Exposure to HCl vapors or liquid can cause severe respiratory issues, skin burns, and eye damage. Implementing comprehensive personal protective equipment (PPE) protocols and ensuring strict adherence to safety procedures are ongoing challenges for organizations. Training programs must be regularly updated to reflect the latest safety standards and best practices, which can be resource-intensive.
Environmental compliance presents additional hurdles in HCl handling. Strict regulations govern the disposal of HCl and its byproducts, necessitating sophisticated waste management systems. Neutralization processes must be carefully controlled to prevent the release of harmful substances into the environment. Monitoring and reporting emissions and effluents in accordance with local and national regulations require advanced tracking systems and expertise.
The transportation of HCl is another area fraught with compliance challenges. Stringent regulations govern the packaging, labeling, and shipping of HCl, with requirements varying across different jurisdictions. Ensuring that all transportation activities meet these diverse standards demands meticulous planning and documentation.
Emergency response preparedness is a critical aspect of HCl handling compliance. Organizations must develop and maintain comprehensive emergency plans, including spill response procedures and evacuation protocols. Regular drills and training exercises are necessary to ensure readiness, but coordinating these activities across large facilities or multiple sites can be logistically challenging.
Keeping up with evolving regulations and industry standards poses an ongoing challenge for HCl handlers. Regulatory frameworks are subject to frequent updates, requiring constant vigilance and adaptability. Implementing changes to comply with new standards often involves significant investments in equipment upgrades, process modifications, and staff training.
Lastly, the documentation and record-keeping requirements associated with HCl handling compliance are extensive. Maintaining accurate and up-to-date records of safety protocols, incident reports, training logs, and environmental monitoring data is crucial for demonstrating compliance during audits and inspections. Developing robust systems to manage this information effectively while ensuring data integrity and accessibility remains a persistent challenge for many organizations.
Existing HCl Compliance Solutions
01 Storage and handling of hydrochloric acid
Proper storage and handling of hydrochloric acid is crucial for compliance. This includes using corrosion-resistant materials for storage tanks and containers, implementing safety measures for transportation, and ensuring proper ventilation in storage areas to prevent the accumulation of fumes.- Storage and handling of hydrochloric acid: Proper storage and handling of hydrochloric acid is crucial for compliance. This includes using corrosion-resistant materials for storage tanks and containers, implementing safety measures for transportation, and ensuring proper ventilation in storage areas to prevent the accumulation of fumes.
- Waste treatment and neutralization: Compliance with environmental regulations requires proper treatment and neutralization of hydrochloric acid waste. This may involve using neutralizing agents, implementing scrubbing systems to remove acid fumes, and ensuring proper disposal of neutralized waste in accordance with local regulations.
- Safety equipment and protective measures: Ensuring worker safety is a key aspect of hydrochloric acid compliance. This includes providing appropriate personal protective equipment (PPE), installing emergency showers and eyewash stations, and implementing proper training programs for handling and emergency procedures.
- Monitoring and control systems: Implementing monitoring and control systems is essential for maintaining compliance in hydrochloric acid handling. This may include pH monitoring, leak detection systems, and automated shut-off valves to prevent spills or releases.
- Purification and quality control: Ensuring the purity and quality of hydrochloric acid is important for compliance in various industries. This involves implementing purification processes, such as distillation or membrane separation, and establishing quality control measures to meet industry standards and regulatory requirements.
02 Waste treatment and neutralization
Compliance with environmental regulations requires proper treatment and neutralization of hydrochloric acid waste. This involves implementing processes for neutralizing the acid before disposal, using appropriate treatment methods, and ensuring that effluents meet regulatory standards for pH and chemical composition.Expand Specific Solutions03 Safety equipment and protective measures
Ensuring worker safety is a key aspect of hydrochloric acid compliance. This includes providing appropriate personal protective equipment (PPE), installing emergency showers and eyewash stations, and implementing proper training programs for handling and emergency response procedures.Expand Specific Solutions04 Monitoring and control systems
Implementing robust monitoring and control systems is essential for maintaining compliance in hydrochloric acid handling. This involves using sensors and automated systems to monitor acid concentration, pH levels, and potential leaks, as well as implementing process control measures to prevent accidents and ensure consistent quality.Expand Specific Solutions05 Purification and quality control
Ensuring the purity and quality of hydrochloric acid is important for compliance in various industries. This includes implementing purification processes to remove impurities, conducting regular quality control tests, and maintaining proper documentation of acid specifications and batch information.Expand Specific Solutions
Key Players in HCl Industry
The competitive landscape for ensuring compliance when using hydrochloric acid is characterized by a mature market with established players and growing regulatory pressures. The global market for hydrochloric acid compliance solutions is expanding, driven by increasing industrial applications and stringent environmental regulations. Key players like Abbott Laboratories, AbbVie, and Vertex Pharmaceuticals have developed advanced compliance systems, leveraging their extensive experience in pharmaceutical manufacturing. Companies such as Arkema France SA and Covestro Deutschland AG are focusing on innovative safety technologies and sustainable practices. The market is seeing a trend towards integrated compliance management solutions, with firms like Schlumberger and Halliburton Energy Services incorporating advanced monitoring and control systems in their operations to ensure adherence to safety standards and environmental regulations.
Abbott Laboratories
Technical Solution: Abbott Laboratories has implemented a multi-faceted approach to ensure compliance when using hydrochloric acid in pharmaceutical manufacturing. Their strategy includes the use of advanced containment systems with multiple redundancies to prevent accidental releases. Abbott has developed proprietary software for tracking acid usage, storage conditions, and disposal processes, ensuring full traceability and regulatory compliance. They employ automated neutralization systems that can rapidly respond to potential spills or excess acid, minimizing environmental impact. Abbott also focuses on personnel safety, using advanced personal protective equipment (PPE) and implementing rigorous training programs that cover both handling procedures and emergency response protocols[7].
Strengths: Comprehensive approach tailored to pharmaceutical industry needs; strong focus on traceability and personnel safety. Weaknesses: Solutions may be highly specialized for pharmaceutical use, potentially limiting applicability in other industries.
Arkema France SA
Technical Solution: Arkema has developed a range of innovative solutions for safe handling and use of hydrochloric acid. Their approach includes the development of specialized corrosion-resistant materials for storage and transport containers, reducing the risk of leaks and spills. Arkema has also created advanced polymer linings that can be applied to existing infrastructure to improve chemical resistance. Their compliance strategy incorporates smart sensors and IoT technology to monitor acid concentrations, pH levels, and container integrity in real-time[5]. Additionally, Arkema offers eco-friendly alternatives to traditional hydrochloric acid for certain applications, helping companies reduce their overall acid usage and associated compliance burden[6].
Strengths: Comprehensive approach addressing multiple aspects of acid handling; innovative materials science solutions. Weaknesses: Some solutions may require significant infrastructure upgrades, which could be costly for smaller operations.
Innovative HCl Safety Technologies
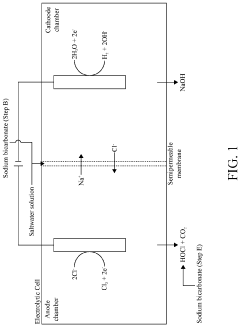
Apparatus and method for the electrolytic production of hypochlorous acid
PatentPendingUS20230313389A1
Innovation
- An apparatus and method using electrolysis of a sodium chloride solution with an acidic solution in a reaction loop, controlled by a system that monitors and adjusts pH to produce a stable HOCl solution, allowing for on-demand production with variable scale and remote monitoring.
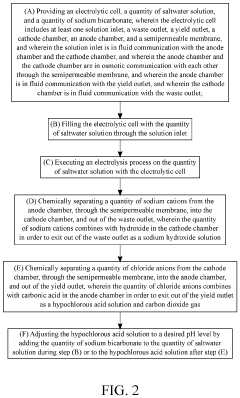
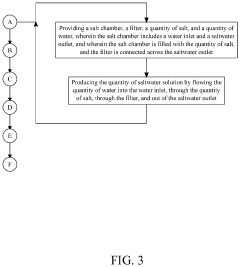
System and Method for Making Hypochlorous Acid Using Saltwater with Sodium Bicarbonate
PatentActiveUS20210395904A1
Innovation
- Incorporating sodium bicarbonate into the saltwater electrolysis process using a semipermeable membrane to separate the solutions, which forms purer hypochlorous acid by reacting chloride ions with carbonic acid, thereby maintaining a higher pH and eliminating strong hydrochloric acid.
Regulatory Framework for HCl Usage
The regulatory framework for hydrochloric acid (HCl) usage is complex and multifaceted, encompassing various national and international regulations, standards, and guidelines. At the federal level in the United States, the Occupational Safety and Health Administration (OSHA) plays a crucial role in regulating HCl use in workplaces. OSHA's Permissible Exposure Limit (PEL) for HCl is set at 5 parts per million (ppm) as a ceiling limit, meaning this concentration should never be exceeded during any part of the workday.
The Environmental Protection Agency (EPA) also regulates HCl under the Clean Air Act, classifying it as a Hazardous Air Pollutant (HAP). Facilities that emit HCl above certain thresholds must comply with stringent reporting and emission control requirements. Additionally, the EPA's Resource Conservation and Recovery Act (RCRA) governs the disposal of HCl waste, mandating proper handling and disposal procedures.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, HCl is classified as a corrosive substance, requiring specific labeling and safety data sheet information.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes additional requirements on manufacturers, importers, and users of HCl within the EU market. This includes registration of substances, safety assessments, and communication of risk management measures throughout the supply chain.
Industry-specific regulations also apply to HCl usage. For instance, in the food industry, the Food and Drug Administration (FDA) regulates HCl as a food additive and sets limits on its use in food processing. Similarly, the pharmaceutical industry must adhere to Good Manufacturing Practice (GMP) guidelines, which include specific provisions for handling and using HCl in drug production.
Local and state regulations may impose additional requirements or restrictions on HCl usage, storage, and transportation. These can vary significantly between jurisdictions and may include zoning restrictions, local permitting processes, or more stringent exposure limits.
To ensure compliance with this complex regulatory framework, organizations must implement comprehensive management systems that address all applicable regulations. This typically involves regular training programs, robust documentation practices, periodic audits, and continuous monitoring of regulatory changes. Staying informed about updates to regulations and industry best practices is crucial for maintaining compliance in the dynamic landscape of chemical safety and environmental protection.
The Environmental Protection Agency (EPA) also regulates HCl under the Clean Air Act, classifying it as a Hazardous Air Pollutant (HAP). Facilities that emit HCl above certain thresholds must comply with stringent reporting and emission control requirements. Additionally, the EPA's Resource Conservation and Recovery Act (RCRA) governs the disposal of HCl waste, mandating proper handling and disposal procedures.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under GHS, HCl is classified as a corrosive substance, requiring specific labeling and safety data sheet information.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation imposes additional requirements on manufacturers, importers, and users of HCl within the EU market. This includes registration of substances, safety assessments, and communication of risk management measures throughout the supply chain.
Industry-specific regulations also apply to HCl usage. For instance, in the food industry, the Food and Drug Administration (FDA) regulates HCl as a food additive and sets limits on its use in food processing. Similarly, the pharmaceutical industry must adhere to Good Manufacturing Practice (GMP) guidelines, which include specific provisions for handling and using HCl in drug production.
Local and state regulations may impose additional requirements or restrictions on HCl usage, storage, and transportation. These can vary significantly between jurisdictions and may include zoning restrictions, local permitting processes, or more stringent exposure limits.
To ensure compliance with this complex regulatory framework, organizations must implement comprehensive management systems that address all applicable regulations. This typically involves regular training programs, robust documentation practices, periodic audits, and continuous monitoring of regulatory changes. Staying informed about updates to regulations and industry best practices is crucial for maintaining compliance in the dynamic landscape of chemical safety and environmental protection.
Environmental Impact Assessment
The environmental impact assessment of hydrochloric acid usage is a critical component in ensuring compliance with regulations and minimizing ecological harm. Hydrochloric acid, a strong and corrosive mineral acid, poses significant risks to the environment if not properly managed. Its potential impacts include soil acidification, water pollution, and air quality degradation.
When released into soil, hydrochloric acid can alter pH levels, affecting soil fertility and microbial activity. This can lead to reduced plant growth and ecosystem disruption. In aquatic environments, even small amounts of hydrochloric acid can cause severe damage to aquatic life by altering water pH and increasing the solubility of heavy metals. The acid's corrosive nature can also damage aquatic habitats and infrastructure.
Air emissions containing hydrochloric acid can contribute to acid rain formation, impacting both terrestrial and aquatic ecosystems over large areas. Additionally, these emissions can cause respiratory issues in humans and animals, as well as corrode buildings and infrastructure.
To mitigate these environmental risks, comprehensive control measures must be implemented. Proper storage and handling procedures are essential to prevent accidental spills or leaks. This includes using corrosion-resistant containers, implementing secondary containment systems, and regularly inspecting storage facilities.
Wastewater treatment is crucial when hydrochloric acid is used in industrial processes. Neutralization techniques, such as adding alkaline substances, should be employed before discharge. Advanced treatment methods like ion exchange or reverse osmosis may be necessary to remove residual acidity and dissolved metals.
Air emission control technologies, such as scrubbers or absorption systems, should be installed to capture and neutralize acid vapors before release into the atmosphere. Regular monitoring of air quality around facilities using hydrochloric acid is essential to ensure compliance with emission standards.
Proper disposal of hydrochloric acid waste is vital. Neutralization followed by treatment at authorized hazardous waste facilities is typically required. Recycling and reuse options should be explored to minimize waste generation and environmental impact.
Environmental monitoring programs should be established to assess the long-term effects of hydrochloric acid usage on surrounding ecosystems. This includes regular soil and water testing, as well as monitoring of local flora and fauna for signs of acid-related stress or damage.
Employee training and emergency response planning are crucial components of environmental impact mitigation. Staff should be well-versed in proper handling procedures, spill response protocols, and the use of personal protective equipment to minimize the risk of accidental releases.
By implementing these comprehensive measures and continuously assessing their effectiveness, organizations can significantly reduce the environmental impact of hydrochloric acid usage while ensuring compliance with regulatory requirements.
When released into soil, hydrochloric acid can alter pH levels, affecting soil fertility and microbial activity. This can lead to reduced plant growth and ecosystem disruption. In aquatic environments, even small amounts of hydrochloric acid can cause severe damage to aquatic life by altering water pH and increasing the solubility of heavy metals. The acid's corrosive nature can also damage aquatic habitats and infrastructure.
Air emissions containing hydrochloric acid can contribute to acid rain formation, impacting both terrestrial and aquatic ecosystems over large areas. Additionally, these emissions can cause respiratory issues in humans and animals, as well as corrode buildings and infrastructure.
To mitigate these environmental risks, comprehensive control measures must be implemented. Proper storage and handling procedures are essential to prevent accidental spills or leaks. This includes using corrosion-resistant containers, implementing secondary containment systems, and regularly inspecting storage facilities.
Wastewater treatment is crucial when hydrochloric acid is used in industrial processes. Neutralization techniques, such as adding alkaline substances, should be employed before discharge. Advanced treatment methods like ion exchange or reverse osmosis may be necessary to remove residual acidity and dissolved metals.
Air emission control technologies, such as scrubbers or absorption systems, should be installed to capture and neutralize acid vapors before release into the atmosphere. Regular monitoring of air quality around facilities using hydrochloric acid is essential to ensure compliance with emission standards.
Proper disposal of hydrochloric acid waste is vital. Neutralization followed by treatment at authorized hazardous waste facilities is typically required. Recycling and reuse options should be explored to minimize waste generation and environmental impact.
Environmental monitoring programs should be established to assess the long-term effects of hydrochloric acid usage on surrounding ecosystems. This includes regular soil and water testing, as well as monitoring of local flora and fauna for signs of acid-related stress or damage.
Employee training and emergency response planning are crucial components of environmental impact mitigation. Staff should be well-versed in proper handling procedures, spill response protocols, and the use of personal protective equipment to minimize the risk of accidental releases.
By implementing these comprehensive measures and continuously assessing their effectiveness, organizations can significantly reduce the environmental impact of hydrochloric acid usage while ensuring compliance with regulatory requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!