Hydrochloric Acid's Role in Oil and Gas Extraction
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl in O&G: Background
Hydrochloric acid (HCl) has played a pivotal role in the oil and gas industry for decades, particularly in the extraction and production processes. Its significance stems from its unique chemical properties and versatile applications in enhancing well productivity and reservoir management.
The use of HCl in oil and gas extraction can be traced back to the early 20th century when operators discovered its effectiveness in stimulating carbonate formations. Since then, it has become an indispensable tool in the industry, evolving alongside technological advancements and environmental considerations.
HCl's primary function in oil and gas extraction is acidizing, a well stimulation technique that involves injecting acid into the formation to dissolve minerals and organic deposits. This process creates new channels or enlarges existing ones, improving the flow of hydrocarbons from the reservoir to the wellbore. Acidizing is particularly effective in carbonate formations, such as limestone and dolomite, which are common in many oil and gas-producing regions worldwide.
In addition to acidizing, HCl serves various other purposes in the oil and gas industry. It is used in well cleaning operations to remove scale, rust, and other debris that can accumulate in wellbores and production equipment. HCl also plays a crucial role in hydraulic fracturing operations, where it is used as a component in fracturing fluids to help dissolve minerals and initiate cracks in the rock formation.
The effectiveness of HCl in oil and gas extraction is attributed to its strong acidic properties and rapid reaction with carbonate minerals. When HCl reacts with calcium carbonate (CaCO3), for example, it produces calcium chloride, water, and carbon dioxide. This reaction not only dissolves the rock but also creates pressure that helps to further stimulate the formation.
Over time, the application of HCl in the oil and gas industry has become more sophisticated. Operators now use various acid blends and additives to optimize performance and address specific formation characteristics. These advancements have led to improved efficiency, reduced environmental impact, and enhanced safety in acid treatments.
Despite its benefits, the use of HCl in oil and gas extraction also presents challenges. These include potential corrosion of well equipment, environmental concerns related to acid disposal, and health and safety risks associated with handling strong acids. As a result, the industry has developed numerous safeguards and alternative technologies to mitigate these issues while maintaining the benefits of acid treatments.
The use of HCl in oil and gas extraction can be traced back to the early 20th century when operators discovered its effectiveness in stimulating carbonate formations. Since then, it has become an indispensable tool in the industry, evolving alongside technological advancements and environmental considerations.
HCl's primary function in oil and gas extraction is acidizing, a well stimulation technique that involves injecting acid into the formation to dissolve minerals and organic deposits. This process creates new channels or enlarges existing ones, improving the flow of hydrocarbons from the reservoir to the wellbore. Acidizing is particularly effective in carbonate formations, such as limestone and dolomite, which are common in many oil and gas-producing regions worldwide.
In addition to acidizing, HCl serves various other purposes in the oil and gas industry. It is used in well cleaning operations to remove scale, rust, and other debris that can accumulate in wellbores and production equipment. HCl also plays a crucial role in hydraulic fracturing operations, where it is used as a component in fracturing fluids to help dissolve minerals and initiate cracks in the rock formation.
The effectiveness of HCl in oil and gas extraction is attributed to its strong acidic properties and rapid reaction with carbonate minerals. When HCl reacts with calcium carbonate (CaCO3), for example, it produces calcium chloride, water, and carbon dioxide. This reaction not only dissolves the rock but also creates pressure that helps to further stimulate the formation.
Over time, the application of HCl in the oil and gas industry has become more sophisticated. Operators now use various acid blends and additives to optimize performance and address specific formation characteristics. These advancements have led to improved efficiency, reduced environmental impact, and enhanced safety in acid treatments.
Despite its benefits, the use of HCl in oil and gas extraction also presents challenges. These include potential corrosion of well equipment, environmental concerns related to acid disposal, and health and safety risks associated with handling strong acids. As a result, the industry has developed numerous safeguards and alternative technologies to mitigate these issues while maintaining the benefits of acid treatments.
Market Demand Analysis
The market demand for hydrochloric acid in oil and gas extraction has been steadily increasing over the past decade, driven by the growing global energy needs and the expansion of unconventional oil and gas production methods. Hydrochloric acid plays a crucial role in well stimulation and enhanced oil recovery techniques, particularly in hydraulic fracturing and matrix acidizing processes.
In the hydraulic fracturing sector, hydrochloric acid is used to dissolve limestone, dolomite, and calcite cement between rock grains, creating new pathways for oil and gas to flow more freely. This application has seen significant growth, especially in regions with shale gas and tight oil formations, such as North America and parts of Europe and Asia.
The demand for hydrochloric acid in conventional oil fields has also remained strong, as it is essential for well maintenance and stimulation. Acid treatments are regularly performed to remove scale buildup and improve well productivity, contributing to the sustained demand for hydrochloric acid in mature oilfields worldwide.
Geographically, North America dominates the market for hydrochloric acid in oil and gas extraction, primarily due to the extensive shale gas and tight oil operations in the United States and Canada. The Middle East and North Africa region follows, with its vast conventional oil reserves requiring regular acid treatments for enhanced recovery.
The market size for hydrochloric acid in the oil and gas sector is substantial, with annual consumption reaching millions of tons. This demand is expected to continue growing, albeit at a more moderate pace, as the industry focuses on optimizing existing wells and exploring new reserves.
However, the market is not without challenges. Environmental concerns and regulatory pressures regarding the use of chemicals in oil and gas extraction have led to increased scrutiny of hydrochloric acid applications. This has spurred research into more environmentally friendly alternatives and improved acid formulations with reduced environmental impact.
The cyclical nature of the oil and gas industry also affects the demand for hydrochloric acid. During periods of low oil prices, drilling activities may decrease, temporarily reducing the demand for well stimulation chemicals. Conversely, when oil prices are high, increased exploration and production activities drive up the demand for hydrochloric acid and related products.
Looking ahead, the market for hydrochloric acid in oil and gas extraction is expected to evolve with the industry's focus on sustainability and efficiency. Innovations in acid formulations, such as the development of self-diverting acids and environmentally friendly additives, are likely to shape future demand patterns and market growth trajectories.
In the hydraulic fracturing sector, hydrochloric acid is used to dissolve limestone, dolomite, and calcite cement between rock grains, creating new pathways for oil and gas to flow more freely. This application has seen significant growth, especially in regions with shale gas and tight oil formations, such as North America and parts of Europe and Asia.
The demand for hydrochloric acid in conventional oil fields has also remained strong, as it is essential for well maintenance and stimulation. Acid treatments are regularly performed to remove scale buildup and improve well productivity, contributing to the sustained demand for hydrochloric acid in mature oilfields worldwide.
Geographically, North America dominates the market for hydrochloric acid in oil and gas extraction, primarily due to the extensive shale gas and tight oil operations in the United States and Canada. The Middle East and North Africa region follows, with its vast conventional oil reserves requiring regular acid treatments for enhanced recovery.
The market size for hydrochloric acid in the oil and gas sector is substantial, with annual consumption reaching millions of tons. This demand is expected to continue growing, albeit at a more moderate pace, as the industry focuses on optimizing existing wells and exploring new reserves.
However, the market is not without challenges. Environmental concerns and regulatory pressures regarding the use of chemicals in oil and gas extraction have led to increased scrutiny of hydrochloric acid applications. This has spurred research into more environmentally friendly alternatives and improved acid formulations with reduced environmental impact.
The cyclical nature of the oil and gas industry also affects the demand for hydrochloric acid. During periods of low oil prices, drilling activities may decrease, temporarily reducing the demand for well stimulation chemicals. Conversely, when oil prices are high, increased exploration and production activities drive up the demand for hydrochloric acid and related products.
Looking ahead, the market for hydrochloric acid in oil and gas extraction is expected to evolve with the industry's focus on sustainability and efficiency. Innovations in acid formulations, such as the development of self-diverting acids and environmentally friendly additives, are likely to shape future demand patterns and market growth trajectories.
Technical Challenges
The use of hydrochloric acid in oil and gas extraction faces several significant technical challenges. One of the primary issues is the corrosive nature of the acid, which can severely damage well equipment and infrastructure. This corrosion not only leads to increased maintenance costs but also poses safety risks and potential environmental hazards. Developing acid-resistant materials and protective coatings that can withstand prolonged exposure to hydrochloric acid remains an ongoing challenge for the industry.
Another major technical hurdle is controlling the reaction rate of hydrochloric acid with formation rocks. In some cases, the acid may react too quickly, leading to inefficient stimulation of the reservoir and potential formation damage. Conversely, if the reaction is too slow, it may result in incomplete treatment and reduced production gains. Achieving the optimal balance in reaction kinetics requires sophisticated modeling and real-time monitoring techniques, which are still being refined.
The heterogeneous nature of reservoir formations presents additional complexities. Different rock types and varying mineral compositions within the same well can lead to unpredictable acid-rock interactions. This variability makes it challenging to design a one-size-fits-all acid treatment, necessitating the development of adaptive and customizable acid formulations. Furthermore, the presence of high temperatures and pressures in deep reservoirs can alter the behavior of hydrochloric acid, affecting its effectiveness and stability.
Acid placement and distribution within the reservoir is another critical challenge. Ensuring that the acid reaches the intended zones and achieves uniform coverage is essential for successful stimulation. However, factors such as natural fractures, high-permeability streaks, and fluid loss can lead to uneven acid distribution. Advanced diversion techniques and real-time monitoring systems are needed to address this issue effectively.
Environmental concerns associated with hydrochloric acid usage pose significant technical challenges. The potential for groundwater contamination and surface spills requires robust containment and treatment systems. Developing environmentally friendly alternatives or methods to neutralize and safely dispose of spent acid fluids remains a priority for the industry. Additionally, the transportation and handling of large volumes of hydrochloric acid present logistical and safety challenges that require innovative solutions.
Lastly, the integration of hydrochloric acid treatments with other enhanced oil recovery techniques presents technical complexities. Optimizing the sequencing and compatibility of acid treatments with methods such as hydraulic fracturing, chemical flooding, or thermal recovery requires a deep understanding of fluid-rock interactions and reservoir dynamics. Developing integrated approaches that maximize the synergistic effects of these techniques while minimizing potential conflicts is an ongoing area of research and development in the oil and gas industry.
Another major technical hurdle is controlling the reaction rate of hydrochloric acid with formation rocks. In some cases, the acid may react too quickly, leading to inefficient stimulation of the reservoir and potential formation damage. Conversely, if the reaction is too slow, it may result in incomplete treatment and reduced production gains. Achieving the optimal balance in reaction kinetics requires sophisticated modeling and real-time monitoring techniques, which are still being refined.
The heterogeneous nature of reservoir formations presents additional complexities. Different rock types and varying mineral compositions within the same well can lead to unpredictable acid-rock interactions. This variability makes it challenging to design a one-size-fits-all acid treatment, necessitating the development of adaptive and customizable acid formulations. Furthermore, the presence of high temperatures and pressures in deep reservoirs can alter the behavior of hydrochloric acid, affecting its effectiveness and stability.
Acid placement and distribution within the reservoir is another critical challenge. Ensuring that the acid reaches the intended zones and achieves uniform coverage is essential for successful stimulation. However, factors such as natural fractures, high-permeability streaks, and fluid loss can lead to uneven acid distribution. Advanced diversion techniques and real-time monitoring systems are needed to address this issue effectively.
Environmental concerns associated with hydrochloric acid usage pose significant technical challenges. The potential for groundwater contamination and surface spills requires robust containment and treatment systems. Developing environmentally friendly alternatives or methods to neutralize and safely dispose of spent acid fluids remains a priority for the industry. Additionally, the transportation and handling of large volumes of hydrochloric acid present logistical and safety challenges that require innovative solutions.
Lastly, the integration of hydrochloric acid treatments with other enhanced oil recovery techniques presents technical complexities. Optimizing the sequencing and compatibility of acid treatments with methods such as hydraulic fracturing, chemical flooding, or thermal recovery requires a deep understanding of fluid-rock interactions and reservoir dynamics. Developing integrated approaches that maximize the synergistic effects of these techniques while minimizing potential conflicts is an ongoing area of research and development in the oil and gas industry.
Current HCl Solutions
01 Production methods of hydrochloric acid
Various methods are employed to produce hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These production methods are optimized for efficiency and purity in industrial settings.- Production methods of hydrochloric acid: Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.
- Purification and concentration techniques: Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for various industrial applications.
- Applications in chemical processing: Hydrochloric acid is widely used in chemical processing, including metal treatment, pH regulation, and as a catalyst in various reactions. It plays a crucial role in the production of pharmaceuticals, plastics, and other industrial chemicals.
- Safety and handling considerations: Proper safety measures and handling procedures are essential when working with hydrochloric acid due to its corrosive nature. This includes the use of specialized storage containers, protective equipment, and neutralization techniques for spills or disposal.
- Environmental impact and recycling: Efforts to mitigate the environmental impact of hydrochloric acid include recycling and recovery processes, as well as the development of alternative production methods with reduced emissions. These approaches aim to minimize waste and promote sustainable use of the acid in industrial applications.
02 Purification and concentration techniques
Hydrochloric acid purification and concentration techniques involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and adjust the acid concentration for specific industrial applications, ensuring high-quality acid production.Expand Specific Solutions03 Industrial applications of hydrochloric acid
Hydrochloric acid finds widespread use in various industries, including metal processing, chemical manufacturing, and water treatment. It is utilized for pH adjustment, metal etching, and as a reagent in numerous chemical processes, showcasing its versatility in industrial applications.Expand Specific Solutions04 Safety and handling procedures
Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes using appropriate personal protective equipment, implementing spill containment strategies, and following specific storage and transportation guidelines to minimize risks associated with acid handling.Expand Specific Solutions05 Environmental impact and waste management
Managing the environmental impact of hydrochloric acid involves proper waste treatment, recycling, and neutralization techniques. Efforts are made to minimize emissions and develop eco-friendly production processes, addressing concerns related to acid disposal and its effects on the environment.Expand Specific Solutions
Key Industry Players
The hydrochloric acid market in oil and gas extraction is in a mature stage, with a significant global market size driven by increasing energy demand. The technology is well-established, with major players like China Petroleum & Chemical Corp., PetroChina, and ExxonMobil leading the field. These companies have extensive research capabilities and operational experience, contributing to the technology's maturity. Emerging players such as Fluid Energy Group and Cleansorb are introducing innovative solutions, focusing on environmentally friendly alternatives and enhanced efficiency. The competitive landscape is characterized by a mix of established oil and gas giants and specialized chemical companies, indicating a diverse and dynamic market.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced techniques for using hydrochloric acid in oil and gas extraction. Their approach involves a multi-stage acid fracturing process, where hydrochloric acid is injected at high pressure to create and enlarge fractures in carbonate reservoirs. This method enhances the permeability of the formation, allowing for increased oil and gas flow. Sinopec has also implemented a novel acid-resistant coating technology for their equipment, extending the lifespan of their extraction infrastructure in acidic environments[1][3]. Additionally, they have developed a proprietary acid mixture that combines hydrochloric acid with organic acids and surfactants, optimizing the acid's penetration and reaction with the formation rock[5].
Strengths: Highly effective in carbonate reservoirs, increased well productivity, and extended equipment lifespan. Weaknesses: Potential environmental concerns and the need for specialized handling and disposal of acid waste.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group Ltd. has pioneered an innovative approach to hydrochloric acid use in oil and gas extraction through their HydroFORM technology. This system utilizes a proprietary blend of hydrochloric acid and organic acids, combined with advanced surfactants and corrosion inhibitors. The HydroFORM technology allows for controlled acid release, reducing the overall acid concentration required while maintaining effectiveness. This method results in a more uniform etching of the formation, leading to improved connectivity and enhanced oil and gas recovery[2]. Fluid Energy Group has also developed a real-time monitoring system that adjusts acid injection rates based on downhole conditions, optimizing the acid treatment process[4].
Strengths: Reduced environmental impact, improved acid efficiency, and real-time optimization capabilities. Weaknesses: Higher initial implementation costs and the need for specialized training for operators.
HCl Innovations
Synthetic acid compositions alternatives to conventional acids in the oil and gas industry
PatentActiveCA2961794A1
Innovation
- A synthetic acid composition comprising urea and hydrogen chloride in a specific molar ratio, combined with cinnamaldehyde and phosphonic acid derivatives, which reduces corrosion rates, is non-fuming, non-toxic, and biodegradable, with controlled reactivity and high salinity tolerance, allowing for deeper formation penetration and reduced equipment damage.
Using Synthetic Acid Compositions as Alternatives to Conventional Acids in The Oil And Gas Industry
PatentActiveUS20230086463A1
Innovation
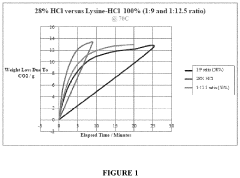
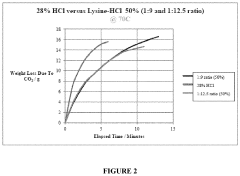
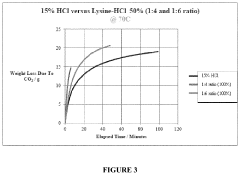
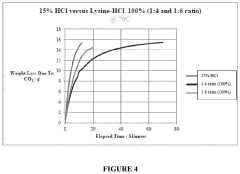
- An aqueous synthetic acid composition comprising lysine and hydrogen chloride in specific molar ratios, which provides low corrosion rates, biodegradability, controlled reaction rates, and thermal stability up to 220°C, reducing toxicity and environmental impact while maintaining the effectiveness of hydrochloric acid.
Environmental Impact
The use of hydrochloric acid in oil and gas extraction, particularly in hydraulic fracturing operations, has raised significant environmental concerns. The primary issue stems from the potential contamination of groundwater and surface water resources. When hydrochloric acid is injected into wells, there is a risk of leakage or improper disposal, which can lead to soil and water pollution. This contamination can have far-reaching effects on local ecosystems and human health.
One of the most pressing environmental impacts is the alteration of soil and water pH levels. Hydrochloric acid, being a strong acid, can dramatically lower the pH of surrounding environments, creating acidic conditions that are harmful to plant and animal life. This change in pH can disrupt delicate ecological balances, potentially leading to the loss of biodiversity in affected areas.
Furthermore, the acid can mobilize heavy metals and other toxic substances naturally present in rock formations. As the acid dissolves these materials, it can release them into groundwater aquifers, potentially contaminating drinking water sources for nearby communities. This mobilization of contaminants poses long-term risks to both environmental and public health.
The transportation and storage of hydrochloric acid also present environmental hazards. Accidental spills during transport or at drilling sites can cause immediate and severe damage to local flora and fauna. The corrosive nature of the acid can destroy vegetation and harm wildlife upon contact. In aquatic environments, even small spills can have devastating effects on fish populations and other aquatic organisms.
Air quality is another concern associated with the use of hydrochloric acid in oil and gas extraction. During the fracturing process, some of the acid may vaporize, releasing hydrogen chloride gas into the atmosphere. This can contribute to the formation of acid rain and potentially impact air quality in surrounding areas.
The disposal of wastewater containing hydrochloric acid and its byproducts presents additional environmental challenges. Improper treatment or disposal of this wastewater can lead to the contamination of surface waters and soil. The high salinity and potential presence of toxic compounds in the wastewater can have long-lasting impacts on ecosystems and agricultural lands.
To mitigate these environmental risks, the oil and gas industry has been developing and implementing various strategies. These include improved well casing designs to prevent leakage, more efficient acid use to reduce the overall volume required, and advanced wastewater treatment technologies. Additionally, regulatory bodies have imposed stricter guidelines on the handling, use, and disposal of hydrochloric acid in extraction processes.
One of the most pressing environmental impacts is the alteration of soil and water pH levels. Hydrochloric acid, being a strong acid, can dramatically lower the pH of surrounding environments, creating acidic conditions that are harmful to plant and animal life. This change in pH can disrupt delicate ecological balances, potentially leading to the loss of biodiversity in affected areas.
Furthermore, the acid can mobilize heavy metals and other toxic substances naturally present in rock formations. As the acid dissolves these materials, it can release them into groundwater aquifers, potentially contaminating drinking water sources for nearby communities. This mobilization of contaminants poses long-term risks to both environmental and public health.
The transportation and storage of hydrochloric acid also present environmental hazards. Accidental spills during transport or at drilling sites can cause immediate and severe damage to local flora and fauna. The corrosive nature of the acid can destroy vegetation and harm wildlife upon contact. In aquatic environments, even small spills can have devastating effects on fish populations and other aquatic organisms.
Air quality is another concern associated with the use of hydrochloric acid in oil and gas extraction. During the fracturing process, some of the acid may vaporize, releasing hydrogen chloride gas into the atmosphere. This can contribute to the formation of acid rain and potentially impact air quality in surrounding areas.
The disposal of wastewater containing hydrochloric acid and its byproducts presents additional environmental challenges. Improper treatment or disposal of this wastewater can lead to the contamination of surface waters and soil. The high salinity and potential presence of toxic compounds in the wastewater can have long-lasting impacts on ecosystems and agricultural lands.
To mitigate these environmental risks, the oil and gas industry has been developing and implementing various strategies. These include improved well casing designs to prevent leakage, more efficient acid use to reduce the overall volume required, and advanced wastewater treatment technologies. Additionally, regulatory bodies have imposed stricter guidelines on the handling, use, and disposal of hydrochloric acid in extraction processes.
Regulatory Framework
The regulatory framework surrounding the use of hydrochloric acid in oil and gas extraction is complex and multifaceted, reflecting the need to balance economic interests with environmental and safety concerns. At the federal level in the United States, the Environmental Protection Agency (EPA) plays a crucial role in overseeing the use of chemicals in hydraulic fracturing operations. The EPA's regulations focus on protecting groundwater resources and ensuring proper disposal of wastewater containing hydrochloric acid and other chemicals.
The Safe Drinking Water Act (SDWA) and the Clean Water Act (CWA) are two primary federal statutes that govern the use of hydrochloric acid in oil and gas extraction. Under the SDWA, the EPA has established Underground Injection Control (UIC) programs to prevent contamination of underground sources of drinking water. The CWA regulates the discharge of pollutants into surface waters, including wastewater from oil and gas operations.
At the state level, regulations vary widely, with some states imposing stricter controls than others. For example, California has implemented comprehensive regulations requiring operators to disclose the chemicals used in hydraulic fracturing, including hydrochloric acid. Texas, on the other hand, has less stringent disclosure requirements but still mandates reporting of chemical use to the Railroad Commission of Texas.
Internationally, the regulatory landscape is equally diverse. The European Union has adopted a precautionary approach, with some member states banning hydraulic fracturing altogether. In contrast, countries like China and Argentina have embraced the technology, albeit with varying degrees of regulatory oversight.
Industry self-regulation also plays a significant role in shaping the use of hydrochloric acid in oil and gas extraction. Many companies have voluntarily adopted best practices and safety protocols that go beyond regulatory requirements. These include measures such as using safer alternatives to hydrochloric acid where possible, implementing robust spill prevention and response plans, and investing in advanced treatment technologies for wastewater.
The regulatory framework is continually evolving as new scientific evidence emerges and public awareness of environmental issues grows. Recent trends indicate a move towards more stringent regulations, particularly in areas such as chemical disclosure, well integrity standards, and wastewater management. This evolving landscape presents both challenges and opportunities for the oil and gas industry, driving innovation in extraction techniques and environmental protection measures.
The Safe Drinking Water Act (SDWA) and the Clean Water Act (CWA) are two primary federal statutes that govern the use of hydrochloric acid in oil and gas extraction. Under the SDWA, the EPA has established Underground Injection Control (UIC) programs to prevent contamination of underground sources of drinking water. The CWA regulates the discharge of pollutants into surface waters, including wastewater from oil and gas operations.
At the state level, regulations vary widely, with some states imposing stricter controls than others. For example, California has implemented comprehensive regulations requiring operators to disclose the chemicals used in hydraulic fracturing, including hydrochloric acid. Texas, on the other hand, has less stringent disclosure requirements but still mandates reporting of chemical use to the Railroad Commission of Texas.
Internationally, the regulatory landscape is equally diverse. The European Union has adopted a precautionary approach, with some member states banning hydraulic fracturing altogether. In contrast, countries like China and Argentina have embraced the technology, albeit with varying degrees of regulatory oversight.
Industry self-regulation also plays a significant role in shaping the use of hydrochloric acid in oil and gas extraction. Many companies have voluntarily adopted best practices and safety protocols that go beyond regulatory requirements. These include measures such as using safer alternatives to hydrochloric acid where possible, implementing robust spill prevention and response plans, and investing in advanced treatment technologies for wastewater.
The regulatory framework is continually evolving as new scientific evidence emerges and public awareness of environmental issues grows. Recent trends indicate a move towards more stringent regulations, particularly in areas such as chemical disclosure, well integrity standards, and wastewater management. This evolving landscape presents both challenges and opportunities for the oil and gas industry, driving innovation in extraction techniques and environmental protection measures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!