How to Expand Hydrochloric Acid Proficiency Within Teams?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Expertise Goals
Expanding hydrochloric acid (HCl) proficiency within teams is crucial for organizations operating in industries where this chemical plays a significant role. The primary goal is to enhance the overall understanding, handling, and application of HCl across various departments and personnel levels. This objective encompasses several key areas of focus.
Firstly, teams must strive to develop a comprehensive knowledge base regarding HCl's chemical properties, reactivity, and potential hazards. This includes understanding its corrosive nature, its behavior in different concentrations, and its interactions with various materials. By establishing this foundational knowledge, teams can ensure safer and more efficient handling of HCl in laboratory and industrial settings.
Secondly, there is a need to improve practical skills in HCl handling and usage. This involves mastering techniques for proper storage, transportation, and disposal of HCl, as well as developing proficiency in its application in various processes. Teams should aim to become adept at preparing HCl solutions of different concentrations and understanding how to use HCl effectively in different chemical reactions and industrial applications.
Another critical goal is to enhance safety protocols and emergency response procedures related to HCl. This includes training team members on proper personal protective equipment (PPE) usage, spill containment and cleanup procedures, and first aid measures in case of exposure. Developing a culture of safety and vigilance around HCl handling is paramount to preventing accidents and minimizing risks.
Furthermore, teams should focus on expanding their knowledge of HCl's role in specific industry applications. This could involve understanding its use in metal processing, water treatment, oil well acidizing, or pharmaceutical manufacturing, depending on the organization's focus. By deepening this specialized knowledge, teams can optimize HCl usage in their particular field and potentially innovate new applications or processes.
Lastly, there should be an emphasis on staying current with regulatory requirements and best practices related to HCl. This includes keeping abreast of changes in environmental regulations, transportation guidelines, and industry standards. Teams should aim to not only comply with these regulations but also to implement best practices that go beyond minimum requirements, positioning the organization as a leader in responsible HCl management.
Firstly, teams must strive to develop a comprehensive knowledge base regarding HCl's chemical properties, reactivity, and potential hazards. This includes understanding its corrosive nature, its behavior in different concentrations, and its interactions with various materials. By establishing this foundational knowledge, teams can ensure safer and more efficient handling of HCl in laboratory and industrial settings.
Secondly, there is a need to improve practical skills in HCl handling and usage. This involves mastering techniques for proper storage, transportation, and disposal of HCl, as well as developing proficiency in its application in various processes. Teams should aim to become adept at preparing HCl solutions of different concentrations and understanding how to use HCl effectively in different chemical reactions and industrial applications.
Another critical goal is to enhance safety protocols and emergency response procedures related to HCl. This includes training team members on proper personal protective equipment (PPE) usage, spill containment and cleanup procedures, and first aid measures in case of exposure. Developing a culture of safety and vigilance around HCl handling is paramount to preventing accidents and minimizing risks.
Furthermore, teams should focus on expanding their knowledge of HCl's role in specific industry applications. This could involve understanding its use in metal processing, water treatment, oil well acidizing, or pharmaceutical manufacturing, depending on the organization's focus. By deepening this specialized knowledge, teams can optimize HCl usage in their particular field and potentially innovate new applications or processes.
Lastly, there should be an emphasis on staying current with regulatory requirements and best practices related to HCl. This includes keeping abreast of changes in environmental regulations, transportation guidelines, and industry standards. Teams should aim to not only comply with these regulations but also to implement best practices that go beyond minimum requirements, positioning the organization as a leader in responsible HCl management.
HCl Market Analysis
The global hydrochloric acid market has been experiencing steady growth, driven by increasing demand from various industries such as steel pickling, oil well acidizing, and chemical manufacturing. The market size was valued at approximately $7.8 billion in 2020 and is projected to reach $9.5 billion by 2026, growing at a CAGR of 3.5% during the forecast period.
The demand for hydrochloric acid is closely tied to industrial production and economic growth. Developing economies, particularly in Asia-Pacific, are witnessing rapid industrialization, which is fueling the demand for hydrochloric acid in various applications. China remains the largest consumer and producer of hydrochloric acid, accounting for nearly 40% of global production.
In terms of end-use industries, the steel sector continues to be the primary consumer of hydrochloric acid, using it for pickling and descaling processes. The growing construction and infrastructure development activities worldwide are driving the demand for steel, consequently boosting the hydrochloric acid market.
The oil and gas industry is another significant consumer of hydrochloric acid, particularly in well acidizing operations. With the recovery of oil prices and increasing exploration activities, the demand for hydrochloric acid in this sector is expected to grow steadily.
Environmental regulations and safety concerns are influencing market dynamics. Stringent regulations regarding the production, handling, and disposal of hydrochloric acid are prompting manufacturers to invest in advanced technologies and sustainable production methods. This trend is likely to drive innovation in the industry and create opportunities for eco-friendly alternatives.
The market is characterized by the presence of several large-scale manufacturers and numerous regional players. Key market players are focusing on expanding their production capacities and geographical presence to gain a competitive edge. Mergers, acquisitions, and strategic partnerships are common strategies employed by companies to strengthen their market position.
Pricing trends in the hydrochloric acid market are influenced by raw material costs, energy prices, and supply-demand dynamics. The market has witnessed price fluctuations in recent years due to changes in production costs and varying demand across different regions.
Looking ahead, the hydrochloric acid market is expected to continue its growth trajectory, driven by increasing industrial applications and technological advancements. However, challenges such as environmental concerns and the development of alternative technologies may impact market growth in the long term. Companies that focus on sustainable production methods and diversification of applications are likely to succeed in this evolving market landscape.
The demand for hydrochloric acid is closely tied to industrial production and economic growth. Developing economies, particularly in Asia-Pacific, are witnessing rapid industrialization, which is fueling the demand for hydrochloric acid in various applications. China remains the largest consumer and producer of hydrochloric acid, accounting for nearly 40% of global production.
In terms of end-use industries, the steel sector continues to be the primary consumer of hydrochloric acid, using it for pickling and descaling processes. The growing construction and infrastructure development activities worldwide are driving the demand for steel, consequently boosting the hydrochloric acid market.
The oil and gas industry is another significant consumer of hydrochloric acid, particularly in well acidizing operations. With the recovery of oil prices and increasing exploration activities, the demand for hydrochloric acid in this sector is expected to grow steadily.
Environmental regulations and safety concerns are influencing market dynamics. Stringent regulations regarding the production, handling, and disposal of hydrochloric acid are prompting manufacturers to invest in advanced technologies and sustainable production methods. This trend is likely to drive innovation in the industry and create opportunities for eco-friendly alternatives.
The market is characterized by the presence of several large-scale manufacturers and numerous regional players. Key market players are focusing on expanding their production capacities and geographical presence to gain a competitive edge. Mergers, acquisitions, and strategic partnerships are common strategies employed by companies to strengthen their market position.
Pricing trends in the hydrochloric acid market are influenced by raw material costs, energy prices, and supply-demand dynamics. The market has witnessed price fluctuations in recent years due to changes in production costs and varying demand across different regions.
Looking ahead, the hydrochloric acid market is expected to continue its growth trajectory, driven by increasing industrial applications and technological advancements. However, challenges such as environmental concerns and the development of alternative technologies may impact market growth in the long term. Companies that focus on sustainable production methods and diversification of applications are likely to succeed in this evolving market landscape.
HCl Handling Challenges
Handling hydrochloric acid (HCl) presents numerous challenges for teams working in various industries, from chemical manufacturing to research laboratories. The corrosive nature of HCl demands strict safety protocols and specialized equipment, which can be daunting for inexperienced personnel. One of the primary challenges is ensuring proper personal protective equipment (PPE) usage, including chemical-resistant gloves, goggles, and face shields, to prevent skin and eye contact with the acid.
Storage and transportation of HCl require careful consideration due to its reactivity with many materials. Teams must be proficient in selecting appropriate containers and storage facilities that can withstand the acid's corrosive properties. Additionally, proper labeling and segregation from incompatible substances are crucial to prevent accidental mixing and potential hazardous reactions.
Ventilation is another critical concern when handling HCl. The acid releases toxic fumes that can cause severe respiratory issues if inhaled. Teams need to be well-versed in operating fume hoods and maintaining adequate ventilation systems to minimize exposure risks. This challenge extends to the proper disposal of HCl waste, which requires neutralization and adherence to environmental regulations.
Spill management is a significant challenge that teams must be prepared to address. Quick and effective response to HCl spills is essential to prevent injuries and environmental damage. This requires knowledge of spill containment techniques, neutralization procedures, and the use of appropriate absorbent materials.
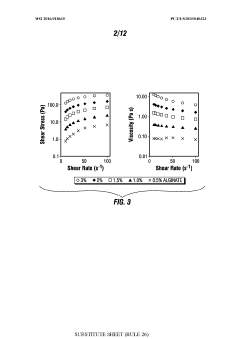
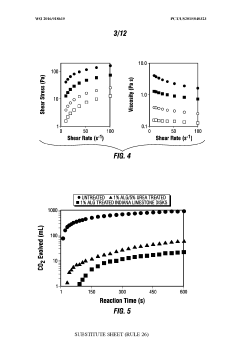
The concentration of HCl solutions presents varying levels of risk, and teams must be proficient in handling different concentrations safely. Dilution procedures, when necessary, demand precise calculations and careful execution to avoid dangerous exothermic reactions.
Maintaining and calibrating pH meters and other analytical instruments used in conjunction with HCl is another challenge. Accurate measurements are crucial for many applications, and teams need to understand the intricacies of these devices to ensure reliable results.
Lastly, the documentation and record-keeping associated with HCl handling pose administrative challenges. Teams must maintain detailed logs of usage, storage, and disposal, as well as keep up-to-date safety data sheets (SDS) and standard operating procedures (SOPs) readily available.
Addressing these challenges requires comprehensive training programs, regular safety drills, and a culture of continuous improvement in HCl handling practices. By focusing on these areas, teams can enhance their proficiency and minimize the risks associated with hydrochloric acid use in their operations.
Storage and transportation of HCl require careful consideration due to its reactivity with many materials. Teams must be proficient in selecting appropriate containers and storage facilities that can withstand the acid's corrosive properties. Additionally, proper labeling and segregation from incompatible substances are crucial to prevent accidental mixing and potential hazardous reactions.
Ventilation is another critical concern when handling HCl. The acid releases toxic fumes that can cause severe respiratory issues if inhaled. Teams need to be well-versed in operating fume hoods and maintaining adequate ventilation systems to minimize exposure risks. This challenge extends to the proper disposal of HCl waste, which requires neutralization and adherence to environmental regulations.
Spill management is a significant challenge that teams must be prepared to address. Quick and effective response to HCl spills is essential to prevent injuries and environmental damage. This requires knowledge of spill containment techniques, neutralization procedures, and the use of appropriate absorbent materials.
The concentration of HCl solutions presents varying levels of risk, and teams must be proficient in handling different concentrations safely. Dilution procedures, when necessary, demand precise calculations and careful execution to avoid dangerous exothermic reactions.
Maintaining and calibrating pH meters and other analytical instruments used in conjunction with HCl is another challenge. Accurate measurements are crucial for many applications, and teams need to understand the intricacies of these devices to ensure reliable results.
Lastly, the documentation and record-keeping associated with HCl handling pose administrative challenges. Teams must maintain detailed logs of usage, storage, and disposal, as well as keep up-to-date safety data sheets (SDS) and standard operating procedures (SOPs) readily available.
Addressing these challenges requires comprehensive training programs, regular safety drills, and a culture of continuous improvement in HCl handling practices. By focusing on these areas, teams can enhance their proficiency and minimize the risks associated with hydrochloric acid use in their operations.
Current HCl Training Methods
01 Production and purification of hydrochloric acid
Various methods and systems for producing and purifying hydrochloric acid, including processes for improving yield and quality. This involves techniques for synthesizing, concentrating, and refining hydrochloric acid to meet industrial standards and specific application requirements.- Production and purification of hydrochloric acid: Various methods and systems for producing and purifying hydrochloric acid, including processes for removing impurities and increasing concentration. These techniques aim to improve the quality and efficiency of hydrochloric acid production for industrial applications.
- Hydrochloric acid handling and storage systems: Specialized equipment and containers designed for the safe handling, storage, and transportation of hydrochloric acid. These systems often include corrosion-resistant materials and safety features to prevent leaks and accidents during use and storage.
- Applications of hydrochloric acid in industrial processes: Various industrial applications of hydrochloric acid, including its use in chemical manufacturing, metal processing, and water treatment. These processes leverage the acid's properties for tasks such as pH adjustment, metal etching, and scale removal.
- Safety measures and environmental considerations: Techniques and equipment designed to enhance safety and reduce environmental impact when working with hydrochloric acid. This includes containment systems, neutralization processes, and methods for treating hydrochloric acid waste to minimize environmental harm.
- Analytical methods for hydrochloric acid: Techniques and instruments used for analyzing the concentration, purity, and properties of hydrochloric acid. These methods are crucial for quality control and ensuring the acid meets specific standards for various applications.
02 Handling and storage of hydrochloric acid
Specialized equipment and procedures for safely handling and storing hydrochloric acid. This includes corrosion-resistant containers, safety measures, and storage systems designed to maintain the acid's purity and prevent environmental contamination.Expand Specific Solutions03 Applications of hydrochloric acid in industrial processes
Utilization of hydrochloric acid in various industrial applications, such as metal processing, chemical manufacturing, and water treatment. This covers innovative uses of the acid in different sectors and techniques for optimizing its effectiveness in specific processes.Expand Specific Solutions04 Environmental and safety considerations in hydrochloric acid use
Methods and systems for mitigating environmental impacts and ensuring safety in the production, use, and disposal of hydrochloric acid. This includes emission control technologies, waste treatment processes, and safety protocols for handling and transportation.Expand Specific Solutions05 Analytical techniques for hydrochloric acid quality control
Advanced analytical methods and instruments for assessing the purity, concentration, and overall quality of hydrochloric acid. This encompasses spectroscopic techniques, titration methods, and automated analysis systems for ensuring consistent acid quality in industrial settings.Expand Specific Solutions
Key HCl Industry Players
The hydrochloric acid proficiency expansion within teams is currently in a growth phase, with increasing market demand driven by industrial applications and research needs. The global market size for hydrochloric acid is projected to grow significantly in the coming years. Technologically, the field is moderately mature, with established production methods and safety protocols. However, there is ongoing innovation in purification techniques and handling processes. Companies like Schlumberger, Halliburton, and Akzo Nobel Chemicals are leading players in this space, leveraging their expertise in chemical engineering and industrial applications to enhance team proficiency and develop advanced solutions for hydrochloric acid management and utilization.
Schlumberger Canada Ltd.
Technical Solution: Schlumberger, as a leading oilfield services company, has developed comprehensive training programs to expand hydrochloric acid proficiency within teams. Their approach includes a combination of theoretical and practical training modules. They utilize advanced simulation software to create virtual scenarios for handling hydrochloric acid in various oilfield applications, such as well stimulation and matrix acidizing[1]. The company has also implemented a mentorship program where experienced personnel guide newer team members through real-world acid handling situations. Additionally, Schlumberger has developed proprietary safety protocols and equipment specifically designed for hydrochloric acid operations, which are integrated into their training curriculum[2].
Strengths: Extensive industry experience, advanced simulation technology, and comprehensive safety protocols. Weaknesses: Training may be overly focused on oilfield applications, potentially limiting broader chemical handling skills.
Halliburton Energy Services, Inc.
Technical Solution: Halliburton has implemented a multi-faceted approach to expand hydrochloric acid proficiency within their teams. They have developed a modular training program that covers all aspects of hydrochloric acid handling, from basic chemistry to advanced application techniques in well stimulation and hydraulic fracturing. The company utilizes state-of-the-art e-learning platforms to deliver theoretical content, complemented by hands-on training sessions in specially designed facilities[3]. Halliburton also emphasizes the importance of environmental safety, incorporating modules on proper disposal and neutralization techniques. They have introduced a certification program that requires team members to demonstrate proficiency in hydrochloric acid handling through practical assessments and written exams[4].
Strengths: Comprehensive modular training, integration of e-learning and hands-on experience, strong focus on environmental safety. Weaknesses: Training may be primarily tailored to energy sector applications, potentially limiting versatility in other industries.
HCl Safety Innovations
Methods for treating subterranean formations
PatentWO2016018615A1
Innovation
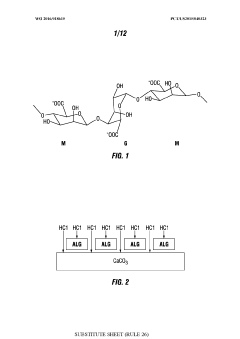
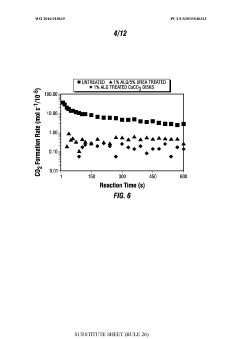
- The use of treatment fluids comprising multivalent-cation reacting polymers, fatty acids with ethanolamine, insoluble calcium salts, urea derivatives, and polyvinylpyrrolidone (PVP) to form protective films on carbonate surfaces, allowing controlled acid penetration and reaction, thereby optimizing acid distribution and reducing corrosion.
Fluid systems and methods for treating subterranean formations
PatentWO2016018614A1
Innovation
- The use of treatment fluids comprising multivalent-cation reacting polymers, fatty acids with ethanolamine, insoluble calcium salt compounds, urea derivatives, and polyvinylpyrrolidone (PVP) to form protective films on carbonate surfaces, allowing controlled acid penetration and stimulation, reducing acid consumption and equipment damage.
HCl Regulatory Compliance
Regulatory compliance is a critical aspect of expanding hydrochloric acid (HCl) proficiency within teams. The handling, storage, and use of HCl are subject to stringent regulations due to its corrosive and hazardous nature. To ensure compliance, organizations must implement comprehensive training programs and establish robust safety protocols.
One of the primary regulatory frameworks governing HCl is the Occupational Safety and Health Administration (OSHA) standards. These standards mandate specific requirements for personal protective equipment (PPE), workplace ventilation, and emergency response procedures. Teams working with HCl must be thoroughly trained on these regulations and understand the importance of adhering to them.
The Environmental Protection Agency (EPA) also plays a crucial role in HCl regulatory compliance. The EPA regulates the storage, transportation, and disposal of HCl under the Resource Conservation and Recovery Act (RCRA). Teams must be well-versed in proper waste management practices and understand the reporting requirements for any accidental releases or spills.
In addition to federal regulations, state and local laws may impose additional requirements for HCl handling. It is essential for organizations to stay informed about these regional variations and ensure that their teams are trained accordingly. This may involve regular updates to training materials and procedures to reflect changes in local regulations.
Proper documentation and record-keeping are integral components of HCl regulatory compliance. Teams should be trained on maintaining accurate logs of HCl usage, storage conditions, and any incidents or near-misses. These records not only demonstrate compliance during inspections but also serve as valuable tools for continuous improvement of safety practices.
To expand HCl proficiency, organizations should consider implementing a tiered training program. This approach allows for progressive skill development, starting with basic safety protocols and gradually advancing to more complex handling procedures. Regular assessments and refresher courses can help maintain and improve team members' knowledge and skills over time.
Collaboration with industry associations and regulatory bodies can provide valuable insights into best practices and emerging compliance requirements. Participating in workshops, seminars, and online training modules offered by these organizations can help teams stay current with the latest regulatory developments and industry standards.
Finally, fostering a culture of safety and compliance is crucial for expanding HCl proficiency. This involves encouraging open communication about safety concerns, recognizing and rewarding compliance efforts, and empowering team members to take an active role in maintaining a safe working environment. By prioritizing regulatory compliance as a fundamental aspect of HCl proficiency, organizations can ensure the safety of their teams and the surrounding community while meeting their operational objectives.
One of the primary regulatory frameworks governing HCl is the Occupational Safety and Health Administration (OSHA) standards. These standards mandate specific requirements for personal protective equipment (PPE), workplace ventilation, and emergency response procedures. Teams working with HCl must be thoroughly trained on these regulations and understand the importance of adhering to them.
The Environmental Protection Agency (EPA) also plays a crucial role in HCl regulatory compliance. The EPA regulates the storage, transportation, and disposal of HCl under the Resource Conservation and Recovery Act (RCRA). Teams must be well-versed in proper waste management practices and understand the reporting requirements for any accidental releases or spills.
In addition to federal regulations, state and local laws may impose additional requirements for HCl handling. It is essential for organizations to stay informed about these regional variations and ensure that their teams are trained accordingly. This may involve regular updates to training materials and procedures to reflect changes in local regulations.
Proper documentation and record-keeping are integral components of HCl regulatory compliance. Teams should be trained on maintaining accurate logs of HCl usage, storage conditions, and any incidents or near-misses. These records not only demonstrate compliance during inspections but also serve as valuable tools for continuous improvement of safety practices.
To expand HCl proficiency, organizations should consider implementing a tiered training program. This approach allows for progressive skill development, starting with basic safety protocols and gradually advancing to more complex handling procedures. Regular assessments and refresher courses can help maintain and improve team members' knowledge and skills over time.
Collaboration with industry associations and regulatory bodies can provide valuable insights into best practices and emerging compliance requirements. Participating in workshops, seminars, and online training modules offered by these organizations can help teams stay current with the latest regulatory developments and industry standards.
Finally, fostering a culture of safety and compliance is crucial for expanding HCl proficiency. This involves encouraging open communication about safety concerns, recognizing and rewarding compliance efforts, and empowering team members to take an active role in maintaining a safe working environment. By prioritizing regulatory compliance as a fundamental aspect of HCl proficiency, organizations can ensure the safety of their teams and the surrounding community while meeting their operational objectives.
HCl Environmental Impact
The environmental impact of hydrochloric acid (HCl) is a critical consideration for teams expanding their proficiency in its use. HCl, a strong and corrosive acid, can have significant effects on ecosystems and human health if not properly managed. When released into the environment, HCl can lower the pH of water bodies, potentially harming aquatic life and disrupting ecological balance. It can also contribute to soil acidification, affecting plant growth and soil microorganisms.
Air emissions of HCl can lead to the formation of acid rain, which has far-reaching consequences for both natural environments and man-made structures. Acid rain can damage forests, crops, and aquatic ecosystems, as well as accelerate the corrosion of buildings and infrastructure. In urban areas, HCl emissions can contribute to smog formation, exacerbating air quality issues and potentially impacting human respiratory health.
The production and transportation of HCl also carry environmental risks. Industrial processes that generate HCl as a byproduct or use it as a reagent may release emissions if not properly controlled. Accidental spills during transport or storage can lead to localized environmental damage and pose immediate health hazards to nearby populations.
To mitigate these environmental impacts, teams must focus on implementing robust safety protocols and containment measures. This includes proper storage and handling procedures, regular equipment maintenance to prevent leaks, and the use of appropriate personal protective equipment (PPE) for workers. Additionally, teams should be trained in spill response and emergency procedures to minimize environmental damage in case of accidents.
Waste management is another crucial aspect of HCl environmental impact. Proper neutralization and disposal of HCl-containing waste are essential to prevent contamination of water sources and soil. Teams should be well-versed in local regulations regarding hazardous waste disposal and implement best practices for waste reduction and recycling where possible.
Monitoring and control systems play a vital role in managing HCl's environmental impact. Continuous emission monitoring systems (CEMS) can help teams track and control HCl releases, ensuring compliance with environmental regulations. Regular environmental assessments and audits can identify potential areas of improvement in handling and containment practices.
As teams expand their HCl proficiency, they should also explore alternatives and technologies that can reduce its environmental footprint. This may include investigating less hazardous substitutes, implementing closed-loop systems to minimize releases, or adopting more efficient processes that require less HCl. By prioritizing environmental considerations alongside technical proficiency, teams can ensure responsible and sustainable use of hydrochloric acid in their operations.
Air emissions of HCl can lead to the formation of acid rain, which has far-reaching consequences for both natural environments and man-made structures. Acid rain can damage forests, crops, and aquatic ecosystems, as well as accelerate the corrosion of buildings and infrastructure. In urban areas, HCl emissions can contribute to smog formation, exacerbating air quality issues and potentially impacting human respiratory health.
The production and transportation of HCl also carry environmental risks. Industrial processes that generate HCl as a byproduct or use it as a reagent may release emissions if not properly controlled. Accidental spills during transport or storage can lead to localized environmental damage and pose immediate health hazards to nearby populations.
To mitigate these environmental impacts, teams must focus on implementing robust safety protocols and containment measures. This includes proper storage and handling procedures, regular equipment maintenance to prevent leaks, and the use of appropriate personal protective equipment (PPE) for workers. Additionally, teams should be trained in spill response and emergency procedures to minimize environmental damage in case of accidents.
Waste management is another crucial aspect of HCl environmental impact. Proper neutralization and disposal of HCl-containing waste are essential to prevent contamination of water sources and soil. Teams should be well-versed in local regulations regarding hazardous waste disposal and implement best practices for waste reduction and recycling where possible.
Monitoring and control systems play a vital role in managing HCl's environmental impact. Continuous emission monitoring systems (CEMS) can help teams track and control HCl releases, ensuring compliance with environmental regulations. Regular environmental assessments and audits can identify potential areas of improvement in handling and containment practices.
As teams expand their HCl proficiency, they should also explore alternatives and technologies that can reduce its environmental footprint. This may include investigating less hazardous substitutes, implementing closed-loop systems to minimize releases, or adopting more efficient processes that require less HCl. By prioritizing environmental considerations alongside technical proficiency, teams can ensure responsible and sustainable use of hydrochloric acid in their operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!