Hydrochloric Acid: A Catalyst for Innovative Research
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Catalysis Background and Objectives
Hydrochloric acid (HCl) has long been recognized as a versatile and powerful catalyst in various chemical processes. Its role in catalysis dates back to the early days of industrial chemistry, where it was first utilized in large-scale production of organic compounds. The evolution of HCl as a catalyst has been closely tied to the development of the chemical industry, particularly in areas such as petrochemicals, pharmaceuticals, and materials science.
The primary objective of research on HCl as a catalyst for innovative applications is to explore and expand its potential beyond traditional uses. This includes investigating its effectiveness in novel reaction pathways, enhancing its catalytic efficiency, and developing more sustainable and environmentally friendly processes. Researchers aim to harness the unique properties of HCl, such as its strong acidity and ability to form complexes with various metals, to catalyze reactions that were previously challenging or impossible.
One of the key trends in HCl catalysis research is the development of supported HCl catalysts. These systems aim to combine the high activity of HCl with the advantages of heterogeneous catalysis, such as ease of separation and reusability. This approach addresses some of the drawbacks associated with homogeneous HCl catalysis, including corrosion and difficulty in product separation.
Another significant area of focus is the application of HCl catalysis in green chemistry. Researchers are exploring ways to use HCl as a catalyst in more environmentally benign processes, such as biomass conversion and the synthesis of biodegradable polymers. The goal is to leverage HCl's catalytic prowess while minimizing its environmental impact and reducing the reliance on more toxic or expensive catalysts.
The field of HCl catalysis is also witnessing advancements in the understanding of reaction mechanisms at the molecular level. This deeper insight is crucial for designing more efficient and selective catalytic systems. Researchers are employing advanced spectroscopic techniques and computational modeling to elucidate the intricate interactions between HCl, substrates, and reaction intermediates.
Furthermore, there is growing interest in exploring the synergistic effects of combining HCl with other catalysts or additives. This approach, known as cooperative catalysis, aims to achieve enhanced reactivity and selectivity by leveraging the complementary properties of different catalytic species. Such innovations could potentially lead to breakthroughs in challenging transformations and open up new avenues for chemical synthesis.
As research in HCl catalysis progresses, the ultimate aim is to develop more efficient, sustainable, and versatile catalytic systems that can address current and future challenges in chemical manufacturing, energy production, and environmental remediation. The ongoing efforts in this field are expected to contribute significantly to the advancement of catalytic science and its practical applications across various industries.
The primary objective of research on HCl as a catalyst for innovative applications is to explore and expand its potential beyond traditional uses. This includes investigating its effectiveness in novel reaction pathways, enhancing its catalytic efficiency, and developing more sustainable and environmentally friendly processes. Researchers aim to harness the unique properties of HCl, such as its strong acidity and ability to form complexes with various metals, to catalyze reactions that were previously challenging or impossible.
One of the key trends in HCl catalysis research is the development of supported HCl catalysts. These systems aim to combine the high activity of HCl with the advantages of heterogeneous catalysis, such as ease of separation and reusability. This approach addresses some of the drawbacks associated with homogeneous HCl catalysis, including corrosion and difficulty in product separation.
Another significant area of focus is the application of HCl catalysis in green chemistry. Researchers are exploring ways to use HCl as a catalyst in more environmentally benign processes, such as biomass conversion and the synthesis of biodegradable polymers. The goal is to leverage HCl's catalytic prowess while minimizing its environmental impact and reducing the reliance on more toxic or expensive catalysts.
The field of HCl catalysis is also witnessing advancements in the understanding of reaction mechanisms at the molecular level. This deeper insight is crucial for designing more efficient and selective catalytic systems. Researchers are employing advanced spectroscopic techniques and computational modeling to elucidate the intricate interactions between HCl, substrates, and reaction intermediates.
Furthermore, there is growing interest in exploring the synergistic effects of combining HCl with other catalysts or additives. This approach, known as cooperative catalysis, aims to achieve enhanced reactivity and selectivity by leveraging the complementary properties of different catalytic species. Such innovations could potentially lead to breakthroughs in challenging transformations and open up new avenues for chemical synthesis.
As research in HCl catalysis progresses, the ultimate aim is to develop more efficient, sustainable, and versatile catalytic systems that can address current and future challenges in chemical manufacturing, energy production, and environmental remediation. The ongoing efforts in this field are expected to contribute significantly to the advancement of catalytic science and its practical applications across various industries.
Market Demand Analysis for HCl Catalysts
The market demand for hydrochloric acid (HCl) as a catalyst has been steadily growing across various industries, driven by its versatility and cost-effectiveness. In the chemical industry, HCl catalysts play a crucial role in numerous processes, including the production of pharmaceuticals, agrochemicals, and specialty chemicals. The pharmaceutical sector, in particular, has shown a significant increase in demand for HCl catalysts due to the rising need for complex drug molecules and the expansion of generic drug manufacturing.
The petrochemical industry represents another major market for HCl catalysts, where they are extensively used in alkylation processes for the production of high-octane gasoline components. As global energy demands continue to rise, the market for HCl catalysts in this sector is expected to expand further. Additionally, the growing focus on sustainable and environmentally friendly processes has led to increased research and development efforts in using HCl catalysts for biomass conversion and renewable energy applications.
In the electronics industry, HCl catalysts are essential for the production of high-purity silicon wafers used in semiconductor manufacturing. With the ongoing digital transformation and the proliferation of smart devices, the demand for HCl catalysts in this sector is projected to experience substantial growth. The automotive industry also contributes to the market demand, particularly in the production of advanced materials and coatings for vehicle components.
Geographically, Asia-Pacific has emerged as the largest market for HCl catalysts, driven by rapid industrialization and economic growth in countries like China and India. North America and Europe follow closely, with established chemical and pharmaceutical industries contributing significantly to the demand. Emerging economies in Latin America and Africa are also showing increased interest in HCl catalysts as they expand their industrial capabilities.
The market demand for HCl catalysts is further influenced by ongoing research into novel applications and improved catalytic systems. Innovations in nanocatalysis and the development of supported HCl catalysts are opening up new possibilities for more efficient and selective chemical transformations. This continuous innovation is expected to drive market growth and create new opportunities across various industrial sectors.
However, the market also faces challenges, such as environmental concerns related to the corrosive nature of HCl and the need for safer handling practices. These factors are driving research into greener alternatives and more sustainable catalytic processes, which may impact the future market dynamics for HCl catalysts.
The petrochemical industry represents another major market for HCl catalysts, where they are extensively used in alkylation processes for the production of high-octane gasoline components. As global energy demands continue to rise, the market for HCl catalysts in this sector is expected to expand further. Additionally, the growing focus on sustainable and environmentally friendly processes has led to increased research and development efforts in using HCl catalysts for biomass conversion and renewable energy applications.
In the electronics industry, HCl catalysts are essential for the production of high-purity silicon wafers used in semiconductor manufacturing. With the ongoing digital transformation and the proliferation of smart devices, the demand for HCl catalysts in this sector is projected to experience substantial growth. The automotive industry also contributes to the market demand, particularly in the production of advanced materials and coatings for vehicle components.
Geographically, Asia-Pacific has emerged as the largest market for HCl catalysts, driven by rapid industrialization and economic growth in countries like China and India. North America and Europe follow closely, with established chemical and pharmaceutical industries contributing significantly to the demand. Emerging economies in Latin America and Africa are also showing increased interest in HCl catalysts as they expand their industrial capabilities.
The market demand for HCl catalysts is further influenced by ongoing research into novel applications and improved catalytic systems. Innovations in nanocatalysis and the development of supported HCl catalysts are opening up new possibilities for more efficient and selective chemical transformations. This continuous innovation is expected to drive market growth and create new opportunities across various industrial sectors.
However, the market also faces challenges, such as environmental concerns related to the corrosive nature of HCl and the need for safer handling practices. These factors are driving research into greener alternatives and more sustainable catalytic processes, which may impact the future market dynamics for HCl catalysts.
Current State and Challenges in HCl Catalysis
Hydrochloric acid (HCl) catalysis has emerged as a significant area of research in recent years, with applications spanning various industries. The current state of HCl catalysis is characterized by a blend of established processes and ongoing innovations. In industrial settings, HCl catalysis is widely employed in petrochemical processes, particularly in the production of vinyl chloride monomer and chlorinated hydrocarbons. These applications have been refined over decades, resulting in highly efficient and economically viable processes.
However, the field faces several challenges that hinder its further development and broader adoption. One of the primary concerns is the corrosive nature of HCl, which necessitates the use of specialized equipment and materials. This not only increases the initial capital investment but also raises maintenance costs, potentially limiting the scalability of HCl-catalyzed processes. Additionally, the handling and disposal of HCl present environmental and safety concerns, requiring stringent protocols and regulatory compliance.
Another significant challenge lies in the selectivity of HCl-catalyzed reactions. While HCl is an effective catalyst for many transformations, achieving high selectivity for desired products remains a hurdle in certain applications. This is particularly evident in complex organic syntheses where multiple reaction pathways are possible. Researchers are actively working on developing more selective catalytic systems, often involving the use of HCl in combination with other catalysts or support materials.
The recovery and recycling of HCl in catalytic processes also present technical challenges. Efficient recovery systems are crucial for both economic and environmental reasons, but current technologies often struggle to achieve high recovery rates without significant energy input. This aspect is particularly important in large-scale industrial applications where the volume of HCl used is substantial.
From a geographical perspective, the development and application of HCl catalysis technology show notable regional variations. Advanced research in this field is predominantly conducted in North America, Western Europe, and East Asia, with these regions also hosting the majority of industrial applications. However, emerging economies are increasingly investing in HCl catalysis research, driven by the growing demand for chemical products and the need for more efficient manufacturing processes.
Looking ahead, the field of HCl catalysis is poised for further advancements. Researchers are exploring novel approaches to mitigate the corrosive effects of HCl, such as the development of more resistant materials and innovative reactor designs. Additionally, there is a growing focus on green chemistry principles, with efforts to develop HCl-catalyzed processes that are more environmentally friendly and sustainable. These developments, coupled with ongoing research into reaction mechanisms and catalyst design, are expected to address many of the current challenges and expand the applications of HCl catalysis in the coming years.
However, the field faces several challenges that hinder its further development and broader adoption. One of the primary concerns is the corrosive nature of HCl, which necessitates the use of specialized equipment and materials. This not only increases the initial capital investment but also raises maintenance costs, potentially limiting the scalability of HCl-catalyzed processes. Additionally, the handling and disposal of HCl present environmental and safety concerns, requiring stringent protocols and regulatory compliance.
Another significant challenge lies in the selectivity of HCl-catalyzed reactions. While HCl is an effective catalyst for many transformations, achieving high selectivity for desired products remains a hurdle in certain applications. This is particularly evident in complex organic syntheses where multiple reaction pathways are possible. Researchers are actively working on developing more selective catalytic systems, often involving the use of HCl in combination with other catalysts or support materials.
The recovery and recycling of HCl in catalytic processes also present technical challenges. Efficient recovery systems are crucial for both economic and environmental reasons, but current technologies often struggle to achieve high recovery rates without significant energy input. This aspect is particularly important in large-scale industrial applications where the volume of HCl used is substantial.
From a geographical perspective, the development and application of HCl catalysis technology show notable regional variations. Advanced research in this field is predominantly conducted in North America, Western Europe, and East Asia, with these regions also hosting the majority of industrial applications. However, emerging economies are increasingly investing in HCl catalysis research, driven by the growing demand for chemical products and the need for more efficient manufacturing processes.
Looking ahead, the field of HCl catalysis is poised for further advancements. Researchers are exploring novel approaches to mitigate the corrosive effects of HCl, such as the development of more resistant materials and innovative reactor designs. Additionally, there is a growing focus on green chemistry principles, with efforts to develop HCl-catalyzed processes that are more environmentally friendly and sustainable. These developments, coupled with ongoing research into reaction mechanisms and catalyst design, are expected to address many of the current challenges and expand the applications of HCl catalysis in the coming years.
Existing HCl Catalytic Solutions
01 Production methods of hydrochloric acid
Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.- Production methods of hydrochloric acid: Various methods are employed to produce hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These production methods are optimized for efficiency and purity in industrial settings.
- Purification and concentration techniques: Hydrochloric acid purification and concentration techniques involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and adjust the acid concentration for specific industrial applications, ensuring high-quality product output.
- Industrial applications of hydrochloric acid: Hydrochloric acid finds widespread use in various industries, including metal processing, chemical manufacturing, and water treatment. It is utilized for pH adjustment, metal etching, and as a reagent in numerous chemical processes, showcasing its versatility in industrial applications.
- Safety measures and handling procedures: Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes using appropriate personal protective equipment, implementing spill containment strategies, and following strict storage and transportation guidelines to minimize risks.
- Environmental impact and waste management: Managing the environmental impact of hydrochloric acid involves proper waste treatment, recycling, and neutralization techniques. Efforts are made to minimize emissions and develop eco-friendly alternatives, addressing concerns related to its production and use in various industries.
02 Purification and concentration techniques
Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for various industrial applications.Expand Specific Solutions03 Industrial applications of hydrochloric acid
Hydrochloric acid finds widespread use in various industries, including metal processing, chemical manufacturing, food production, and water treatment. It is utilized for pH adjustment, cleaning, etching, and as a reagent in numerous chemical processes.Expand Specific Solutions04 Safety and handling of hydrochloric acid
Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes the use of appropriate personal protective equipment, storage in compatible containers, and implementation of spill containment systems.Expand Specific Solutions05 Recovery and recycling of hydrochloric acid
Methods for recovering and recycling hydrochloric acid from industrial processes are developed to reduce waste and improve economic efficiency. These techniques often involve absorption, distillation, and membrane separation to reclaim and purify the acid for reuse.Expand Specific Solutions
Key Players in HCl Catalyst Industry
The research on hydrochloric acid as a catalyst for innovative research is in a mature stage, with a competitive landscape dominated by established chemical and pharmaceutical companies. The market size for this technology is substantial, driven by its wide-ranging applications in various industries. Companies like Mitsui Chemicals, Sumitomo Chemical, and Bayer AG are leading players, leveraging their extensive R&D capabilities and global presence. The technology's maturity is evident from the involvement of diverse entities, including academic institutions like the University of Surrey and specialized firms such as Sandoz AG, indicating a well-developed ecosystem. However, ongoing research by universities and emerging players suggests potential for further innovation and market expansion.
Mitsui Chemicals, Inc.
Technical Solution: Mitsui Chemicals has conducted significant research on hydrochloric acid as a catalyst for various chemical processes. They have developed an HCl-catalyzed method for the production of bisphenol A, a key monomer in polycarbonate and epoxy resin synthesis[12]. This process offers improved efficiency and reduced environmental impact compared to traditional methods. The company has also explored the use of HCl catalysis in the synthesis of specialty isocyanates, leveraging their expertise in polyurethane chemistry[13]. Mitsui Chemicals' research extends to the application of HCl-catalyzed reactions in the production of high-performance polymers and advanced materials, demonstrating their commitment to innovation in the chemical industry[14].
Strengths: Broad portfolio of chemical products, strong R&D capabilities, and a global presence in the chemical industry. Weaknesses: Potential challenges in balancing innovation with environmental concerns associated with HCl use, and the need for continuous adaptation to changing regulatory landscapes.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has conducted extensive research on hydrochloric acid as a catalyst for various chemical transformations. They have developed a highly efficient HCl-catalyzed process for the conversion of cellulose into 5-hydroxymethylfurfural (HMF), a promising platform chemical for biofuels and materials[7]. This method achieves high yields under relatively mild conditions, addressing the challenge of biomass valorization. The institute has also investigated the use of HCl as a catalyst in the synthesis of graphene quantum dots from coal, offering a cost-effective route to these advanced materials[8]. Additionally, their researchers have explored HCl-catalyzed reactions for the production of biodiesel from waste cooking oils, contributing to sustainable energy solutions[9].
Strengths: Strong focus on applied research, expertise in catalysis and materials science, and emphasis on sustainable technologies. Weaknesses: Potential challenges in technology transfer and commercialization of research findings.
Core Innovations in HCl Catalysis
Method for producing a solid carrier for hydrochloric acid
PatentWO2003040031A2
Innovation
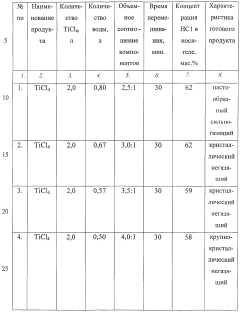
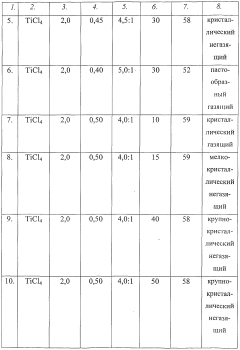
- The interaction of titanium tetrachloride with water in specific ratios (3.0÷4.5) within a sealed apparatus for 15-40 minutes, optimizing the crystallization process and excluding hydrolysis to produce a stable solid carrier of hydrochloric acid in the form of titanium dihydrate crystals.
HYDROCHLORIC ACID RECOVERY AND REGENERATION PROCESS WITH SULFATE PRODUCTION
PatentInactiveBR102022004827A2
Innovation
- A process that regenerates hydrochloric acid by reacting exhausted acid with sulfuric acid, producing zinc sulfate and iron sulfate, utilizing a reactor with heating and agitation, followed by concentration and absorption, allowing the acid to be reused and the sulfates to be used in various industries.
Environmental Impact of HCl Catalysis
The use of hydrochloric acid (HCl) as a catalyst in innovative research has significant environmental implications that warrant careful consideration. While HCl catalysis offers numerous benefits in terms of reaction efficiency and product yield, its potential environmental impact cannot be overlooked.
One of the primary environmental concerns associated with HCl catalysis is the generation of acidic waste streams. These effluents, if not properly treated, can lead to soil and water acidification, causing detrimental effects on local ecosystems. The release of HCl-containing waste into aquatic environments can disrupt the pH balance, potentially harming fish populations and other aquatic organisms.
Atmospheric emissions during HCl catalysis processes also pose environmental risks. Gaseous HCl releases can contribute to acid rain formation, which has far-reaching consequences for both terrestrial and aquatic ecosystems. Moreover, the corrosive nature of HCl vapors can damage infrastructure and vegetation in the vicinity of industrial facilities employing this catalytic method.
The production and transportation of HCl for catalytic purposes present additional environmental challenges. The manufacturing process of HCl often involves energy-intensive operations and the potential for accidental releases. Transportation accidents involving HCl can result in localized environmental contamination and pose risks to human health and safety.
However, it is important to note that advancements in process engineering and environmental technologies have led to significant improvements in mitigating the environmental impact of HCl catalysis. The implementation of closed-loop systems, where HCl is recycled and reused within the process, has greatly reduced waste generation and emissions. Additionally, the development of efficient scrubbing technologies has enabled better control of atmospheric releases.
Furthermore, research into alternative catalysts and greener chemistry approaches has gained momentum in recent years. These efforts aim to reduce reliance on HCl and other potentially harmful catalysts, exploring more environmentally benign options such as solid acid catalysts or biocatalysts.
The regulatory landscape surrounding HCl use in industrial processes has also evolved, with stricter environmental standards and monitoring requirements being implemented globally. This has driven industries to adopt more sustainable practices and invest in pollution control technologies.
In conclusion, while the environmental impact of HCl catalysis remains a concern, ongoing research and technological advancements are continuously improving the sustainability of these processes. Balancing the benefits of HCl catalysis with environmental protection requires a holistic approach, incorporating innovative technologies, responsible management practices, and regulatory compliance.
One of the primary environmental concerns associated with HCl catalysis is the generation of acidic waste streams. These effluents, if not properly treated, can lead to soil and water acidification, causing detrimental effects on local ecosystems. The release of HCl-containing waste into aquatic environments can disrupt the pH balance, potentially harming fish populations and other aquatic organisms.
Atmospheric emissions during HCl catalysis processes also pose environmental risks. Gaseous HCl releases can contribute to acid rain formation, which has far-reaching consequences for both terrestrial and aquatic ecosystems. Moreover, the corrosive nature of HCl vapors can damage infrastructure and vegetation in the vicinity of industrial facilities employing this catalytic method.
The production and transportation of HCl for catalytic purposes present additional environmental challenges. The manufacturing process of HCl often involves energy-intensive operations and the potential for accidental releases. Transportation accidents involving HCl can result in localized environmental contamination and pose risks to human health and safety.
However, it is important to note that advancements in process engineering and environmental technologies have led to significant improvements in mitigating the environmental impact of HCl catalysis. The implementation of closed-loop systems, where HCl is recycled and reused within the process, has greatly reduced waste generation and emissions. Additionally, the development of efficient scrubbing technologies has enabled better control of atmospheric releases.
Furthermore, research into alternative catalysts and greener chemistry approaches has gained momentum in recent years. These efforts aim to reduce reliance on HCl and other potentially harmful catalysts, exploring more environmentally benign options such as solid acid catalysts or biocatalysts.
The regulatory landscape surrounding HCl use in industrial processes has also evolved, with stricter environmental standards and monitoring requirements being implemented globally. This has driven industries to adopt more sustainable practices and invest in pollution control technologies.
In conclusion, while the environmental impact of HCl catalysis remains a concern, ongoing research and technological advancements are continuously improving the sustainability of these processes. Balancing the benefits of HCl catalysis with environmental protection requires a holistic approach, incorporating innovative technologies, responsible management practices, and regulatory compliance.
Safety Protocols in HCl Catalyst Handling
The safe handling of hydrochloric acid (HCl) as a catalyst in innovative research requires stringent safety protocols to protect researchers and the environment. These protocols encompass personal protective equipment (PPE), proper storage and handling procedures, emergency response plans, and waste management strategies.
Appropriate PPE is crucial when working with HCl. Researchers must wear chemical-resistant gloves, safety goggles or face shields, and lab coats or acid-resistant aprons. In cases where HCl vapors may be present, respiratory protection such as a properly fitted respirator with acid gas cartridges is necessary. Closed-toe shoes and long pants are also required to minimize skin exposure.
Proper storage of HCl is essential to prevent accidents and maintain its catalytic properties. HCl should be stored in a well-ventilated area, away from heat sources and direct sunlight. Containers must be clearly labeled and kept tightly sealed when not in use. Incompatible materials, such as strong bases, metals, and oxidizing agents, should be stored separately to prevent dangerous reactions.
Handling procedures for HCl catalysts require careful attention to detail. Always work in a fume hood when using or transferring HCl to minimize exposure to vapors. Use only compatible materials for containers and equipment, such as glass, polyethylene, or certain grades of stainless steel. When diluting HCl, always add acid to water slowly while stirring to prevent splashing and excessive heat generation.
Emergency response plans are critical for addressing potential spills or exposures. Laboratories should be equipped with easily accessible eyewash stations and safety showers. Spill kits containing neutralizing agents, absorbents, and appropriate PPE must be readily available. All researchers should be trained in proper spill response procedures and the use of emergency equipment.
Waste management is an integral part of HCl catalyst safety protocols. Neutralization of waste HCl should be performed carefully, typically using sodium bicarbonate or sodium hydroxide, while monitoring pH levels. Disposal must comply with local, state, and federal regulations, often requiring specialized waste handling services.
Regular safety training and refresher courses are essential to ensure all personnel are up-to-date on the latest safety protocols and best practices. This includes proper documentation of safety procedures, regular safety audits, and continuous improvement of safety measures based on new research and industry standards.
Implementing these comprehensive safety protocols not only protects researchers and the environment but also ensures the integrity of the research process. By maintaining a safe working environment, innovative research using HCl as a catalyst can proceed efficiently and effectively, contributing to scientific advancement while minimizing risks.
Appropriate PPE is crucial when working with HCl. Researchers must wear chemical-resistant gloves, safety goggles or face shields, and lab coats or acid-resistant aprons. In cases where HCl vapors may be present, respiratory protection such as a properly fitted respirator with acid gas cartridges is necessary. Closed-toe shoes and long pants are also required to minimize skin exposure.
Proper storage of HCl is essential to prevent accidents and maintain its catalytic properties. HCl should be stored in a well-ventilated area, away from heat sources and direct sunlight. Containers must be clearly labeled and kept tightly sealed when not in use. Incompatible materials, such as strong bases, metals, and oxidizing agents, should be stored separately to prevent dangerous reactions.
Handling procedures for HCl catalysts require careful attention to detail. Always work in a fume hood when using or transferring HCl to minimize exposure to vapors. Use only compatible materials for containers and equipment, such as glass, polyethylene, or certain grades of stainless steel. When diluting HCl, always add acid to water slowly while stirring to prevent splashing and excessive heat generation.
Emergency response plans are critical for addressing potential spills or exposures. Laboratories should be equipped with easily accessible eyewash stations and safety showers. Spill kits containing neutralizing agents, absorbents, and appropriate PPE must be readily available. All researchers should be trained in proper spill response procedures and the use of emergency equipment.
Waste management is an integral part of HCl catalyst safety protocols. Neutralization of waste HCl should be performed carefully, typically using sodium bicarbonate or sodium hydroxide, while monitoring pH levels. Disposal must comply with local, state, and federal regulations, often requiring specialized waste handling services.
Regular safety training and refresher courses are essential to ensure all personnel are up-to-date on the latest safety protocols and best practices. This includes proper documentation of safety procedures, regular safety audits, and continuous improvement of safety measures based on new research and industry standards.
Implementing these comprehensive safety protocols not only protects researchers and the environment but also ensures the integrity of the research process. By maintaining a safe working environment, innovative research using HCl as a catalyst can proceed efficiently and effectively, contributing to scientific advancement while minimizing risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!