How to Enhance Efficiency in Hydrochloric Acid Neutralization?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Neutralization Background and Objectives
Hydrochloric acid neutralization is a fundamental process in chemical engineering, with applications spanning various industries. The efficiency of this process has become increasingly crucial as industries strive for cost-effectiveness and environmental sustainability. The evolution of HCl neutralization techniques can be traced back to the early 20th century, with significant advancements occurring in recent decades.
The primary objective of enhancing efficiency in hydrochloric acid neutralization is to achieve complete neutralization while minimizing resource consumption and environmental impact. This involves optimizing factors such as reaction time, reagent usage, energy input, and waste generation. As global industrial processes continue to expand, the demand for more efficient neutralization methods has grown exponentially.
Current technological trends in HCl neutralization focus on developing innovative reactor designs, exploring novel neutralizing agents, and implementing advanced process control systems. These advancements aim to address the limitations of traditional batch processes, such as long reaction times and high energy consumption. Continuous flow reactors and microfluidic systems have emerged as promising alternatives, offering improved mixing efficiency and reduced reaction times.
The environmental aspect of HCl neutralization has gained significant attention in recent years. Regulatory pressures and corporate sustainability initiatives have driven research towards greener neutralization processes. This includes the exploration of bio-based neutralizing agents and the development of closed-loop systems that minimize waste generation and maximize resource recovery.
Another key technological goal is the integration of real-time monitoring and control systems. Advanced sensors and data analytics are being employed to optimize neutralization processes dynamically, ensuring precise pH control and reagent dosing. This not only enhances efficiency but also improves product quality and consistency.
The pursuit of enhanced efficiency in HCl neutralization aligns with broader industry trends towards process intensification and smart manufacturing. As such, research efforts are increasingly focused on developing modular, scalable solutions that can be easily integrated into existing industrial setups. This approach aims to make advanced neutralization technologies more accessible to a wider range of industries, from small-scale operations to large chemical plants.
The primary objective of enhancing efficiency in hydrochloric acid neutralization is to achieve complete neutralization while minimizing resource consumption and environmental impact. This involves optimizing factors such as reaction time, reagent usage, energy input, and waste generation. As global industrial processes continue to expand, the demand for more efficient neutralization methods has grown exponentially.
Current technological trends in HCl neutralization focus on developing innovative reactor designs, exploring novel neutralizing agents, and implementing advanced process control systems. These advancements aim to address the limitations of traditional batch processes, such as long reaction times and high energy consumption. Continuous flow reactors and microfluidic systems have emerged as promising alternatives, offering improved mixing efficiency and reduced reaction times.
The environmental aspect of HCl neutralization has gained significant attention in recent years. Regulatory pressures and corporate sustainability initiatives have driven research towards greener neutralization processes. This includes the exploration of bio-based neutralizing agents and the development of closed-loop systems that minimize waste generation and maximize resource recovery.
Another key technological goal is the integration of real-time monitoring and control systems. Advanced sensors and data analytics are being employed to optimize neutralization processes dynamically, ensuring precise pH control and reagent dosing. This not only enhances efficiency but also improves product quality and consistency.
The pursuit of enhanced efficiency in HCl neutralization aligns with broader industry trends towards process intensification and smart manufacturing. As such, research efforts are increasingly focused on developing modular, scalable solutions that can be easily integrated into existing industrial setups. This approach aims to make advanced neutralization technologies more accessible to a wider range of industries, from small-scale operations to large chemical plants.
Market Analysis for Efficient Neutralization Processes
The market for efficient hydrochloric acid neutralization processes is experiencing significant growth, driven by increasing environmental regulations and the need for cost-effective industrial waste management solutions. The global market for acid neutralization technologies is projected to reach $5.2 billion by 2025, with a compound annual growth rate of 4.8% from 2020 to 2025. This growth is primarily fueled by the expanding chemical, pharmaceutical, and mining industries, which generate large volumes of acidic waste requiring treatment.
In the chemical industry, which accounts for approximately 40% of the market share, there is a growing demand for advanced neutralization processes that can handle high concentrations of hydrochloric acid while minimizing reagent consumption and waste generation. The pharmaceutical sector, representing about 25% of the market, requires precise and efficient neutralization methods to meet stringent quality standards and reduce production costs.
The mining industry, contributing roughly 20% to the market, is increasingly adopting innovative neutralization technologies to address environmental concerns and comply with stricter regulations on acid mine drainage. The remaining 15% of the market is distributed among various industries, including food processing, textile manufacturing, and water treatment facilities.
Geographically, Asia-Pacific dominates the market with a 35% share, followed by North America (30%) and Europe (25%). The rapid industrialization in countries like China and India is driving the demand for efficient neutralization processes in the Asia-Pacific region. North America and Europe, on the other hand, are focusing on developing advanced technologies to meet more stringent environmental regulations.
Key market trends include the adoption of continuous flow neutralization systems, which offer improved efficiency and reduced chemical consumption compared to batch processes. There is also a growing interest in the development of smart neutralization systems that utilize real-time monitoring and automated control to optimize the process and reduce operational costs.
The market is characterized by intense competition among established players and new entrants. Major companies are investing heavily in research and development to create innovative solutions that offer higher neutralization efficiency, lower reagent consumption, and reduced environmental impact. Collaborations between industry players and research institutions are becoming more common, aiming to accelerate the development of next-generation neutralization technologies.
Customer demand is shifting towards integrated solutions that not only neutralize hydrochloric acid but also recover valuable by-products and minimize waste generation. This trend is creating new opportunities for companies that can offer comprehensive waste management solutions tailored to specific industry needs.
In the chemical industry, which accounts for approximately 40% of the market share, there is a growing demand for advanced neutralization processes that can handle high concentrations of hydrochloric acid while minimizing reagent consumption and waste generation. The pharmaceutical sector, representing about 25% of the market, requires precise and efficient neutralization methods to meet stringent quality standards and reduce production costs.
The mining industry, contributing roughly 20% to the market, is increasingly adopting innovative neutralization technologies to address environmental concerns and comply with stricter regulations on acid mine drainage. The remaining 15% of the market is distributed among various industries, including food processing, textile manufacturing, and water treatment facilities.
Geographically, Asia-Pacific dominates the market with a 35% share, followed by North America (30%) and Europe (25%). The rapid industrialization in countries like China and India is driving the demand for efficient neutralization processes in the Asia-Pacific region. North America and Europe, on the other hand, are focusing on developing advanced technologies to meet more stringent environmental regulations.
Key market trends include the adoption of continuous flow neutralization systems, which offer improved efficiency and reduced chemical consumption compared to batch processes. There is also a growing interest in the development of smart neutralization systems that utilize real-time monitoring and automated control to optimize the process and reduce operational costs.
The market is characterized by intense competition among established players and new entrants. Major companies are investing heavily in research and development to create innovative solutions that offer higher neutralization efficiency, lower reagent consumption, and reduced environmental impact. Collaborations between industry players and research institutions are becoming more common, aiming to accelerate the development of next-generation neutralization technologies.
Customer demand is shifting towards integrated solutions that not only neutralize hydrochloric acid but also recover valuable by-products and minimize waste generation. This trend is creating new opportunities for companies that can offer comprehensive waste management solutions tailored to specific industry needs.
Current Challenges in HCl Neutralization
The neutralization of hydrochloric acid (HCl) is a fundamental process in various industries, yet it faces several significant challenges that hinder its efficiency. One of the primary issues is the exothermic nature of the reaction, which can lead to rapid temperature increases. This heat generation not only poses safety risks but also affects the reaction kinetics, potentially reducing the overall efficiency of the neutralization process.
Another challenge lies in the corrosive nature of HCl, which necessitates the use of specialized equipment and materials. This requirement often results in increased operational costs and maintenance challenges. The corrosion problem is particularly acute in industrial settings where large volumes of HCl are handled, leading to accelerated wear and tear on processing equipment.
The precise control of the neutralization reaction presents another significant hurdle. Achieving the desired pH level consistently and accurately is crucial for many applications, yet it remains a complex task. Fluctuations in acid concentration, variations in the neutralizing agent's properties, and the dynamic nature of the reaction all contribute to this challenge. Overcoming these issues often requires sophisticated monitoring and control systems, which can be both expensive and complex to implement.
Furthermore, the disposal of byproducts from HCl neutralization poses environmental concerns. The resulting salt solutions, often containing various impurities, require proper treatment and disposal. This aspect not only adds to the operational costs but also necessitates compliance with increasingly stringent environmental regulations.
The efficiency of HCl neutralization is also impacted by mass transfer limitations, particularly in large-scale operations. Ensuring uniform mixing and contact between the acid and the neutralizing agent throughout the reaction vessel is crucial for optimal efficiency. However, achieving this in industrial-scale reactors can be challenging, often leading to localized areas of incomplete neutralization or excess reagent use.
Lastly, the selection of an appropriate neutralizing agent presents its own set of challenges. While common bases like sodium hydroxide are effective, they may introduce unwanted ions into the final product. Alternative neutralizing agents may offer benefits in terms of product quality or environmental impact, but often at the cost of increased complexity or expense in the neutralization process.
Addressing these challenges requires a multifaceted approach, combining innovations in reactor design, advanced process control strategies, and the development of novel neutralizing agents. Enhancing the efficiency of HCl neutralization remains a critical area of research and development, with significant implications for various industries relying on this fundamental chemical process.
Another challenge lies in the corrosive nature of HCl, which necessitates the use of specialized equipment and materials. This requirement often results in increased operational costs and maintenance challenges. The corrosion problem is particularly acute in industrial settings where large volumes of HCl are handled, leading to accelerated wear and tear on processing equipment.
The precise control of the neutralization reaction presents another significant hurdle. Achieving the desired pH level consistently and accurately is crucial for many applications, yet it remains a complex task. Fluctuations in acid concentration, variations in the neutralizing agent's properties, and the dynamic nature of the reaction all contribute to this challenge. Overcoming these issues often requires sophisticated monitoring and control systems, which can be both expensive and complex to implement.
Furthermore, the disposal of byproducts from HCl neutralization poses environmental concerns. The resulting salt solutions, often containing various impurities, require proper treatment and disposal. This aspect not only adds to the operational costs but also necessitates compliance with increasingly stringent environmental regulations.
The efficiency of HCl neutralization is also impacted by mass transfer limitations, particularly in large-scale operations. Ensuring uniform mixing and contact between the acid and the neutralizing agent throughout the reaction vessel is crucial for optimal efficiency. However, achieving this in industrial-scale reactors can be challenging, often leading to localized areas of incomplete neutralization or excess reagent use.
Lastly, the selection of an appropriate neutralizing agent presents its own set of challenges. While common bases like sodium hydroxide are effective, they may introduce unwanted ions into the final product. Alternative neutralizing agents may offer benefits in terms of product quality or environmental impact, but often at the cost of increased complexity or expense in the neutralization process.
Addressing these challenges requires a multifaceted approach, combining innovations in reactor design, advanced process control strategies, and the development of novel neutralizing agents. Enhancing the efficiency of HCl neutralization remains a critical area of research and development, with significant implications for various industries relying on this fundamental chemical process.
Existing HCl Neutralization Methods
01 Neutralization using alkaline substances
Hydrochloric acid can be efficiently neutralized using various alkaline substances. This process involves the reaction between the acid and base to form salt and water. Common alkaline substances used include sodium hydroxide, calcium hydroxide, and magnesium hydroxide. The efficiency of neutralization depends on factors such as the concentration of the alkaline substance, reaction time, and mixing conditions.- Neutralization using alkaline substances: Alkaline substances such as calcium carbonate, sodium hydroxide, or magnesium hydroxide are commonly used to neutralize hydrochloric acid. These materials react with the acid to form salt and water, effectively increasing the pH of the solution. The efficiency of neutralization depends on factors such as the concentration of the alkaline substance, reaction time, and mixing conditions.
- Continuous neutralization systems: Continuous neutralization systems are designed to efficiently neutralize hydrochloric acid in industrial processes. These systems often involve a series of reaction chambers or columns where the acid is gradually neutralized as it flows through. Continuous monitoring and adjustment of pH levels ensure optimal neutralization efficiency throughout the process.
- Use of ion exchange resins: Ion exchange resins can be employed to neutralize hydrochloric acid efficiently. These resins contain functional groups that can exchange ions with the acid, effectively removing the hydrogen ions and neutralizing the solution. The efficiency of this method depends on the type of resin used, its capacity, and regeneration frequency.
- Neutralization with gas injection: Gaseous alkaline substances, such as ammonia or carbon dioxide, can be injected into hydrochloric acid solutions for neutralization. This method allows for precise control of the neutralization process and can be particularly efficient for large-scale applications. The efficiency is influenced by factors like gas flow rate, bubble size, and mixing conditions.
- Electrochemical neutralization: Electrochemical methods can be used to neutralize hydrochloric acid efficiently. These techniques involve the use of electrodes to generate hydroxide ions or to directly reduce the concentration of hydrogen ions in the solution. The efficiency of electrochemical neutralization depends on factors such as electrode material, current density, and solution conductivity.
02 Continuous flow neutralization systems
Continuous flow systems can be employed for efficient neutralization of hydrochloric acid. These systems typically involve a series of reaction chambers or tanks where the acid is gradually neutralized as it flows through. The use of continuous flow systems allows for better control of the neutralization process, improved efficiency, and the ability to handle large volumes of acid.Expand Specific Solutions03 Neutralization with gas injection
Gas injection methods can be used to neutralize hydrochloric acid efficiently. This technique involves injecting alkaline gases, such as ammonia, into the acid solution. The gas reacts with the acid, neutralizing it in the process. This method can be particularly effective for treating large volumes of acid and can offer advantages in terms of reaction speed and control.Expand Specific Solutions04 Monitoring and control systems for neutralization
Advanced monitoring and control systems can significantly improve the efficiency of hydrochloric acid neutralization. These systems typically involve pH sensors, flow meters, and automated dosing equipment. By continuously monitoring the pH and adjusting the addition of neutralizing agents, these systems can optimize the neutralization process, reduce waste, and ensure consistent results.Expand Specific Solutions05 Use of catalysts in neutralization
Catalysts can be employed to enhance the efficiency of hydrochloric acid neutralization. These substances can accelerate the reaction between the acid and the neutralizing agent without being consumed in the process. The use of catalysts can lead to faster neutralization rates, reduced energy requirements, and improved overall efficiency of the neutralization process.Expand Specific Solutions
Key Industry Players in Chemical Neutralization
The hydrochloric acid neutralization market is in a mature stage, with established players like Akzo Nobel Chemicals, Arkema France, and Covestro Deutschland dominating the industry. The global market size is substantial, driven by industrial applications in chemical processing, water treatment, and manufacturing. Technological advancements focus on improving efficiency and environmental sustainability. Companies such as Kurita Water Industries and Element Biosciences are investing in innovative solutions to enhance neutralization processes. The technology's maturity is evident, with ongoing research aimed at optimizing reaction kinetics, reducing waste, and developing more cost-effective methods. Emerging players like Annihilare Medical Systems are introducing novel approaches, potentially disrupting traditional neutralization techniques.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has developed an innovative approach to enhance efficiency in hydrochloric acid neutralization through their Advanced Oxidation Process (AOP) technology. This method combines hydrogen peroxide with UV light or ozone to generate highly reactive hydroxyl radicals, which rapidly oxidize and neutralize hydrochloric acid [1]. The process is optimized using sophisticated control systems that adjust reagent dosing based on real-time pH and oxidation-reduction potential measurements. Additionally, Akzo Nobel has implemented a closed-loop recirculation system that maximizes contact time between the acid and neutralizing agents, significantly improving reaction efficiency and reducing chemical consumption [3].
Strengths: Highly efficient neutralization, reduced chemical usage, and adaptable to various acid concentrations. Weaknesses: Higher initial equipment costs and energy requirements for UV or ozone generation.
Arkema France SA
Technical Solution: Arkema has developed a novel membrane-based technology for hydrochloric acid neutralization. Their system utilizes specially designed ion-exchange membranes that selectively allow the passage of hydrogen ions while retaining chloride ions. This separation process enables the efficient neutralization of hydrochloric acid without the need for additional chemicals. The technology incorporates a bipolar membrane electrodialysis unit, which generates hydroxide ions from water splitting, effectively neutralizing the acid [2]. Arkema's process also includes a heat recovery system that captures and reuses the exothermic heat generated during neutralization, further enhancing overall efficiency [4].
Strengths: Chemical-free neutralization, energy-efficient due to heat recovery, and high purity of recovered products. Weaknesses: Membrane fouling may occur over time, requiring periodic maintenance.
Innovative Approaches in Acid Neutralization
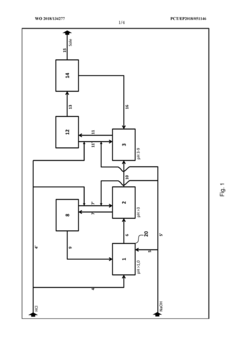
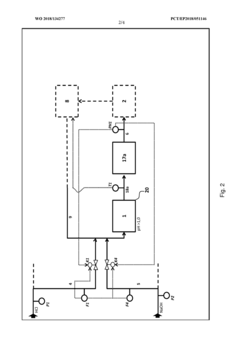
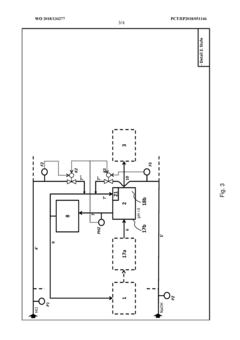
Method and device for the continuous neutralization of hydrochloric acid
PatentWO2018134277A1
Innovation
- A three-stage neutralization process using cooled, recirculated partial streams of the reaction mixture, with static mixers and mixing nozzles for homogenization, and duplicated dosing valves for precise pH adjustment, allowing for continuous operation and heat dissipation through cooling water circuits.
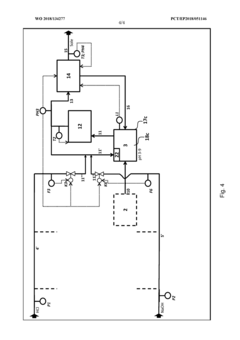
Method and device for the continuous neutralization of hydrochloric acid
PatentActiveEP3571168A1
Innovation
- A three-stage neutralization process using cooled, recirculated partial streams of the reaction mixture, with static mixers and mixing nozzles for homogenization, and duplicated dosing valves for precise pH control, allowing for continuous operation with sodium hydroxide solution, managing pH and temperature while compensating for pressure fluctuations.
Environmental Impact Assessment
The environmental impact assessment of hydrochloric acid neutralization processes is a critical aspect of enhancing efficiency in this chemical operation. The neutralization of hydrochloric acid, while necessary for many industrial applications, can have significant environmental consequences if not managed properly. The primary environmental concerns include the potential release of harmful gases, the generation of waste heat, and the production of byproducts that may affect local ecosystems.
One of the key environmental impacts to consider is the potential for air pollution. During the neutralization process, there is a risk of releasing chlorine gas, which is highly toxic and can have severe effects on both human health and the environment. Proper ventilation systems and gas scrubbers are essential to mitigate this risk and ensure that any emissions are within acceptable limits set by environmental regulations.
Water pollution is another significant concern in hydrochloric acid neutralization. The process often involves the use of large quantities of water, which can become contaminated with residual acid, neutralizing agents, and byproducts. Proper treatment and disposal of this wastewater are crucial to prevent contamination of local water bodies and groundwater resources. Implementing closed-loop systems and water recycling technologies can help reduce the overall water consumption and minimize the risk of environmental contamination.
The generation of waste heat during the neutralization process can also have environmental implications. Excessive heat release can contribute to thermal pollution in nearby water bodies if not properly managed. Implementing heat recovery systems can not only improve the overall energy efficiency of the process but also reduce the environmental impact associated with waste heat discharge.
Soil contamination is another potential environmental risk, particularly in cases of accidental spills or improper disposal of neutralization byproducts. The use of containment systems, spill prevention measures, and proper waste management protocols are essential to mitigate this risk and protect soil quality in the surrounding areas.
The choice of neutralizing agents can also influence the environmental impact of the process. Some neutralizing agents may introduce additional chemical compounds into the environment, potentially affecting local ecosystems. Selecting environmentally friendly neutralizing agents and optimizing their use can help minimize these impacts while maintaining process efficiency.
Lastly, the overall carbon footprint of the neutralization process should be considered in the environmental impact assessment. This includes evaluating the energy consumption of the process, the sourcing of raw materials, and the transportation of chemicals and waste products. Implementing energy-efficient technologies and exploring renewable energy sources can help reduce the carbon emissions associated with hydrochloric acid neutralization.
One of the key environmental impacts to consider is the potential for air pollution. During the neutralization process, there is a risk of releasing chlorine gas, which is highly toxic and can have severe effects on both human health and the environment. Proper ventilation systems and gas scrubbers are essential to mitigate this risk and ensure that any emissions are within acceptable limits set by environmental regulations.
Water pollution is another significant concern in hydrochloric acid neutralization. The process often involves the use of large quantities of water, which can become contaminated with residual acid, neutralizing agents, and byproducts. Proper treatment and disposal of this wastewater are crucial to prevent contamination of local water bodies and groundwater resources. Implementing closed-loop systems and water recycling technologies can help reduce the overall water consumption and minimize the risk of environmental contamination.
The generation of waste heat during the neutralization process can also have environmental implications. Excessive heat release can contribute to thermal pollution in nearby water bodies if not properly managed. Implementing heat recovery systems can not only improve the overall energy efficiency of the process but also reduce the environmental impact associated with waste heat discharge.
Soil contamination is another potential environmental risk, particularly in cases of accidental spills or improper disposal of neutralization byproducts. The use of containment systems, spill prevention measures, and proper waste management protocols are essential to mitigate this risk and protect soil quality in the surrounding areas.
The choice of neutralizing agents can also influence the environmental impact of the process. Some neutralizing agents may introduce additional chemical compounds into the environment, potentially affecting local ecosystems. Selecting environmentally friendly neutralizing agents and optimizing their use can help minimize these impacts while maintaining process efficiency.
Lastly, the overall carbon footprint of the neutralization process should be considered in the environmental impact assessment. This includes evaluating the energy consumption of the process, the sourcing of raw materials, and the transportation of chemicals and waste products. Implementing energy-efficient technologies and exploring renewable energy sources can help reduce the carbon emissions associated with hydrochloric acid neutralization.
Safety Protocols and Regulations
Safety protocols and regulations play a crucial role in enhancing the efficiency of hydrochloric acid neutralization processes while ensuring the protection of personnel and the environment. The handling and neutralization of hydrochloric acid require strict adherence to established safety guidelines and regulatory frameworks.
Proper personal protective equipment (PPE) is essential for all personnel involved in the neutralization process. This includes chemical-resistant gloves, goggles, face shields, and appropriate protective clothing. The selection of PPE must be based on the concentration of the acid and the specific tasks being performed. Regular inspection and maintenance of PPE are necessary to ensure its effectiveness.
Ventilation systems are critical in controlling exposure to acid fumes and vapors. Adequate local exhaust ventilation should be installed in areas where hydrochloric acid is handled or neutralized. The ventilation system must be designed to effectively remove acid vapors and maintain air quality within acceptable limits. Regular monitoring of air quality and ventilation system performance is essential to maintain a safe working environment.
Emergency response procedures must be clearly defined and communicated to all personnel. This includes the location and proper use of emergency showers, eyewash stations, and spill containment equipment. Regular drills and training sessions should be conducted to ensure that all employees are familiar with emergency protocols and can respond effectively in case of accidents or spills.
Proper storage and handling of hydrochloric acid and neutralizing agents are critical for safety and efficiency. Acid-resistant containers should be used for storage, and incompatible materials must be kept separate. Clear labeling and proper documentation of all chemicals are essential for preventing accidents and ensuring proper handling.
Waste management and disposal procedures must comply with local and national regulations. Neutralized acid waste should be properly treated and disposed of according to environmental guidelines. Regular audits and inspections should be conducted to ensure compliance with waste management regulations.
Training and education programs are fundamental to maintaining a safe and efficient neutralization process. All personnel involved in handling hydrochloric acid or performing neutralization tasks must receive comprehensive training on safety protocols, proper handling techniques, and emergency procedures. Regular refresher courses and updates on new regulations or best practices should be provided.
Implementing a robust safety management system is essential for continuous improvement and risk reduction. This system should include regular safety audits, incident reporting and investigation procedures, and mechanisms for implementing corrective actions. By fostering a culture of safety and continuous improvement, organizations can enhance both the efficiency and safety of their hydrochloric acid neutralization processes.
Proper personal protective equipment (PPE) is essential for all personnel involved in the neutralization process. This includes chemical-resistant gloves, goggles, face shields, and appropriate protective clothing. The selection of PPE must be based on the concentration of the acid and the specific tasks being performed. Regular inspection and maintenance of PPE are necessary to ensure its effectiveness.
Ventilation systems are critical in controlling exposure to acid fumes and vapors. Adequate local exhaust ventilation should be installed in areas where hydrochloric acid is handled or neutralized. The ventilation system must be designed to effectively remove acid vapors and maintain air quality within acceptable limits. Regular monitoring of air quality and ventilation system performance is essential to maintain a safe working environment.
Emergency response procedures must be clearly defined and communicated to all personnel. This includes the location and proper use of emergency showers, eyewash stations, and spill containment equipment. Regular drills and training sessions should be conducted to ensure that all employees are familiar with emergency protocols and can respond effectively in case of accidents or spills.
Proper storage and handling of hydrochloric acid and neutralizing agents are critical for safety and efficiency. Acid-resistant containers should be used for storage, and incompatible materials must be kept separate. Clear labeling and proper documentation of all chemicals are essential for preventing accidents and ensuring proper handling.
Waste management and disposal procedures must comply with local and national regulations. Neutralized acid waste should be properly treated and disposed of according to environmental guidelines. Regular audits and inspections should be conducted to ensure compliance with waste management regulations.
Training and education programs are fundamental to maintaining a safe and efficient neutralization process. All personnel involved in handling hydrochloric acid or performing neutralization tasks must receive comprehensive training on safety protocols, proper handling techniques, and emergency procedures. Regular refresher courses and updates on new regulations or best practices should be provided.
Implementing a robust safety management system is essential for continuous improvement and risk reduction. This system should include regular safety audits, incident reporting and investigation procedures, and mechanisms for implementing corrective actions. By fostering a culture of safety and continuous improvement, organizations can enhance both the efficiency and safety of their hydrochloric acid neutralization processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!