Best Practices for Storing Hydrochloric Acid Safely
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Storage Background and Objectives
Hydrochloric acid (HCl) is a widely used industrial chemical with applications ranging from metal processing to food production. Its corrosive nature and potential health hazards necessitate careful handling and storage practices. The evolution of HCl storage techniques has been driven by the need to balance safety, efficiency, and cost-effectiveness.
Historically, HCl storage methods have progressed from simple glass or ceramic containers to more sophisticated systems involving specialized materials and engineering controls. Early industrial use relied on glass carboys, which were fragile and limited in capacity. As demand grew, larger-scale storage solutions became necessary, leading to the development of rubber-lined steel tanks and eventually to the use of advanced polymers and composite materials.
The primary objective of HCl storage is to maintain the chemical's integrity while preventing any release that could harm personnel, equipment, or the environment. This involves addressing several key challenges, including material compatibility, pressure management, and containment of vapors. The corrosive nature of HCl requires storage materials that can withstand long-term exposure without degradation, while also considering factors such as temperature fluctuations and concentration levels.
Recent technological advancements have focused on improving safety features, such as double-walled tanks, leak detection systems, and automated monitoring. These innovations aim to minimize the risk of accidental releases and provide early warning of potential issues. Additionally, there has been a growing emphasis on developing storage solutions that are not only safe but also sustainable and energy-efficient.
The global trend towards stricter environmental regulations and workplace safety standards has further shaped the evolution of HCl storage practices. Industries are now required to implement more robust containment measures, emergency response plans, and regular inspection protocols. This regulatory landscape has spurred research into novel materials and designs that can meet or exceed these stringent requirements.
Looking forward, the field of HCl storage is likely to see continued innovation in areas such as smart monitoring systems, predictive maintenance technologies, and more environmentally friendly storage solutions. The integration of Internet of Things (IoT) devices and artificial intelligence may lead to more proactive and efficient management of HCl storage facilities, further enhancing safety and operational efficiency.
Historically, HCl storage methods have progressed from simple glass or ceramic containers to more sophisticated systems involving specialized materials and engineering controls. Early industrial use relied on glass carboys, which were fragile and limited in capacity. As demand grew, larger-scale storage solutions became necessary, leading to the development of rubber-lined steel tanks and eventually to the use of advanced polymers and composite materials.
The primary objective of HCl storage is to maintain the chemical's integrity while preventing any release that could harm personnel, equipment, or the environment. This involves addressing several key challenges, including material compatibility, pressure management, and containment of vapors. The corrosive nature of HCl requires storage materials that can withstand long-term exposure without degradation, while also considering factors such as temperature fluctuations and concentration levels.
Recent technological advancements have focused on improving safety features, such as double-walled tanks, leak detection systems, and automated monitoring. These innovations aim to minimize the risk of accidental releases and provide early warning of potential issues. Additionally, there has been a growing emphasis on developing storage solutions that are not only safe but also sustainable and energy-efficient.
The global trend towards stricter environmental regulations and workplace safety standards has further shaped the evolution of HCl storage practices. Industries are now required to implement more robust containment measures, emergency response plans, and regular inspection protocols. This regulatory landscape has spurred research into novel materials and designs that can meet or exceed these stringent requirements.
Looking forward, the field of HCl storage is likely to see continued innovation in areas such as smart monitoring systems, predictive maintenance technologies, and more environmentally friendly storage solutions. The integration of Internet of Things (IoT) devices and artificial intelligence may lead to more proactive and efficient management of HCl storage facilities, further enhancing safety and operational efficiency.
Industrial Demand for HCl Storage Solutions
The industrial demand for hydrochloric acid (HCl) storage solutions has been steadily increasing due to the widespread use of HCl in various sectors. Chemical manufacturing, metal processing, oil and gas industries, and water treatment facilities are among the primary consumers of HCl, driving the need for safe and efficient storage solutions.
In the chemical manufacturing sector, HCl is a crucial raw material for producing various chemicals, including PVC, pharmaceuticals, and agrochemicals. The growing demand for these end products has led to an increased requirement for HCl storage capacity. Metal processing industries utilize HCl for pickling and descaling processes, necessitating large-scale storage facilities to ensure a continuous supply for their operations.
The oil and gas industry has seen a significant uptick in HCl usage for well stimulation and hydraulic fracturing processes. This has created a substantial market for portable and on-site HCl storage solutions that can withstand harsh environmental conditions and meet stringent safety standards. Water treatment plants also contribute to the demand for HCl storage, as the acid is used in pH adjustment and water purification processes.
The market for HCl storage solutions is characterized by a growing emphasis on safety and environmental compliance. Regulatory bodies worldwide have implemented strict guidelines for the handling and storage of hazardous chemicals, including HCl. This has led to increased demand for advanced storage systems equipped with leak detection, containment features, and corrosion-resistant materials.
Technological advancements in storage tank design and materials have also shaped market demand. Fiberglass-reinforced plastic (FRP) tanks have gained popularity due to their excellent chemical resistance and durability. Similarly, lined steel tanks with specialized coatings have found widespread adoption in industries requiring large-volume storage.
The global HCl storage solutions market is expected to grow significantly in the coming years. Factors such as industrialization in developing countries, expansion of chemical manufacturing capacities, and increasing focus on water treatment are driving this growth. Additionally, the trend towards modular and customizable storage systems is gaining traction, allowing businesses to scale their storage capabilities as needed.
As environmental concerns continue to rise, there is a growing demand for eco-friendly storage solutions that minimize the risk of spills and emissions. This has led to innovations in closed-loop systems and vapor recovery technologies, further expanding the market for specialized HCl storage equipment.
In the chemical manufacturing sector, HCl is a crucial raw material for producing various chemicals, including PVC, pharmaceuticals, and agrochemicals. The growing demand for these end products has led to an increased requirement for HCl storage capacity. Metal processing industries utilize HCl for pickling and descaling processes, necessitating large-scale storage facilities to ensure a continuous supply for their operations.
The oil and gas industry has seen a significant uptick in HCl usage for well stimulation and hydraulic fracturing processes. This has created a substantial market for portable and on-site HCl storage solutions that can withstand harsh environmental conditions and meet stringent safety standards. Water treatment plants also contribute to the demand for HCl storage, as the acid is used in pH adjustment and water purification processes.
The market for HCl storage solutions is characterized by a growing emphasis on safety and environmental compliance. Regulatory bodies worldwide have implemented strict guidelines for the handling and storage of hazardous chemicals, including HCl. This has led to increased demand for advanced storage systems equipped with leak detection, containment features, and corrosion-resistant materials.
Technological advancements in storage tank design and materials have also shaped market demand. Fiberglass-reinforced plastic (FRP) tanks have gained popularity due to their excellent chemical resistance and durability. Similarly, lined steel tanks with specialized coatings have found widespread adoption in industries requiring large-volume storage.
The global HCl storage solutions market is expected to grow significantly in the coming years. Factors such as industrialization in developing countries, expansion of chemical manufacturing capacities, and increasing focus on water treatment are driving this growth. Additionally, the trend towards modular and customizable storage systems is gaining traction, allowing businesses to scale their storage capabilities as needed.
As environmental concerns continue to rise, there is a growing demand for eco-friendly storage solutions that minimize the risk of spills and emissions. This has led to innovations in closed-loop systems and vapor recovery technologies, further expanding the market for specialized HCl storage equipment.
Current HCl Storage Challenges
The storage of hydrochloric acid (HCl) presents significant challenges due to its highly corrosive nature and potential health hazards. One of the primary concerns is the material compatibility of storage containers. HCl can rapidly degrade many common materials, including certain metals and plastics, leading to container failure and dangerous leaks. This necessitates the use of specialized materials such as high-density polyethylene (HDPE) or glass-lined steel, which can withstand long-term exposure to the acid.
Another critical challenge is the management of fumes and vapors. HCl readily releases corrosive vapors that can cause respiratory issues and damage surrounding equipment. Proper ventilation systems and vapor-tight seals are essential to prevent the escape of these harmful emissions. Additionally, the hygroscopic nature of HCl means it can absorb moisture from the air, potentially leading to concentration changes and increased corrosivity over time.
Temperature control poses another significant hurdle in HCl storage. Extreme temperatures can affect the acid's stability and the integrity of storage containers. High temperatures may increase vapor pressure and accelerate corrosion, while low temperatures can lead to freezing and potential container rupture. Maintaining a stable, moderate temperature range is crucial but often challenging in varying environmental conditions.
The risk of accidental spills or leaks is a constant concern in HCl storage. Even small releases can create hazardous situations, requiring immediate response and specialized cleanup procedures. This necessitates robust containment systems, regular inspections, and well-trained personnel to handle emergencies effectively.
Regulatory compliance adds another layer of complexity to HCl storage. Stringent regulations govern the storage, handling, and transportation of hazardous materials like HCl. Keeping up with evolving standards and ensuring full compliance across all aspects of storage and handling can be resource-intensive and challenging for many organizations.
Lastly, the long-term storage of large quantities of HCl presents unique challenges in terms of inventory management and turnover. The acid's properties can change over time, potentially affecting its quality and usability. Implementing effective inventory rotation systems and maintaining optimal stock levels while ensuring continuous availability for production needs requires careful planning and management.
Another critical challenge is the management of fumes and vapors. HCl readily releases corrosive vapors that can cause respiratory issues and damage surrounding equipment. Proper ventilation systems and vapor-tight seals are essential to prevent the escape of these harmful emissions. Additionally, the hygroscopic nature of HCl means it can absorb moisture from the air, potentially leading to concentration changes and increased corrosivity over time.
Temperature control poses another significant hurdle in HCl storage. Extreme temperatures can affect the acid's stability and the integrity of storage containers. High temperatures may increase vapor pressure and accelerate corrosion, while low temperatures can lead to freezing and potential container rupture. Maintaining a stable, moderate temperature range is crucial but often challenging in varying environmental conditions.
The risk of accidental spills or leaks is a constant concern in HCl storage. Even small releases can create hazardous situations, requiring immediate response and specialized cleanup procedures. This necessitates robust containment systems, regular inspections, and well-trained personnel to handle emergencies effectively.
Regulatory compliance adds another layer of complexity to HCl storage. Stringent regulations govern the storage, handling, and transportation of hazardous materials like HCl. Keeping up with evolving standards and ensuring full compliance across all aspects of storage and handling can be resource-intensive and challenging for many organizations.
Lastly, the long-term storage of large quantities of HCl presents unique challenges in terms of inventory management and turnover. The acid's properties can change over time, potentially affecting its quality and usability. Implementing effective inventory rotation systems and maintaining optimal stock levels while ensuring continuous availability for production needs requires careful planning and management.
Existing HCl Storage Best Practices
01 Personal protective equipment for handling hydrochloric acid
Proper personal protective equipment (PPE) is crucial when handling hydrochloric acid. This includes acid-resistant gloves, goggles or face shields, and protective clothing. Respiratory protection may also be necessary in certain situations. Using appropriate PPE helps prevent skin contact, inhalation, and eye exposure to the corrosive acid.- Personal protective equipment for handling hydrochloric acid: Proper personal protective equipment (PPE) is crucial when handling hydrochloric acid. This includes acid-resistant gloves, protective eyewear, face shields, and chemical-resistant clothing. Specialized safety equipment such as acid-proof aprons and boots may also be necessary. Adequate ventilation systems should be in place to prevent inhalation of acid fumes.
- Storage and containment of hydrochloric acid: Safe storage of hydrochloric acid is essential to prevent accidents and spills. Acid-resistant containers, such as polyethylene or glass-lined tanks, should be used. Storage areas should be well-ventilated, cool, and dry. Proper labeling and segregation from incompatible materials are necessary. Secondary containment systems can help prevent environmental contamination in case of leaks.
- Emergency response and spill management: Proper emergency response procedures are crucial for hydrochloric acid safety. This includes having readily available neutralizing agents, absorbent materials, and spill kits. Emergency showers and eyewash stations should be installed in areas where acid is handled. Training personnel in spill containment and decontamination procedures is essential for minimizing risks and environmental impact.
- Neutralization and disposal methods: Safe neutralization and disposal of hydrochloric acid are important for environmental protection and worker safety. Controlled neutralization using bases such as sodium hydroxide or calcium carbonate can be employed. Proper dilution and pH adjustment are necessary before disposal. Specialized waste treatment facilities may be required for large-scale acid disposal to ensure compliance with environmental regulations.
- Safety monitoring and detection systems: Implementing safety monitoring and detection systems is crucial for early warning and prevention of hydrochloric acid-related incidents. This includes pH sensors, vapor detectors, and alarm systems to alert personnel of potential leaks or dangerous concentrations. Regular equipment inspections and maintenance of safety systems are essential to ensure their effectiveness in protecting workers and the environment.
02 Storage and containment of hydrochloric acid
Safe storage and containment of hydrochloric acid is essential to prevent spills and accidents. This involves using corrosion-resistant containers, proper labeling, and storing the acid in well-ventilated areas away from incompatible materials. Secondary containment systems can help prevent widespread contamination in case of leaks or spills.Expand Specific Solutions03 Neutralization and disposal methods
Proper neutralization and disposal of hydrochloric acid are important for environmental and safety reasons. This may involve using alkaline substances to neutralize the acid before disposal. Specialized waste treatment facilities may be required for larger quantities. Proper disposal methods help prevent environmental contamination and protect workers from exposure.Expand Specific Solutions04 Emergency response and first aid procedures
Establishing clear emergency response and first aid procedures is crucial when working with hydrochloric acid. This includes having readily available eyewash stations and safety showers, as well as training personnel on proper response to spills or exposure. Immediate flushing with water is typically the first step in case of skin or eye contact.Expand Specific Solutions05 Ventilation and exposure control
Proper ventilation is essential when working with hydrochloric acid to prevent the buildup of harmful vapors. This may include the use of fume hoods, local exhaust ventilation systems, or general room ventilation. Regular air quality monitoring and exposure assessments can help ensure that workers are not exposed to dangerous levels of acid vapors.Expand Specific Solutions
Key Players in Chemical Storage Industry
The market for safe hydrochloric acid storage is in a mature stage, with established practices and regulations in place. The global market size for hydrochloric acid is projected to reach $1.5 billion by 2027, driven by industrial applications. Major players like China Petroleum & Chemical Corp., Sumitomo Metal Mining, and Ecolab USA dominate the market with advanced storage solutions. These companies have developed sophisticated containment systems, corrosion-resistant materials, and safety protocols. Emerging technologies focus on improving leak detection, remote monitoring, and automated handling systems. Smaller firms like Longnan Hongyutai Technology and Green Products & Technologies are innovating in niche areas such as eco-friendly storage solutions and specialized containment for high-purity acid applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced storage solutions for hydrochloric acid, focusing on safety and efficiency. Their approach includes using corrosion-resistant materials like high-density polyethylene (HDPE) or fiberglass-reinforced plastic (FRP) tanks [1]. These tanks are equipped with secondary containment systems and advanced leak detection technology. Sinopec has also implemented automated monitoring systems that continuously track acid concentration, temperature, and pressure [3]. For transportation, they utilize specially designed tanker trucks with multiple safety features, including pressure relief valves and emergency shut-off systems [5].
Strengths: Comprehensive safety measures, advanced monitoring systems, and use of corrosion-resistant materials. Weaknesses: Potentially higher initial costs for implementation and maintenance of sophisticated systems.
Ecolab USA, Inc.
Technical Solution: Ecolab USA, Inc. has developed a multi-faceted approach to hydrochloric acid storage safety. Their system includes specially designed storage tanks with double-wall construction and leak detection systems [2]. They've also created a proprietary chemical management system that integrates real-time monitoring of acid levels, pH, and temperature. Ecolab's solution incorporates automated dispensing systems to minimize human contact during handling [4]. Additionally, they've developed specialized coatings for storage areas to prevent corrosion and enhance containment in case of spills. Their approach also includes comprehensive training programs for personnel handling hydrochloric acid [6].
Strengths: Integrated approach combining hardware solutions with software monitoring and human training. Weaknesses: May require significant changes to existing infrastructure and processes.
Innovative HCl Containment Materials
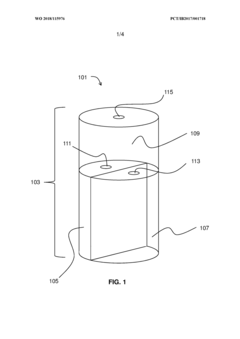
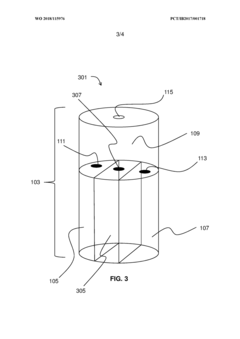
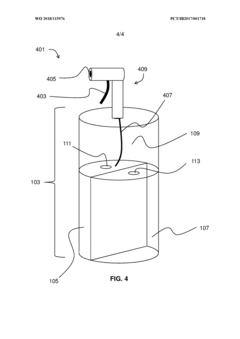
Storage medium for storing hydrogen chloride and method for separating and storing hydrogen chloride HCl from HCl containing gas
PatentWO2023020942A1
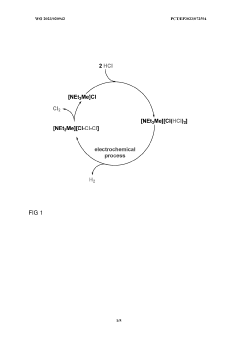
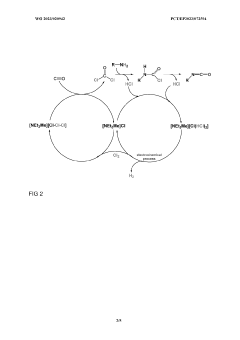
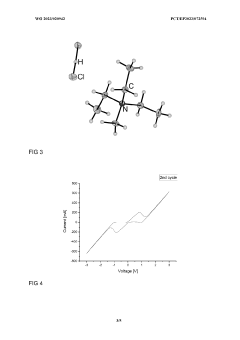
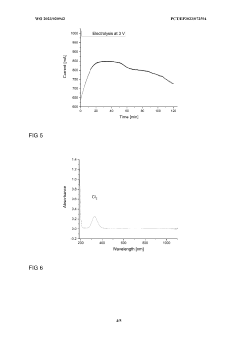
Innovation
- A storage medium comprising an ionic compound with an asymmetrically substituted ammonium cation and poly(HCI)chloride anion, allowing reversible absorption and storage of HCI, with the ability to release hydrogen and chlorine through electrolysis at low cell voltage, and capable of storing chlorine for further synthetic applications.
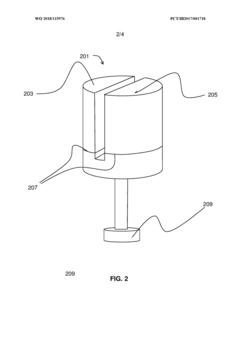
Multi-chambered storage and delivery container
PatentWO2018115976A1
Innovation
- A multi-chambered container system that separates and stabilizes the components for producing hypochlorous acid, using one-way valves and buffering agents to maintain a stable pH and prevent air exposure, allowing for on-site preparation and long-term storage of hypochlorous acid compositions, which are substantially free of chloride and metal ions.
Regulatory Framework for Hazardous Chemical Storage
The regulatory framework for hazardous chemical storage, particularly concerning hydrochloric acid, is a complex and critical aspect of industrial safety. At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a foundation for standardizing hazard communication. This system has been widely adopted and adapted by various countries, influencing national regulations on chemical storage and handling.
In the United States, the Occupational Safety and Health Administration (OSHA) plays a pivotal role in regulating hazardous chemical storage. OSHA's Hazard Communication Standard (HCS) aligns with the GHS and mandates specific requirements for labeling, safety data sheets, and employee training. The Environmental Protection Agency (EPA) also contributes to the regulatory landscape through the Resource Conservation and Recovery Act (RCRA), which governs the storage and disposal of hazardous waste, including spent hydrochloric acid.
The European Union's regulatory framework is primarily based on the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation and the Classification, Labelling, and Packaging (CLP) regulation. These comprehensive legislations ensure a high level of protection for human health and the environment, while also promoting innovation and competitiveness in the chemical industry.
In Asia, countries like China and Japan have developed their own regulatory systems. China's Regulations on Safe Management of Hazardous Chemicals and Japan's Industrial Safety and Health Act provide specific guidelines for the storage and handling of corrosive substances like hydrochloric acid.
At the facility level, these regulations translate into specific requirements for storage infrastructure, containment systems, ventilation, and emergency response planning. For hydrochloric acid storage, key considerations include the use of corrosion-resistant materials, secondary containment measures, and proper ventilation systems to manage potential fumes.
Compliance with these regulations often necessitates regular inspections, meticulous record-keeping, and ongoing employee training programs. Many jurisdictions require facilities to develop and maintain comprehensive chemical management plans, which outline procedures for safe storage, handling, and emergency response.
As the understanding of chemical hazards evolves and new technologies emerge, regulatory frameworks continue to adapt. Recent trends include an increased focus on risk-based approaches to chemical management and the integration of digital technologies for real-time monitoring and compliance tracking. These developments aim to enhance safety while balancing the need for operational efficiency in industrial settings.
In the United States, the Occupational Safety and Health Administration (OSHA) plays a pivotal role in regulating hazardous chemical storage. OSHA's Hazard Communication Standard (HCS) aligns with the GHS and mandates specific requirements for labeling, safety data sheets, and employee training. The Environmental Protection Agency (EPA) also contributes to the regulatory landscape through the Resource Conservation and Recovery Act (RCRA), which governs the storage and disposal of hazardous waste, including spent hydrochloric acid.
The European Union's regulatory framework is primarily based on the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation and the Classification, Labelling, and Packaging (CLP) regulation. These comprehensive legislations ensure a high level of protection for human health and the environment, while also promoting innovation and competitiveness in the chemical industry.
In Asia, countries like China and Japan have developed their own regulatory systems. China's Regulations on Safe Management of Hazardous Chemicals and Japan's Industrial Safety and Health Act provide specific guidelines for the storage and handling of corrosive substances like hydrochloric acid.
At the facility level, these regulations translate into specific requirements for storage infrastructure, containment systems, ventilation, and emergency response planning. For hydrochloric acid storage, key considerations include the use of corrosion-resistant materials, secondary containment measures, and proper ventilation systems to manage potential fumes.
Compliance with these regulations often necessitates regular inspections, meticulous record-keeping, and ongoing employee training programs. Many jurisdictions require facilities to develop and maintain comprehensive chemical management plans, which outline procedures for safe storage, handling, and emergency response.
As the understanding of chemical hazards evolves and new technologies emerge, regulatory frameworks continue to adapt. Recent trends include an increased focus on risk-based approaches to chemical management and the integration of digital technologies for real-time monitoring and compliance tracking. These developments aim to enhance safety while balancing the need for operational efficiency in industrial settings.
Environmental Impact of HCl Storage Methods
The environmental impact of hydrochloric acid (HCl) storage methods is a critical consideration in the safe handling and management of this corrosive substance. Proper storage practices not only ensure the safety of personnel and facilities but also play a crucial role in minimizing potential environmental hazards.
One of the primary environmental concerns associated with HCl storage is the risk of accidental releases or spills. Even small leaks can have significant ecological consequences, particularly if the acid reaches water bodies or soil. HCl can dramatically alter the pH of aquatic environments, leading to the death of fish and other aquatic organisms. In soil, it can disrupt microbial communities and impair plant growth.
To mitigate these risks, modern storage facilities employ various containment systems. Secondary containment structures, such as dikes or berms around storage tanks, are designed to capture and contain any potential leaks. These systems effectively prevent the spread of HCl to the surrounding environment, allowing for proper cleanup and disposal.
The choice of storage container material is another crucial factor in environmental protection. Corrosion-resistant materials like high-density polyethylene (HDPE) or fiberglass-reinforced plastic (FRP) are commonly used for HCl storage tanks. These materials not only prevent leaks but also reduce the need for frequent tank replacements, thereby minimizing waste generation and resource consumption.
Proper ventilation systems in storage areas play a dual role in environmental protection. They help prevent the buildup of potentially harmful vapors inside the facility while also reducing the risk of atmospheric pollution. Advanced scrubber systems can be employed to neutralize any acid vapors before they are released into the atmosphere, further minimizing environmental impact.
The location of storage facilities is another critical consideration. Siting HCl storage away from sensitive ecosystems, water sources, and densely populated areas can significantly reduce the potential environmental impact in case of a major incident. Additionally, strategic placement can facilitate more efficient emergency response and containment efforts.
Regular monitoring and maintenance of storage systems are essential for long-term environmental protection. This includes routine inspections for signs of corrosion or wear, testing of containment systems, and periodic replacement of aging components. Such proactive measures help prevent gradual deterioration that could lead to environmental contamination.
In recent years, there has been a growing emphasis on the lifecycle management of HCl storage systems. This approach considers the environmental impact from the initial installation through to the eventual decommissioning of storage facilities. It includes strategies for the safe disposal or recycling of storage containers and associated equipment, minimizing the long-term environmental footprint of HCl storage operations.
One of the primary environmental concerns associated with HCl storage is the risk of accidental releases or spills. Even small leaks can have significant ecological consequences, particularly if the acid reaches water bodies or soil. HCl can dramatically alter the pH of aquatic environments, leading to the death of fish and other aquatic organisms. In soil, it can disrupt microbial communities and impair plant growth.
To mitigate these risks, modern storage facilities employ various containment systems. Secondary containment structures, such as dikes or berms around storage tanks, are designed to capture and contain any potential leaks. These systems effectively prevent the spread of HCl to the surrounding environment, allowing for proper cleanup and disposal.
The choice of storage container material is another crucial factor in environmental protection. Corrosion-resistant materials like high-density polyethylene (HDPE) or fiberglass-reinforced plastic (FRP) are commonly used for HCl storage tanks. These materials not only prevent leaks but also reduce the need for frequent tank replacements, thereby minimizing waste generation and resource consumption.
Proper ventilation systems in storage areas play a dual role in environmental protection. They help prevent the buildup of potentially harmful vapors inside the facility while also reducing the risk of atmospheric pollution. Advanced scrubber systems can be employed to neutralize any acid vapors before they are released into the atmosphere, further minimizing environmental impact.
The location of storage facilities is another critical consideration. Siting HCl storage away from sensitive ecosystems, water sources, and densely populated areas can significantly reduce the potential environmental impact in case of a major incident. Additionally, strategic placement can facilitate more efficient emergency response and containment efforts.
Regular monitoring and maintenance of storage systems are essential for long-term environmental protection. This includes routine inspections for signs of corrosion or wear, testing of containment systems, and periodic replacement of aging components. Such proactive measures help prevent gradual deterioration that could lead to environmental contamination.
In recent years, there has been a growing emphasis on the lifecycle management of HCl storage systems. This approach considers the environmental impact from the initial installation through to the eventual decommissioning of storage facilities. It includes strategies for the safe disposal or recycling of storage containers and associated equipment, minimizing the long-term environmental footprint of HCl storage operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!