Hydrochloric Acid Stability Requirements in Development Phases
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Stability Background
Hydrochloric acid (HCl) has been a cornerstone in various industrial processes and scientific applications for decades. Its stability is crucial in ensuring the efficacy and safety of numerous products and processes across different development phases. The importance of HCl stability stems from its widespread use in pharmaceuticals, chemical manufacturing, and materials processing.
In the pharmaceutical industry, HCl stability is particularly critical during drug development and formulation. Many active pharmaceutical ingredients (APIs) are formulated as hydrochloride salts to enhance solubility and bioavailability. The stability of these HCl salts directly impacts the shelf life, efficacy, and safety of the final drug products. Unstable HCl formulations can lead to degradation of the API, potentially resulting in reduced therapeutic effect or the formation of harmful by-products.
The chemical manufacturing sector relies heavily on HCl for various processes, including synthesis, pH adjustment, and cleaning. In these applications, the stability of HCl is essential for maintaining consistent product quality and process efficiency. Fluctuations in HCl concentration or purity can lead to unpredictable reactions, yield variations, and quality control issues.
In materials processing, HCl plays a vital role in metal treatment, surface cleaning, and etching processes. The stability of HCl solutions is crucial for achieving precise and reproducible results in these applications. Unstable HCl can lead to inconsistent surface treatments, compromising the integrity and performance of the processed materials.
The stability requirements for HCl vary depending on the specific application and development phase. In early research and development stages, high-purity, stable HCl is necessary for accurate experimentation and reliable results. As products move into pilot-scale production and eventually full-scale manufacturing, the focus shifts to maintaining HCl stability under various environmental conditions and over extended periods.
Factors affecting HCl stability include temperature, light exposure, container materials, and the presence of impurities or other reactive substances. Understanding and controlling these factors is essential for developing robust HCl-based products and processes. Advances in analytical techniques and stability testing protocols have significantly improved our ability to assess and predict HCl stability across different development phases.
As industries continue to evolve, the demand for more stable and precisely controlled HCl formulations grows. This has led to ongoing research into novel stabilization techniques, improved packaging materials, and innovative process control methods. The pursuit of enhanced HCl stability drives innovation in various sectors, contributing to the development of more efficient, safer, and higher-quality products and processes.
In the pharmaceutical industry, HCl stability is particularly critical during drug development and formulation. Many active pharmaceutical ingredients (APIs) are formulated as hydrochloride salts to enhance solubility and bioavailability. The stability of these HCl salts directly impacts the shelf life, efficacy, and safety of the final drug products. Unstable HCl formulations can lead to degradation of the API, potentially resulting in reduced therapeutic effect or the formation of harmful by-products.
The chemical manufacturing sector relies heavily on HCl for various processes, including synthesis, pH adjustment, and cleaning. In these applications, the stability of HCl is essential for maintaining consistent product quality and process efficiency. Fluctuations in HCl concentration or purity can lead to unpredictable reactions, yield variations, and quality control issues.
In materials processing, HCl plays a vital role in metal treatment, surface cleaning, and etching processes. The stability of HCl solutions is crucial for achieving precise and reproducible results in these applications. Unstable HCl can lead to inconsistent surface treatments, compromising the integrity and performance of the processed materials.
The stability requirements for HCl vary depending on the specific application and development phase. In early research and development stages, high-purity, stable HCl is necessary for accurate experimentation and reliable results. As products move into pilot-scale production and eventually full-scale manufacturing, the focus shifts to maintaining HCl stability under various environmental conditions and over extended periods.
Factors affecting HCl stability include temperature, light exposure, container materials, and the presence of impurities or other reactive substances. Understanding and controlling these factors is essential for developing robust HCl-based products and processes. Advances in analytical techniques and stability testing protocols have significantly improved our ability to assess and predict HCl stability across different development phases.
As industries continue to evolve, the demand for more stable and precisely controlled HCl formulations grows. This has led to ongoing research into novel stabilization techniques, improved packaging materials, and innovative process control methods. The pursuit of enhanced HCl stability drives innovation in various sectors, contributing to the development of more efficient, safer, and higher-quality products and processes.
Market Analysis
The market for hydrochloric acid (HCl) is experiencing significant growth, driven by its widespread applications across various industries. The global hydrochloric acid market size was valued at USD 7.8 billion in 2020 and is projected to reach USD 9.4 billion by 2026, growing at a CAGR of 3.2% during the forecast period. This growth is primarily attributed to the increasing demand from end-use industries such as steel pickling, oil well acidizing, and chemical manufacturing.
In the development phases of products and processes involving hydrochloric acid, stability requirements play a crucial role in determining market demand and product quality. Industries such as pharmaceuticals, food processing, and water treatment require high-purity, stable hydrochloric acid to maintain product integrity and meet regulatory standards. This has led to a growing demand for specialized grades of hydrochloric acid with enhanced stability characteristics.
The steel industry remains the largest consumer of hydrochloric acid, accounting for approximately 40% of the global market share. The acid's use in steel pickling processes to remove rust and scale from steel surfaces drives this demand. However, the industry's cyclical nature and environmental concerns have led to the development of more stable and environmentally friendly formulations, creating new market opportunities for innovative HCl products.
The oil and gas sector represents another significant market for hydrochloric acid, particularly in well acidizing applications. As exploration and production activities continue to expand, especially in unconventional oil and gas reserves, the demand for stable HCl formulations that can withstand high temperatures and pressures is increasing. This trend is expected to drive research and development efforts towards more robust acid stability solutions.
In the chemical manufacturing sector, hydrochloric acid serves as a key raw material for producing various chemicals, including vinyl chloride monomer (VCM) and polyvinyl chloride (PVC). The stability of HCl during storage and transportation is critical for these applications, as degradation can lead to reduced product quality and increased production costs. As a result, there is a growing market for stabilized HCl formulations that can maintain their properties over extended periods.
The pharmaceutical industry's stringent quality requirements have created a niche market for ultra-pure, highly stable hydrochloric acid. This segment is expected to witness the fastest growth, with a CAGR of 4.5% from 2021 to 2026. The increasing focus on drug development and the expansion of the generic drug market are key factors driving this growth, emphasizing the need for HCl stability throughout the product lifecycle.
Geographically, Asia-Pacific dominates the hydrochloric acid market, accounting for over 40% of the global consumption. The region's rapid industrialization, particularly in China and India, continues to drive demand across various end-use industries. However, mature markets in North America and Europe are focusing on developing advanced, stable HCl formulations to meet evolving regulatory standards and sustainability goals.
In the development phases of products and processes involving hydrochloric acid, stability requirements play a crucial role in determining market demand and product quality. Industries such as pharmaceuticals, food processing, and water treatment require high-purity, stable hydrochloric acid to maintain product integrity and meet regulatory standards. This has led to a growing demand for specialized grades of hydrochloric acid with enhanced stability characteristics.
The steel industry remains the largest consumer of hydrochloric acid, accounting for approximately 40% of the global market share. The acid's use in steel pickling processes to remove rust and scale from steel surfaces drives this demand. However, the industry's cyclical nature and environmental concerns have led to the development of more stable and environmentally friendly formulations, creating new market opportunities for innovative HCl products.
The oil and gas sector represents another significant market for hydrochloric acid, particularly in well acidizing applications. As exploration and production activities continue to expand, especially in unconventional oil and gas reserves, the demand for stable HCl formulations that can withstand high temperatures and pressures is increasing. This trend is expected to drive research and development efforts towards more robust acid stability solutions.
In the chemical manufacturing sector, hydrochloric acid serves as a key raw material for producing various chemicals, including vinyl chloride monomer (VCM) and polyvinyl chloride (PVC). The stability of HCl during storage and transportation is critical for these applications, as degradation can lead to reduced product quality and increased production costs. As a result, there is a growing market for stabilized HCl formulations that can maintain their properties over extended periods.
The pharmaceutical industry's stringent quality requirements have created a niche market for ultra-pure, highly stable hydrochloric acid. This segment is expected to witness the fastest growth, with a CAGR of 4.5% from 2021 to 2026. The increasing focus on drug development and the expansion of the generic drug market are key factors driving this growth, emphasizing the need for HCl stability throughout the product lifecycle.
Geographically, Asia-Pacific dominates the hydrochloric acid market, accounting for over 40% of the global consumption. The region's rapid industrialization, particularly in China and India, continues to drive demand across various end-use industries. However, mature markets in North America and Europe are focusing on developing advanced, stable HCl formulations to meet evolving regulatory standards and sustainability goals.
Technical Challenges
The development of hydrochloric acid (HCl) with enhanced stability presents several significant technical challenges. One of the primary obstacles is maintaining the acid's concentration and purity over extended periods. HCl is highly reactive and prone to degradation, particularly when exposed to air, moisture, or contaminants. This instability can lead to reduced efficacy and potential safety hazards during storage and transportation.
Another major challenge lies in the containment and handling of HCl. The corrosive nature of the acid necessitates the use of specialized materials for storage and processing equipment. Developing cost-effective, durable materials that can withstand prolonged exposure to HCl without degradation or contamination is a complex engineering task. This challenge extends to the design of seals, valves, and other components that come into contact with the acid.
The environmental impact of HCl production and use also poses significant technical hurdles. Developing processes that minimize emissions and waste while maintaining the acid's stability and effectiveness requires innovative approaches to chemical engineering. This includes exploring new catalysts and reaction pathways that could lead to more environmentally friendly production methods.
Temperature control during various development phases presents another technical challenge. HCl's stability is highly temperature-dependent, and maintaining optimal conditions throughout production, storage, and application processes is crucial. Developing efficient and reliable temperature control systems that can operate in diverse environments is essential for ensuring the acid's stability and safety.
The analytical techniques used to assess HCl stability also face limitations. Current methods may not provide sufficiently accurate or real-time data on the acid's composition and potential degradation products. Developing more sensitive, robust, and non-invasive analytical tools is crucial for monitoring HCl stability throughout its lifecycle.
Scaling up laboratory findings to industrial production levels introduces additional complexities. Maintaining stability in large-scale production and storage facilities requires overcoming challenges related to heat transfer, mixing efficiency, and process control. Bridging the gap between small-scale experiments and full-scale manufacturing while preserving HCl stability is a significant technical hurdle.
Lastly, the development of stabilizing additives or formulations that can enhance HCl's long-term stability without compromising its performance or introducing unwanted side effects remains an ongoing challenge. Finding the right balance between stability enhancement and maintaining the acid's desired properties requires extensive research and testing.
Another major challenge lies in the containment and handling of HCl. The corrosive nature of the acid necessitates the use of specialized materials for storage and processing equipment. Developing cost-effective, durable materials that can withstand prolonged exposure to HCl without degradation or contamination is a complex engineering task. This challenge extends to the design of seals, valves, and other components that come into contact with the acid.
The environmental impact of HCl production and use also poses significant technical hurdles. Developing processes that minimize emissions and waste while maintaining the acid's stability and effectiveness requires innovative approaches to chemical engineering. This includes exploring new catalysts and reaction pathways that could lead to more environmentally friendly production methods.
Temperature control during various development phases presents another technical challenge. HCl's stability is highly temperature-dependent, and maintaining optimal conditions throughout production, storage, and application processes is crucial. Developing efficient and reliable temperature control systems that can operate in diverse environments is essential for ensuring the acid's stability and safety.
The analytical techniques used to assess HCl stability also face limitations. Current methods may not provide sufficiently accurate or real-time data on the acid's composition and potential degradation products. Developing more sensitive, robust, and non-invasive analytical tools is crucial for monitoring HCl stability throughout its lifecycle.
Scaling up laboratory findings to industrial production levels introduces additional complexities. Maintaining stability in large-scale production and storage facilities requires overcoming challenges related to heat transfer, mixing efficiency, and process control. Bridging the gap between small-scale experiments and full-scale manufacturing while preserving HCl stability is a significant technical hurdle.
Lastly, the development of stabilizing additives or formulations that can enhance HCl's long-term stability without compromising its performance or introducing unwanted side effects remains an ongoing challenge. Finding the right balance between stability enhancement and maintaining the acid's desired properties requires extensive research and testing.
Current Solutions
01 Stabilization of hydrochloric acid using additives
Various additives can be used to enhance the stability of hydrochloric acid. These additives may include organic compounds, inorganic salts, or other chemical agents that help maintain the acid's concentration and prevent degradation over time. The specific additives used can vary depending on the intended application and storage conditions of the hydrochloric acid.- Stabilization of hydrochloric acid using additives: Various additives can be used to enhance the stability of hydrochloric acid. These additives may include organic compounds, inorganic salts, or specific chemical agents that help maintain the acid's concentration and prevent degradation over time. The stabilizers can improve the shelf life and performance of hydrochloric acid in different applications.
- Storage and packaging solutions for hydrochloric acid: Proper storage and packaging play a crucial role in maintaining the stability of hydrochloric acid. This includes using corrosion-resistant materials for containers, implementing appropriate sealing methods, and controlling environmental factors such as temperature and humidity. Specialized packaging designs can help prevent contamination and minimize exposure to air or moisture.
- Purification techniques to improve acid stability: Various purification methods can be employed to remove impurities and contaminants from hydrochloric acid, thereby enhancing its stability. These techniques may include distillation, ion exchange, or advanced filtration processes. Purified hydrochloric acid tends to have improved stability and a longer shelf life.
- Temperature control for maintaining acid stability: Controlling the temperature during storage and handling of hydrochloric acid is crucial for maintaining its stability. This may involve using specialized cooling systems, insulated containers, or temperature-controlled storage facilities. Proper temperature management can prevent degradation and maintain the acid's concentration over extended periods.
- Monitoring and quality control methods: Implementing effective monitoring and quality control methods is essential for ensuring the stability of hydrochloric acid. This may include regular testing of acid concentration, pH levels, and impurity content. Advanced analytical techniques and automated monitoring systems can be used to detect any changes in the acid's composition or stability over time.
02 Storage and packaging solutions for hydrochloric acid
Proper storage and packaging play a crucial role in maintaining the stability of hydrochloric acid. This may involve using specialized containers made from corrosion-resistant materials, implementing temperature control measures, or designing storage systems that minimize exposure to air and contaminants. Effective packaging solutions can significantly extend the shelf life of hydrochloric acid.Expand Specific Solutions03 Purification methods to improve hydrochloric acid stability
Various purification techniques can be employed to remove impurities and contaminants that may affect the stability of hydrochloric acid. These methods may include distillation, ion exchange, or other separation processes. By reducing the presence of destabilizing agents, the overall stability and quality of the hydrochloric acid can be improved.Expand Specific Solutions04 Temperature control for hydrochloric acid stability
Maintaining appropriate temperature conditions is essential for preserving the stability of hydrochloric acid. This may involve implementing cooling systems, insulation measures, or temperature-controlled storage facilities. Proper temperature management can help prevent degradation, maintain concentration, and extend the usable life of hydrochloric acid.Expand Specific Solutions05 Monitoring and quality control of hydrochloric acid stability
Implementing effective monitoring and quality control measures is crucial for ensuring the long-term stability of hydrochloric acid. This may include regular testing of acid concentration, pH levels, and impurity content. Advanced analytical techniques and automated monitoring systems can be employed to maintain consistent quality and detect any potential stability issues early on.Expand Specific Solutions
Industry Players
The competitive landscape for hydrochloric acid stability in development phases is characterized by a mature market with established players and ongoing innovation. The industry is in a growth phase, driven by increasing demand in various sectors such as pharmaceuticals, water treatment, and chemical manufacturing. Market size is substantial, with key players like Schlumberger, Industrie De Nora, and SK Chemicals contributing to technological advancements. The technology maturity varies, with companies like Astex Therapeutics and Alvotech focusing on specialized applications in drug development, while others like Aquaox and Contec Cleanroom concentrate on water treatment and cleanroom solutions. Overall, the field is competitive, with ongoing research and development efforts to improve acid stability and performance across different applications.
Schlumberger Canada Ltd.
Technical Solution: Schlumberger Canada Ltd. has developed innovative solutions for hydrochloric acid stability in oilfield services, particularly for well stimulation and acidizing treatments. Their approach involves the use of advanced chemical additives that enhance HCl stability under high-temperature and high-pressure reservoir conditions[1]. The company has implemented a proprietary blend of corrosion inhibitors and stabilizers that extend the effective life of HCl solutions during well treatments[3]. Schlumberger's research has led to the development of "smart" acid systems that can self-adjust their properties based on downhole conditions, maintaining optimal stability and performance[5]. Additionally, they have developed novel fluid delivery systems that minimize HCl degradation during pumping and injection processes, ensuring consistent acid strength at the target formation[7].
Strengths: Specialized solutions for oilfield applications, "smart" acid systems. Weaknesses: May have limited applicability outside of oil and gas industry.
Industrie De Nora SpA
Technical Solution: Industrie De Nora SpA has focused on developing stable hydrochloric acid solutions for electrochemical applications, particularly in chlor-alkali production. Their approach involves the use of high-purity membrane cell technology that produces HCl as a by-product with inherent stability[2]. The company has implemented advanced process control systems that maintain precise concentrations and temperatures throughout the production cycle, ensuring consistent HCl stability[4]. De Nora has also developed specialized materials for electrodes and cell components that resist corrosion from HCl, thereby enhancing the overall stability of the production process[6]. Their research extends to the development of novel catalysts that improve the efficiency of HCl synthesis while maintaining stability under various operating conditions[8].
Strengths: Expertise in electrochemical HCl production, advanced process control systems. Weaknesses: Solutions may be primarily focused on large-scale industrial applications.
Key Innovations
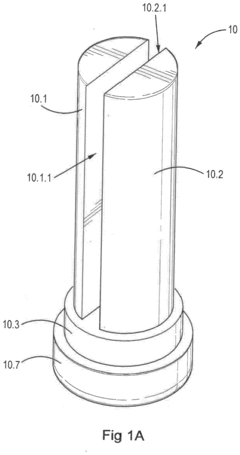
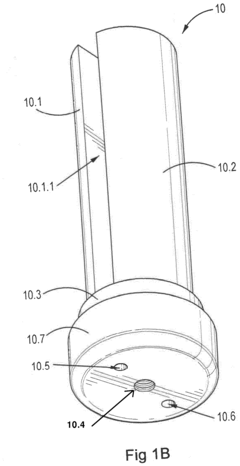
Hypochlorous acid
PatentActiveEP3312305A1
Innovation
- A solution of hypochlorous acid and water comprising magnesium chloride or strontium chloride, with controlled pH and electrolysis to achieve concentrations above 100 mg/L while minimizing chlorine gas and hypochlorite contamination, using magnesium or strontium carbonate to stabilize and buffer the solution.
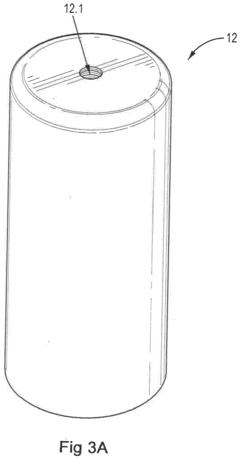
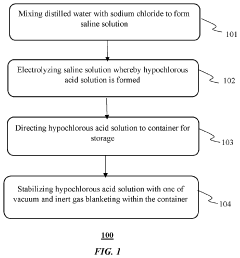
Method for stabilization of hypochlorous acid solution
PatentInactiveUS20200080208A1
Innovation
- A method involving the electrolysis of a saline solution using a uniflow membrane to produce hypochlorous acid, followed by storage in containers with vacuum or inert gas blanketing and specific gas and moisture transmission rates to maintain stability, and optionally coating with Titanium Dioxide.
Regulatory Framework
The regulatory framework surrounding hydrochloric acid stability requirements in development phases is a critical aspect that pharmaceutical companies must navigate to ensure compliance and product safety. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other international authorities have established guidelines and standards for the stability testing of drug substances and drug products.
These regulatory requirements typically mandate comprehensive stability studies throughout the drug development process, from early-stage formulation to commercial production. The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q1A(R2) on Stability Testing of New Drug Substances and Products, provide a harmonized approach to stability testing that is widely adopted by regulatory agencies worldwide.
For hydrochloric acid, which is often used as an excipient or in the formulation of drug products, stability requirements focus on maintaining its chemical integrity and ensuring it does not adversely affect the active pharmaceutical ingredient (API) or other components of the formulation. Regulatory bodies require manufacturers to conduct stability studies under various environmental conditions, including temperature, humidity, and light exposure, to simulate potential storage and transportation scenarios.
The stability data generated during these studies must demonstrate that the hydrochloric acid remains within specified limits throughout the proposed shelf life of the drug product. This includes monitoring pH levels, chemical purity, and potential degradation products. Regulatory agencies expect manufacturers to establish appropriate acceptance criteria for these parameters based on scientific rationale and risk assessment.
In addition to long-term stability studies, accelerated stability testing is often required to predict potential degradation pathways and support shelf-life claims. For products containing hydrochloric acid, special attention may be given to its potential for corrosion and interaction with packaging materials, necessitating compatibility studies as part of the regulatory submission.
Regulatory frameworks also emphasize the importance of stability-indicating analytical methods for monitoring hydrochloric acid stability. These methods must be validated according to ICH Q2(R1) guidelines on Validation of Analytical Procedures. The validation data, along with detailed stability protocols and results, form a crucial part of the regulatory dossier submitted for drug approval.
Furthermore, regulatory agencies require ongoing stability monitoring throughout the product lifecycle, including annual stability testing of commercial batches. This ensures that the stability profile of hydrochloric acid in the drug product remains consistent with the approved specifications and supports any proposed changes to storage conditions or shelf life.
These regulatory requirements typically mandate comprehensive stability studies throughout the drug development process, from early-stage formulation to commercial production. The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q1A(R2) on Stability Testing of New Drug Substances and Products, provide a harmonized approach to stability testing that is widely adopted by regulatory agencies worldwide.
For hydrochloric acid, which is often used as an excipient or in the formulation of drug products, stability requirements focus on maintaining its chemical integrity and ensuring it does not adversely affect the active pharmaceutical ingredient (API) or other components of the formulation. Regulatory bodies require manufacturers to conduct stability studies under various environmental conditions, including temperature, humidity, and light exposure, to simulate potential storage and transportation scenarios.
The stability data generated during these studies must demonstrate that the hydrochloric acid remains within specified limits throughout the proposed shelf life of the drug product. This includes monitoring pH levels, chemical purity, and potential degradation products. Regulatory agencies expect manufacturers to establish appropriate acceptance criteria for these parameters based on scientific rationale and risk assessment.
In addition to long-term stability studies, accelerated stability testing is often required to predict potential degradation pathways and support shelf-life claims. For products containing hydrochloric acid, special attention may be given to its potential for corrosion and interaction with packaging materials, necessitating compatibility studies as part of the regulatory submission.
Regulatory frameworks also emphasize the importance of stability-indicating analytical methods for monitoring hydrochloric acid stability. These methods must be validated according to ICH Q2(R1) guidelines on Validation of Analytical Procedures. The validation data, along with detailed stability protocols and results, form a crucial part of the regulatory dossier submitted for drug approval.
Furthermore, regulatory agencies require ongoing stability monitoring throughout the product lifecycle, including annual stability testing of commercial batches. This ensures that the stability profile of hydrochloric acid in the drug product remains consistent with the approved specifications and supports any proposed changes to storage conditions or shelf life.
Environmental Impact
The environmental impact of hydrochloric acid (HCl) stability requirements in development phases is a critical consideration for industries and researchers. HCl, being a strong and corrosive acid, poses significant risks to ecosystems and human health if not properly managed throughout its lifecycle.
During the development phases, ensuring HCl stability is crucial to minimize potential environmental hazards. Unstable HCl can lead to uncontrolled releases, which may result in soil and water contamination. These releases can drastically alter the pH of affected areas, causing severe damage to flora and fauna. Aquatic ecosystems are particularly vulnerable, as even small changes in acidity can disrupt the delicate balance of marine and freshwater habitats.
Air pollution is another concern associated with HCl stability. Volatile HCl emissions can contribute to the formation of acid rain, which has far-reaching consequences for terrestrial and aquatic ecosystems. The acidification of soil and water bodies can lead to the leaching of toxic metals, further exacerbating environmental degradation.
To mitigate these risks, stringent stability requirements must be implemented during the development phases. This includes the use of appropriate containment materials resistant to HCl corrosion, such as specialized plastics or coated metals. Proper ventilation systems and scrubbers are essential to capture and neutralize any potential HCl vapors, reducing the risk of atmospheric pollution.
Waste management is a critical aspect of environmental protection in HCl-related processes. Neutralization techniques must be employed before disposal, and any byproducts should be carefully treated to prevent secondary environmental impacts. The development of closed-loop systems and recycling processes can significantly reduce the overall environmental footprint of HCl use.
Monitoring and early detection systems play a vital role in maintaining HCl stability and preventing environmental incidents. Regular inspections, real-time pH monitoring, and leak detection technologies should be integrated into development processes to ensure rapid response to potential stability issues.
The long-term environmental effects of HCl stability requirements extend beyond immediate impacts. Chronic low-level exposure can lead to gradual ecosystem changes, affecting biodiversity and ecological succession. Therefore, comprehensive environmental impact assessments should be conducted throughout the development phases to identify and address potential long-term consequences.
During the development phases, ensuring HCl stability is crucial to minimize potential environmental hazards. Unstable HCl can lead to uncontrolled releases, which may result in soil and water contamination. These releases can drastically alter the pH of affected areas, causing severe damage to flora and fauna. Aquatic ecosystems are particularly vulnerable, as even small changes in acidity can disrupt the delicate balance of marine and freshwater habitats.
Air pollution is another concern associated with HCl stability. Volatile HCl emissions can contribute to the formation of acid rain, which has far-reaching consequences for terrestrial and aquatic ecosystems. The acidification of soil and water bodies can lead to the leaching of toxic metals, further exacerbating environmental degradation.
To mitigate these risks, stringent stability requirements must be implemented during the development phases. This includes the use of appropriate containment materials resistant to HCl corrosion, such as specialized plastics or coated metals. Proper ventilation systems and scrubbers are essential to capture and neutralize any potential HCl vapors, reducing the risk of atmospheric pollution.
Waste management is a critical aspect of environmental protection in HCl-related processes. Neutralization techniques must be employed before disposal, and any byproducts should be carefully treated to prevent secondary environmental impacts. The development of closed-loop systems and recycling processes can significantly reduce the overall environmental footprint of HCl use.
Monitoring and early detection systems play a vital role in maintaining HCl stability and preventing environmental incidents. Regular inspections, real-time pH monitoring, and leak detection technologies should be integrated into development processes to ensure rapid response to potential stability issues.
The long-term environmental effects of HCl stability requirements extend beyond immediate impacts. Chronic low-level exposure can lead to gradual ecosystem changes, affecting biodiversity and ecological succession. Therefore, comprehensive environmental impact assessments should be conducted throughout the development phases to identify and address potential long-term consequences.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!