How to Advance Hydrochloric Acid Integration in New Applications?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Integration Background and Objectives
Hydrochloric acid (HCl) has been a cornerstone in various industrial applications for decades, primarily in the chemical, metallurgical, and food processing sectors. However, the landscape of HCl integration is evolving rapidly, driven by technological advancements and the pressing need for sustainable solutions across industries. This technological evolution presents both challenges and opportunities for expanding HCl's role in novel applications.
The primary objective of advancing HCl integration in new applications is to leverage its unique chemical properties while addressing the limitations that have historically confined its use. These properties include its strong acidity, ability to dissolve many metals and minerals, and its role as a crucial reagent in numerous chemical reactions. By exploring innovative integration methods, we aim to unlock HCl's potential in emerging fields such as advanced materials manufacturing, environmental remediation, and energy storage technologies.
One of the key drivers behind this push for innovation is the growing demand for more efficient and environmentally friendly industrial processes. As global sustainability initiatives gain momentum, there is an increasing focus on developing circular economy models where waste products, including HCl, can be repurposed or recycled. This shift in perspective opens up new avenues for HCl integration, particularly in waste treatment and resource recovery applications.
The historical trajectory of HCl usage provides valuable insights into its potential future applications. From its initial discovery and industrial production in the early 19th century to its widespread use in steel pickling and chemical synthesis, HCl has demonstrated remarkable versatility. Recent technological trends suggest that the next frontier for HCl integration lies in nanotechnology, advanced catalysis, and smart materials development.
To achieve the goal of advancing HCl integration, it is crucial to address several technical challenges. These include improving handling and storage safety, developing corrosion-resistant materials for HCl-based processes, and optimizing reaction conditions to enhance efficiency and reduce environmental impact. Additionally, there is a need to explore novel synthesis routes that could lead to the production of high-purity HCl with minimal byproducts.
The objectives of this technological exploration extend beyond mere process improvements. They encompass the creation of entirely new applications that could revolutionize industries or solve pressing global challenges. For instance, the potential use of HCl in hydrogen production processes or its role in developing next-generation energy storage solutions aligns with the global push towards renewable energy and decarbonization.
The primary objective of advancing HCl integration in new applications is to leverage its unique chemical properties while addressing the limitations that have historically confined its use. These properties include its strong acidity, ability to dissolve many metals and minerals, and its role as a crucial reagent in numerous chemical reactions. By exploring innovative integration methods, we aim to unlock HCl's potential in emerging fields such as advanced materials manufacturing, environmental remediation, and energy storage technologies.
One of the key drivers behind this push for innovation is the growing demand for more efficient and environmentally friendly industrial processes. As global sustainability initiatives gain momentum, there is an increasing focus on developing circular economy models where waste products, including HCl, can be repurposed or recycled. This shift in perspective opens up new avenues for HCl integration, particularly in waste treatment and resource recovery applications.
The historical trajectory of HCl usage provides valuable insights into its potential future applications. From its initial discovery and industrial production in the early 19th century to its widespread use in steel pickling and chemical synthesis, HCl has demonstrated remarkable versatility. Recent technological trends suggest that the next frontier for HCl integration lies in nanotechnology, advanced catalysis, and smart materials development.
To achieve the goal of advancing HCl integration, it is crucial to address several technical challenges. These include improving handling and storage safety, developing corrosion-resistant materials for HCl-based processes, and optimizing reaction conditions to enhance efficiency and reduce environmental impact. Additionally, there is a need to explore novel synthesis routes that could lead to the production of high-purity HCl with minimal byproducts.
The objectives of this technological exploration extend beyond mere process improvements. They encompass the creation of entirely new applications that could revolutionize industries or solve pressing global challenges. For instance, the potential use of HCl in hydrogen production processes or its role in developing next-generation energy storage solutions aligns with the global push towards renewable energy and decarbonization.
Market Demand Analysis for HCl Applications
The market demand for hydrochloric acid (HCl) applications continues to expand across various industries, driven by its versatile chemical properties and increasing industrial applications. The global hydrochloric acid market is experiencing steady growth, with projections indicating a compound annual growth rate of 5.2% from 2021 to 2026. This growth is primarily fueled by the rising demand in key sectors such as chemical manufacturing, steel pickling, oil and gas, and water treatment.
In the chemical manufacturing sector, HCl serves as a crucial raw material for producing various chemicals, including vinyl chloride, chlorinated solvents, and high-purity silicon. The expanding chemical industry, particularly in emerging economies, is significantly contributing to the increased demand for HCl. Additionally, the steel industry's growth, especially in developing countries, is boosting the demand for HCl in steel pickling processes, where it is used to remove rust and scale from steel surfaces.
The oil and gas industry represents another significant market for HCl applications. In hydraulic fracturing operations, HCl is utilized to dissolve minerals and create cracks in rock formations, enhancing oil and gas extraction efficiency. As unconventional oil and gas exploration continues to grow, the demand for HCl in this sector is expected to rise correspondingly.
Water treatment is an emerging application area for HCl, with increasing emphasis on water purification and wastewater management globally. HCl is used in pH adjustment, metal removal, and regeneration of ion exchange resins in water treatment processes. The growing awareness of water scarcity and stringent environmental regulations are driving the adoption of advanced water treatment technologies, thereby boosting the demand for HCl in this sector.
The food industry also contributes to the market demand for HCl, where it is used in food processing for pH control, flavor enhancement, and as a preservative. The expanding food and beverage sector, coupled with increasing consumer preferences for processed foods, is expected to drive further growth in HCl applications within this industry.
Geographically, Asia-Pacific dominates the global HCl market, accounting for the largest share of consumption. This is attributed to the rapid industrialization, urbanization, and economic growth in countries like China and India. North America and Europe follow, with steady demand from established industries and increasing focus on water treatment and environmental applications.
Despite the positive market outlook, challenges such as environmental concerns and stringent regulations regarding HCl handling and disposal may impact market growth. However, these challenges also present opportunities for developing eco-friendly production methods and exploring new applications that align with sustainability goals, potentially opening up new market segments for HCl integration.
In the chemical manufacturing sector, HCl serves as a crucial raw material for producing various chemicals, including vinyl chloride, chlorinated solvents, and high-purity silicon. The expanding chemical industry, particularly in emerging economies, is significantly contributing to the increased demand for HCl. Additionally, the steel industry's growth, especially in developing countries, is boosting the demand for HCl in steel pickling processes, where it is used to remove rust and scale from steel surfaces.
The oil and gas industry represents another significant market for HCl applications. In hydraulic fracturing operations, HCl is utilized to dissolve minerals and create cracks in rock formations, enhancing oil and gas extraction efficiency. As unconventional oil and gas exploration continues to grow, the demand for HCl in this sector is expected to rise correspondingly.
Water treatment is an emerging application area for HCl, with increasing emphasis on water purification and wastewater management globally. HCl is used in pH adjustment, metal removal, and regeneration of ion exchange resins in water treatment processes. The growing awareness of water scarcity and stringent environmental regulations are driving the adoption of advanced water treatment technologies, thereby boosting the demand for HCl in this sector.
The food industry also contributes to the market demand for HCl, where it is used in food processing for pH control, flavor enhancement, and as a preservative. The expanding food and beverage sector, coupled with increasing consumer preferences for processed foods, is expected to drive further growth in HCl applications within this industry.
Geographically, Asia-Pacific dominates the global HCl market, accounting for the largest share of consumption. This is attributed to the rapid industrialization, urbanization, and economic growth in countries like China and India. North America and Europe follow, with steady demand from established industries and increasing focus on water treatment and environmental applications.
Despite the positive market outlook, challenges such as environmental concerns and stringent regulations regarding HCl handling and disposal may impact market growth. However, these challenges also present opportunities for developing eco-friendly production methods and exploring new applications that align with sustainability goals, potentially opening up new market segments for HCl integration.
Current HCl Integration Challenges
The integration of hydrochloric acid (HCl) into new applications faces several significant challenges that hinder its widespread adoption and utilization. One of the primary obstacles is the corrosive nature of HCl, which poses substantial risks to equipment and infrastructure. This corrosivity necessitates the use of specialized materials and protective coatings, significantly increasing implementation costs and limiting the range of potential applications.
Another major challenge is the environmental impact associated with HCl production and use. The manufacturing process of HCl often involves the emission of harmful byproducts, raising concerns about air and water pollution. Stringent environmental regulations and public awareness of ecological issues create barriers to expanding HCl integration in various industries.
Safety considerations also present a significant hurdle in advancing HCl applications. The handling and storage of HCl require strict safety protocols and specialized equipment to prevent accidents and protect workers. These safety requirements can be prohibitively expensive for smaller operations and may deter potential users from adopting HCl-based solutions.
The transportation of HCl poses additional challenges due to its hazardous nature. Strict regulations govern the movement of HCl, often resulting in higher logistics costs and complexities in supply chain management. This can make it difficult for businesses to incorporate HCl into their processes, especially if they are located far from production facilities.
Furthermore, the concentration and purity requirements for HCl vary widely across different applications. Achieving the necessary levels of purity and maintaining consistent concentrations can be technically challenging and costly, particularly for high-precision industries such as semiconductor manufacturing or pharmaceutical production.
The lack of standardization in HCl integration techniques across industries also impedes its advancement. Different sectors have developed their own methods and best practices, leading to a fragmented approach that hampers knowledge sharing and technological progress. This fragmentation makes it difficult to establish universal guidelines and streamline integration processes.
Lastly, the perception of HCl as a traditional, well-established chemical sometimes leads to a lack of innovation and research funding. This complacency can slow down the development of new applications and improvements in existing processes, limiting the potential for breakthrough advancements in HCl integration technologies.
Another major challenge is the environmental impact associated with HCl production and use. The manufacturing process of HCl often involves the emission of harmful byproducts, raising concerns about air and water pollution. Stringent environmental regulations and public awareness of ecological issues create barriers to expanding HCl integration in various industries.
Safety considerations also present a significant hurdle in advancing HCl applications. The handling and storage of HCl require strict safety protocols and specialized equipment to prevent accidents and protect workers. These safety requirements can be prohibitively expensive for smaller operations and may deter potential users from adopting HCl-based solutions.
The transportation of HCl poses additional challenges due to its hazardous nature. Strict regulations govern the movement of HCl, often resulting in higher logistics costs and complexities in supply chain management. This can make it difficult for businesses to incorporate HCl into their processes, especially if they are located far from production facilities.
Furthermore, the concentration and purity requirements for HCl vary widely across different applications. Achieving the necessary levels of purity and maintaining consistent concentrations can be technically challenging and costly, particularly for high-precision industries such as semiconductor manufacturing or pharmaceutical production.
The lack of standardization in HCl integration techniques across industries also impedes its advancement. Different sectors have developed their own methods and best practices, leading to a fragmented approach that hampers knowledge sharing and technological progress. This fragmentation makes it difficult to establish universal guidelines and streamline integration processes.
Lastly, the perception of HCl as a traditional, well-established chemical sometimes leads to a lack of innovation and research funding. This complacency can slow down the development of new applications and improvements in existing processes, limiting the potential for breakthrough advancements in HCl integration technologies.
Existing HCl Integration Solutions
01 Production methods of hydrochloric acid
Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.- Production methods of hydrochloric acid: Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.
- Purification and concentration techniques: Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for various industrial applications.
- Applications in chemical processing: Hydrochloric acid is widely used in chemical processing, including metal treatment, pH regulation, and as a catalyst in various reactions. It plays a crucial role in the production of organic and inorganic compounds, as well as in the extraction of certain minerals.
- Safety and handling considerations: Proper safety measures and handling procedures are essential when working with hydrochloric acid due to its corrosive nature. This includes the use of specialized storage containers, protective equipment, and neutralization techniques in case of spills or accidents.
- Environmental impact and waste management: The environmental impact of hydrochloric acid production and use is a significant concern. Strategies for waste management, recycling, and neutralization are implemented to minimize environmental damage and comply with regulations. This includes treatment of acid waste streams and recovery of valuable byproducts.
02 Purification and concentration techniques
Hydrochloric acid purification and concentration techniques involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and adjust the acid concentration for specific industrial applications, ensuring high-quality product output.Expand Specific Solutions03 Applications in chemical processing
Hydrochloric acid is widely used in various chemical processes, including metal treatment, pH regulation, and as a reagent in organic synthesis. Its strong acidic properties make it valuable in industries such as steel production, water treatment, and pharmaceutical manufacturing.Expand Specific Solutions04 Safety and handling procedures
Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes using appropriate personal protective equipment, implementing spill containment strategies, and following strict storage and transportation guidelines to prevent accidents and environmental contamination.Expand Specific Solutions05 Recycling and waste management
Recycling and waste management of hydrochloric acid involve neutralization, recovery, and reuse processes. These methods aim to minimize environmental impact and maximize resource efficiency in industrial settings, often incorporating advanced treatment technologies and closed-loop systems.Expand Specific Solutions
Key Players in HCl Integration
The hydrochloric acid integration market is in a growth phase, driven by increasing applications across various industries. The market size is expanding, with a projected CAGR of 5-6% over the next five years. Technologically, the field is moderately mature, with ongoing innovations focused on improving efficiency and environmental sustainability. Key players like Fluid Energy Group, Vertex Pharmaceuticals, and Akzo Nobel Chemicals are leading the charge in developing advanced integration techniques. Emerging companies such as WIAB WATER INNOVATION AB and Jiangyin Runma Electronic Material are also making significant contributions, particularly in niche applications. The competitive landscape is characterized by a mix of established chemical giants and specialized firms, each leveraging their unique strengths to capture market share in this evolving sector.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group has focused on developing environmentally friendly alternatives to traditional hydrochloric acid applications, particularly in the oil and gas industry. They have created a proprietary line of synthetic acid systems that mimic the performance of hydrochloric acid while significantly reducing environmental impact and improving worker safety[13]. These systems have found applications in well stimulation, scale removal, and enhanced oil recovery. Fluid Energy Group has also developed specialized acid blends that incorporate hydrochloric acid with organic acids and surfactants, optimizing performance in various geological formations[14]. Their research extends to developing acid systems with built-in corrosion inhibitors, reducing the need for additional chemical treatments[15].
Strengths: Specialization in eco-friendly acid systems, strong presence in the oil and gas sector, and focus on worker safety. Weaknesses: Limited diversification outside the energy sector and potential challenges in scaling up production for broader industrial applications.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has developed innovative applications for hydrochloric acid in water treatment and industrial processes. They have created a novel chlorine dioxide generation system that uses hydrochloric acid as a precursor, providing a more stable and efficient method for water disinfection[1]. Additionally, Akzo Nobel has engineered specialized coatings that incorporate hydrochloric acid for enhanced corrosion resistance in harsh industrial environments[2]. Their research also focuses on developing greener production methods for hydrochloric acid, including membrane electrolysis technology that reduces energy consumption and byproduct formation[3].
Strengths: Extensive experience in chemical engineering, strong R&D capabilities, and a global presence. Weaknesses: High production costs and environmental concerns associated with traditional hydrochloric acid manufacturing methods.
Core HCl Integration Innovations
Salt of monochloroacetic acid with chelating agent for delayed acidification in the oil field industry
PatentWO2020002011A1
Innovation
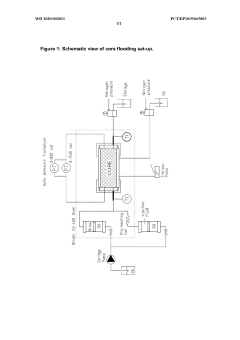
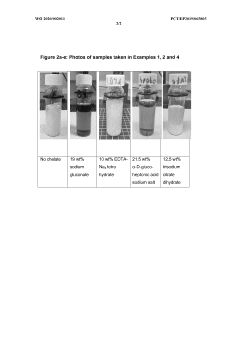
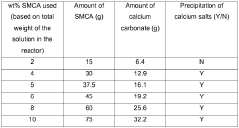
- A process using a monovalent salt of monochloroacetic acid in combination with a chelating agent having a monovalent counterion and a carbon chain with at least five hydroxyl groups, such as sodium gluconate, to inhibit calcium glycolate formation and control acidification, forming stable wormholes without scaling.
Synthetic acid compositions and uses thereof
PatentActiveCA2925142A1
Innovation
- A synthetic acid composition comprising urea and hydrogen chloride in a specific molar ratio, combined with metal iodides, alcohols, and phosphonic acids, which reduces corrosion rates, is non-fuming, non-toxic, and biodegradable, offering improved safety and environmental compatibility.
Environmental Impact of HCl Use
The integration of hydrochloric acid (HCl) in new applications brings significant environmental considerations that must be carefully evaluated and addressed. HCl is a highly corrosive and reactive substance, and its use can have substantial impacts on air quality, water resources, and soil composition. When released into the atmosphere, HCl can contribute to acid rain formation, potentially harming ecosystems and infrastructure. In aquatic environments, even small amounts of HCl can dramatically alter pH levels, endangering marine life and disrupting delicate ecological balances.
The production and transportation of HCl also present environmental challenges. Manufacturing processes often involve energy-intensive methods that contribute to greenhouse gas emissions. Additionally, the risk of accidental spills during transport poses threats to both human health and the environment, requiring stringent safety protocols and emergency response plans.
However, advancements in HCl handling and containment technologies have mitigated some of these risks. Modern industrial processes employ closed-loop systems and scrubbers to minimize atmospheric emissions. Improved storage and transportation methods, such as double-walled tanks and specialized linings, reduce the likelihood of leaks and spills.
In terms of waste management, the disposal of HCl and its byproducts requires careful consideration. Neutralization processes are commonly employed to render HCl waste less harmful, but these methods can generate significant quantities of salt byproducts that may impact soil and water quality if not properly managed.
Despite these challenges, HCl integration in new applications can also offer environmental benefits. In water treatment, for instance, HCl is used to adjust pH levels and remove contaminants, contributing to improved water quality. In the renewable energy sector, HCl plays a role in the production of high-purity silicon for solar panels, indirectly supporting the transition to cleaner energy sources.
As research into green chemistry advances, there are ongoing efforts to develop more environmentally friendly alternatives to HCl or to improve its production and use efficiency. These initiatives include exploring bio-based sources for HCl production, enhancing recycling and recovery processes, and optimizing reaction conditions to minimize waste generation.
To advance HCl integration responsibly, a comprehensive life cycle assessment approach is crucial. This involves evaluating the environmental impacts from raw material extraction through production, use, and disposal. By identifying hotspots in the HCl life cycle, researchers and industry professionals can focus on developing targeted solutions to mitigate environmental risks and enhance sustainability.
The production and transportation of HCl also present environmental challenges. Manufacturing processes often involve energy-intensive methods that contribute to greenhouse gas emissions. Additionally, the risk of accidental spills during transport poses threats to both human health and the environment, requiring stringent safety protocols and emergency response plans.
However, advancements in HCl handling and containment technologies have mitigated some of these risks. Modern industrial processes employ closed-loop systems and scrubbers to minimize atmospheric emissions. Improved storage and transportation methods, such as double-walled tanks and specialized linings, reduce the likelihood of leaks and spills.
In terms of waste management, the disposal of HCl and its byproducts requires careful consideration. Neutralization processes are commonly employed to render HCl waste less harmful, but these methods can generate significant quantities of salt byproducts that may impact soil and water quality if not properly managed.
Despite these challenges, HCl integration in new applications can also offer environmental benefits. In water treatment, for instance, HCl is used to adjust pH levels and remove contaminants, contributing to improved water quality. In the renewable energy sector, HCl plays a role in the production of high-purity silicon for solar panels, indirectly supporting the transition to cleaner energy sources.
As research into green chemistry advances, there are ongoing efforts to develop more environmentally friendly alternatives to HCl or to improve its production and use efficiency. These initiatives include exploring bio-based sources for HCl production, enhancing recycling and recovery processes, and optimizing reaction conditions to minimize waste generation.
To advance HCl integration responsibly, a comprehensive life cycle assessment approach is crucial. This involves evaluating the environmental impacts from raw material extraction through production, use, and disposal. By identifying hotspots in the HCl life cycle, researchers and industry professionals can focus on developing targeted solutions to mitigate environmental risks and enhance sustainability.
Safety Protocols for HCl Handling
The integration of hydrochloric acid (HCl) in new applications necessitates stringent safety protocols to mitigate risks associated with its corrosive and hazardous nature. Proper handling of HCl is crucial to ensure worker safety, environmental protection, and operational efficiency.
Personal protective equipment (PPE) forms the first line of defense in HCl handling. Workers must wear acid-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection, such as acid gas respirators, is essential when working with HCl vapors. Regular inspection and maintenance of PPE are vital to ensure their effectiveness.
Facility design plays a critical role in HCl safety. Dedicated storage areas should be well-ventilated, with corrosion-resistant flooring and containment systems. Emergency showers and eyewash stations must be readily accessible. Proper labeling of containers and pipelines, along with clear signage indicating hazard levels, is mandatory.
Training and education are fundamental components of HCl safety protocols. Comprehensive training programs should cover proper handling techniques, emergency response procedures, and the use of safety equipment. Regular refresher courses and safety drills help maintain a high level of preparedness among personnel.
Spill response protocols are essential for minimizing the impact of accidental releases. Neutralization agents, such as sodium bicarbonate or lime, should be readily available. Spill kits designed specifically for acid containment must be strategically placed throughout the facility. Proper disposal procedures for contaminated materials should be clearly defined and followed.
Ventilation systems play a crucial role in managing HCl vapors. Local exhaust ventilation should be installed at points of potential release, and general ventilation systems must be designed to prevent the accumulation of acid fumes. Regular monitoring of air quality and ventilation system performance is necessary to ensure a safe working environment.
Storage and transportation of HCl require specific safety measures. Containers must be made of compatible materials, such as glass-lined steel or high-density polyethylene. Secondary containment systems should be in place to prevent widespread contamination in case of leaks. Transportation protocols must comply with relevant regulations for hazardous materials.
Emergency response planning is a critical aspect of HCl safety protocols. Detailed procedures for various scenarios, including spills, fires, and personnel exposure, should be developed and regularly updated. Coordination with local emergency services and the establishment of clear communication channels are essential for effective incident management.
Implementing these comprehensive safety protocols for HCl handling is crucial for advancing its integration into new applications. By prioritizing safety, organizations can explore innovative uses of HCl while minimizing risks to personnel, equipment, and the environment.
Personal protective equipment (PPE) forms the first line of defense in HCl handling. Workers must wear acid-resistant gloves, goggles, face shields, and protective clothing. Respiratory protection, such as acid gas respirators, is essential when working with HCl vapors. Regular inspection and maintenance of PPE are vital to ensure their effectiveness.
Facility design plays a critical role in HCl safety. Dedicated storage areas should be well-ventilated, with corrosion-resistant flooring and containment systems. Emergency showers and eyewash stations must be readily accessible. Proper labeling of containers and pipelines, along with clear signage indicating hazard levels, is mandatory.
Training and education are fundamental components of HCl safety protocols. Comprehensive training programs should cover proper handling techniques, emergency response procedures, and the use of safety equipment. Regular refresher courses and safety drills help maintain a high level of preparedness among personnel.
Spill response protocols are essential for minimizing the impact of accidental releases. Neutralization agents, such as sodium bicarbonate or lime, should be readily available. Spill kits designed specifically for acid containment must be strategically placed throughout the facility. Proper disposal procedures for contaminated materials should be clearly defined and followed.
Ventilation systems play a crucial role in managing HCl vapors. Local exhaust ventilation should be installed at points of potential release, and general ventilation systems must be designed to prevent the accumulation of acid fumes. Regular monitoring of air quality and ventilation system performance is necessary to ensure a safe working environment.
Storage and transportation of HCl require specific safety measures. Containers must be made of compatible materials, such as glass-lined steel or high-density polyethylene. Secondary containment systems should be in place to prevent widespread contamination in case of leaks. Transportation protocols must comply with relevant regulations for hazardous materials.
Emergency response planning is a critical aspect of HCl safety protocols. Detailed procedures for various scenarios, including spills, fires, and personnel exposure, should be developed and regularly updated. Coordination with local emergency services and the establishment of clear communication channels are essential for effective incident management.
Implementing these comprehensive safety protocols for HCl handling is crucial for advancing its integration into new applications. By prioritizing safety, organizations can explore innovative uses of HCl while minimizing risks to personnel, equipment, and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!