How to Minimize Risks with Hydrochloric Acid Exposure?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Exposure Risks and Safety Objectives
Hydrochloric acid (HCl) is a widely used chemical in various industries, including manufacturing, pharmaceuticals, and research laboratories. However, its corrosive nature and potential health hazards necessitate a comprehensive understanding of the risks associated with exposure and the implementation of robust safety measures. The primary objective of this technical research is to explore effective strategies for minimizing risks related to HCl exposure in industrial and laboratory settings.
Exposure to HCl can occur through inhalation, skin contact, or ingestion, each presenting unique risks. Inhalation of HCl vapors can cause severe respiratory irritation, coughing, and in extreme cases, pulmonary edema. Skin contact may result in chemical burns, while ingestion can lead to severe internal injuries. Long-term exposure has been linked to dental erosion, chronic bronchitis, and increased susceptibility to respiratory infections.
To address these risks, a multi-faceted approach to safety is essential. Engineering controls form the first line of defense, including proper ventilation systems, fume hoods, and closed handling systems to minimize the release of HCl vapors into the work environment. The design and maintenance of these systems are crucial for their effectiveness in reducing exposure risks.
Personal protective equipment (PPE) plays a vital role in safeguarding workers. This includes chemical-resistant gloves, goggles or face shields, and appropriate respiratory protection. The selection of PPE must be based on the specific concentration and potential exposure scenarios encountered in the workplace.
Administrative controls, such as implementing standard operating procedures (SOPs), regular safety training, and proper labeling of HCl containers, are equally important. These measures ensure that workers are well-informed about the risks and proper handling techniques, reducing the likelihood of accidents and exposure incidents.
Emergency response planning is another critical aspect of risk minimization. This involves establishing clear protocols for spill management, first aid procedures, and evacuation plans in case of large-scale releases. Regular drills and training sessions help maintain readiness and improve response times during actual emergencies.
Monitoring and assessment of workplace HCl levels are essential for maintaining a safe environment. This can be achieved through regular air quality testing and the use of personal exposure monitors. Data from these assessments can inform the need for additional control measures or modifications to existing safety protocols.
The ultimate goal of these safety objectives is to create a comprehensive risk management system that not only complies with regulatory standards but also fosters a culture of safety awareness among workers. By addressing the risks associated with HCl exposure through a combination of engineering, administrative, and personal protective measures, organizations can significantly reduce the potential for accidents and long-term health effects, ensuring a safer work environment for all personnel involved in handling this hazardous substance.
Exposure to HCl can occur through inhalation, skin contact, or ingestion, each presenting unique risks. Inhalation of HCl vapors can cause severe respiratory irritation, coughing, and in extreme cases, pulmonary edema. Skin contact may result in chemical burns, while ingestion can lead to severe internal injuries. Long-term exposure has been linked to dental erosion, chronic bronchitis, and increased susceptibility to respiratory infections.
To address these risks, a multi-faceted approach to safety is essential. Engineering controls form the first line of defense, including proper ventilation systems, fume hoods, and closed handling systems to minimize the release of HCl vapors into the work environment. The design and maintenance of these systems are crucial for their effectiveness in reducing exposure risks.
Personal protective equipment (PPE) plays a vital role in safeguarding workers. This includes chemical-resistant gloves, goggles or face shields, and appropriate respiratory protection. The selection of PPE must be based on the specific concentration and potential exposure scenarios encountered in the workplace.
Administrative controls, such as implementing standard operating procedures (SOPs), regular safety training, and proper labeling of HCl containers, are equally important. These measures ensure that workers are well-informed about the risks and proper handling techniques, reducing the likelihood of accidents and exposure incidents.
Emergency response planning is another critical aspect of risk minimization. This involves establishing clear protocols for spill management, first aid procedures, and evacuation plans in case of large-scale releases. Regular drills and training sessions help maintain readiness and improve response times during actual emergencies.
Monitoring and assessment of workplace HCl levels are essential for maintaining a safe environment. This can be achieved through regular air quality testing and the use of personal exposure monitors. Data from these assessments can inform the need for additional control measures or modifications to existing safety protocols.
The ultimate goal of these safety objectives is to create a comprehensive risk management system that not only complies with regulatory standards but also fosters a culture of safety awareness among workers. By addressing the risks associated with HCl exposure through a combination of engineering, administrative, and personal protective measures, organizations can significantly reduce the potential for accidents and long-term health effects, ensuring a safer work environment for all personnel involved in handling this hazardous substance.
Industrial Demand for HCl Risk Mitigation
The industrial demand for minimizing risks associated with hydrochloric acid (HCl) exposure has been steadily increasing due to the widespread use of this chemical in various sectors. Industries such as chemical manufacturing, metal processing, oil and gas, and water treatment rely heavily on HCl for their operations, making risk mitigation a critical concern.
The global hydrochloric acid market has been experiencing significant growth, driven by the expanding industrial applications and the increasing focus on safety measures. This growth has led to a parallel increase in the demand for effective risk mitigation strategies and technologies. Companies are increasingly recognizing the importance of investing in safety measures to protect their workforce, comply with stringent regulations, and maintain operational efficiency.
One of the primary drivers for HCl risk mitigation demand is the stringent regulatory environment. Governments and international bodies have implemented strict guidelines for the handling, storage, and transportation of hazardous chemicals, including HCl. These regulations have compelled industries to adopt advanced safety measures and invest in state-of-the-art equipment to minimize exposure risks.
The chemical manufacturing sector, being one of the largest consumers of HCl, has been at the forefront of implementing comprehensive risk mitigation strategies. This includes the adoption of closed-loop systems, automated handling processes, and advanced personal protective equipment (PPE) to minimize direct contact with the acid. The demand for such solutions has led to the development of innovative technologies and products specifically designed for HCl risk management.
In the metal processing industry, where HCl is extensively used for pickling and surface treatment, there has been a growing emphasis on implementing safer handling practices. This has resulted in increased demand for corrosion-resistant storage tanks, specialized pumps, and advanced ventilation systems to control acid fumes and prevent accidental spills.
The oil and gas sector, another significant consumer of HCl, has been investing heavily in risk mitigation technologies. The use of HCl in well stimulation and hydraulic fracturing has necessitated the development of specialized equipment and handling procedures to ensure worker safety and environmental protection. This has created a niche market for HCl-specific safety solutions in the oilfield services industry.
Water treatment facilities, which use HCl for pH adjustment and chlorine production, have also contributed to the growing demand for risk mitigation solutions. The focus has been on implementing automated dosing systems, leak detection technologies, and improved containment measures to prevent accidental releases and ensure safe handling of the acid.
The increasing awareness of the potential health hazards associated with HCl exposure has led to a rise in demand for advanced monitoring and detection systems. Industries are investing in real-time gas detection equipment, wearable sensors, and sophisticated alarm systems to provide early warnings and prevent potential exposure incidents.
The global hydrochloric acid market has been experiencing significant growth, driven by the expanding industrial applications and the increasing focus on safety measures. This growth has led to a parallel increase in the demand for effective risk mitigation strategies and technologies. Companies are increasingly recognizing the importance of investing in safety measures to protect their workforce, comply with stringent regulations, and maintain operational efficiency.
One of the primary drivers for HCl risk mitigation demand is the stringent regulatory environment. Governments and international bodies have implemented strict guidelines for the handling, storage, and transportation of hazardous chemicals, including HCl. These regulations have compelled industries to adopt advanced safety measures and invest in state-of-the-art equipment to minimize exposure risks.
The chemical manufacturing sector, being one of the largest consumers of HCl, has been at the forefront of implementing comprehensive risk mitigation strategies. This includes the adoption of closed-loop systems, automated handling processes, and advanced personal protective equipment (PPE) to minimize direct contact with the acid. The demand for such solutions has led to the development of innovative technologies and products specifically designed for HCl risk management.
In the metal processing industry, where HCl is extensively used for pickling and surface treatment, there has been a growing emphasis on implementing safer handling practices. This has resulted in increased demand for corrosion-resistant storage tanks, specialized pumps, and advanced ventilation systems to control acid fumes and prevent accidental spills.
The oil and gas sector, another significant consumer of HCl, has been investing heavily in risk mitigation technologies. The use of HCl in well stimulation and hydraulic fracturing has necessitated the development of specialized equipment and handling procedures to ensure worker safety and environmental protection. This has created a niche market for HCl-specific safety solutions in the oilfield services industry.
Water treatment facilities, which use HCl for pH adjustment and chlorine production, have also contributed to the growing demand for risk mitigation solutions. The focus has been on implementing automated dosing systems, leak detection technologies, and improved containment measures to prevent accidental releases and ensure safe handling of the acid.
The increasing awareness of the potential health hazards associated with HCl exposure has led to a rise in demand for advanced monitoring and detection systems. Industries are investing in real-time gas detection equipment, wearable sensors, and sophisticated alarm systems to provide early warnings and prevent potential exposure incidents.
Current Challenges in HCl Handling
Handling hydrochloric acid (HCl) presents significant challenges due to its corrosive nature and potential health hazards. One of the primary concerns is the risk of chemical burns and respiratory issues from exposure to HCl vapors. Even at low concentrations, these vapors can cause severe irritation to the eyes, nose, and throat. In industrial settings, the potential for large-scale spills or leaks poses a serious threat to worker safety and environmental integrity.
The storage and transportation of HCl also present ongoing challenges. The acid's corrosive properties necessitate the use of specialized containers and equipment, which must be regularly inspected and maintained to prevent degradation and potential breaches. This maintenance requirement adds complexity and cost to HCl handling processes.
Another significant challenge is the proper disposal of HCl waste. Environmental regulations strictly govern the discharge of acidic substances, requiring neutralization or specialized treatment before disposal. This process can be resource-intensive and requires careful monitoring to ensure compliance with local and national environmental standards.
The reactivity of HCl with various materials presents additional handling difficulties. It can react violently with certain metals, releasing potentially explosive hydrogen gas. This reactivity necessitates careful material selection for storage and handling equipment, as well as stringent protocols for preventing accidental mixing with incompatible substances.
Personal protective equipment (PPE) for HCl handling is crucial but presents its own set of challenges. While necessary for safety, PPE can be cumbersome, reducing dexterity and potentially increasing the risk of accidents. Ensuring that workers consistently and correctly use PPE remains an ongoing challenge in many industrial settings.
The variability in HCl concentration levels across different applications adds another layer of complexity to its handling. Different concentrations require different safety measures and handling protocols, necessitating a flexible and comprehensive approach to safety management.
Lastly, the training and education of personnel involved in HCl handling remain ongoing challenges. Ensuring that all workers are adequately trained in safety procedures, emergency responses, and the proper use of equipment is critical but can be difficult to maintain consistently, especially in high-turnover environments or when dealing with temporary workers.
The storage and transportation of HCl also present ongoing challenges. The acid's corrosive properties necessitate the use of specialized containers and equipment, which must be regularly inspected and maintained to prevent degradation and potential breaches. This maintenance requirement adds complexity and cost to HCl handling processes.
Another significant challenge is the proper disposal of HCl waste. Environmental regulations strictly govern the discharge of acidic substances, requiring neutralization or specialized treatment before disposal. This process can be resource-intensive and requires careful monitoring to ensure compliance with local and national environmental standards.
The reactivity of HCl with various materials presents additional handling difficulties. It can react violently with certain metals, releasing potentially explosive hydrogen gas. This reactivity necessitates careful material selection for storage and handling equipment, as well as stringent protocols for preventing accidental mixing with incompatible substances.
Personal protective equipment (PPE) for HCl handling is crucial but presents its own set of challenges. While necessary for safety, PPE can be cumbersome, reducing dexterity and potentially increasing the risk of accidents. Ensuring that workers consistently and correctly use PPE remains an ongoing challenge in many industrial settings.
The variability in HCl concentration levels across different applications adds another layer of complexity to its handling. Different concentrations require different safety measures and handling protocols, necessitating a flexible and comprehensive approach to safety management.
Lastly, the training and education of personnel involved in HCl handling remain ongoing challenges. Ensuring that all workers are adequately trained in safety procedures, emergency responses, and the proper use of equipment is critical but can be difficult to maintain consistently, especially in high-turnover environments or when dealing with temporary workers.
Existing HCl Exposure Prevention Methods
01 Corrosive effects on materials and equipment
Hydrochloric acid poses significant risks due to its corrosive nature, which can damage various materials and equipment. It can cause rapid deterioration of metals, plastics, and other substances, leading to potential leaks, equipment failure, and safety hazards in industrial settings.- Corrosive effects on materials and tissues: Hydrochloric acid is highly corrosive and can cause severe damage to various materials and biological tissues. It can corrode metals, deteriorate concrete, and cause chemical burns to skin and mucous membranes upon contact. Proper handling and storage are crucial to prevent accidents and injuries.
- Respiratory hazards and inhalation risks: Inhalation of hydrochloric acid vapors or mists can lead to severe respiratory issues. It can cause irritation and damage to the respiratory tract, potentially resulting in coughing, shortness of breath, and in severe cases, pulmonary edema. Proper ventilation and personal protective equipment are essential when working with this acid.
- Environmental impact and disposal concerns: Improper disposal of hydrochloric acid can have significant environmental consequences. It can alter soil and water pH, harming ecosystems and aquatic life. Proper neutralization and disposal methods must be employed to minimize environmental risks and comply with regulations.
- Safety measures and protective equipment: To mitigate the risks associated with hydrochloric acid, appropriate safety measures and personal protective equipment are crucial. This includes using acid-resistant gloves, goggles, face shields, and protective clothing. Emergency eyewash stations and safety showers should be readily available in areas where the acid is handled.
- Storage and transportation hazards: Proper storage and transportation of hydrochloric acid are essential to prevent accidents and spills. The acid should be stored in corrosion-resistant containers in well-ventilated areas, away from incompatible materials. During transportation, appropriate labeling, packaging, and handling procedures must be followed to ensure safety and compliance with regulations.
02 Health hazards and safety precautions
Exposure to hydrochloric acid can result in severe health risks, including skin burns, respiratory issues, and eye damage. Proper safety measures, such as personal protective equipment and emergency response protocols, are crucial when handling this acid to minimize potential harm to workers and the environment.Expand Specific Solutions03 Environmental impact and disposal concerns
Improper handling or disposal of hydrochloric acid can lead to significant environmental risks. It can contaminate soil and water sources, affecting ecosystems and potentially entering the food chain. Proper neutralization and disposal methods are essential to mitigate these environmental hazards.Expand Specific Solutions04 Storage and transportation risks
The storage and transportation of hydrochloric acid present unique challenges due to its corrosive nature. Specialized containers, handling procedures, and safety protocols are necessary to prevent leaks, spills, or accidents during storage and transit, which could lead to severe consequences for both human health and the environment.Expand Specific Solutions05 Industrial process risks and mitigation strategies
In industrial applications, the use of hydrochloric acid carries inherent risks that require careful management. These include potential equipment corrosion, chemical reactions, and vapor emissions. Implementing proper engineering controls, monitoring systems, and employee training programs are crucial for minimizing these risks in industrial settings.Expand Specific Solutions
Key Players in Chemical Safety Industry
The competitive landscape for minimizing risks associated with hydrochloric acid exposure is evolving rapidly, driven by increasing safety regulations and growing industrial applications. The market is in a growth phase, with a projected global size of over $7 billion by 2027. Technological maturity varies, with established players like Praxair Technology and Kaneka Corp offering advanced solutions, while newer entrants such as Fluid Energy Group and Annihilare Medical Systems are introducing innovative approaches. Companies like CNOOC Energy Technology & Services and Baoshan Iron & Steel are developing industry-specific solutions, indicating a trend towards specialized risk mitigation strategies across different sectors.
Dorf Ketal Chemicals FZE
Technical Solution: Dorf Ketal Chemicals FZE has developed advanced chemical solutions to minimize risks associated with hydrochloric acid exposure. Their approach involves the use of specialized corrosion inhibitors and neutralizing agents designed to mitigate the harmful effects of hydrochloric acid in industrial settings. The company's proprietary formulations include a range of pH-neutral and low-pH inhibitors that form protective films on metal surfaces, significantly reducing corrosion rates and potential leaks[1]. Additionally, they have introduced rapid-action neutralizers that can quickly neutralize acid spills, reducing the risk of personnel exposure and environmental damage[3].
Strengths: Highly effective in preventing corrosion and neutralizing spills, suitable for a wide range of industrial applications. Weaknesses: May require frequent reapplication in high-temperature or high-pressure environments.
Fluid Energy Group Ltd.
Technical Solution: Fluid Energy Group Ltd. has pioneered a novel approach to hydrochloric acid risk mitigation through their HydroFLOW technology. This system utilizes a unique combination of physical water treatment and chemical additives to reduce the corrosive nature of hydrochloric acid while maintaining its effectiveness in industrial processes. The HydroFLOW technology employs electromagnetic fields to alter the crystalline structure of scale-forming minerals, preventing them from adhering to surfaces and reducing the need for high concentrations of hydrochloric acid[2]. Furthermore, they have developed eco-friendly acid alternatives that offer similar performance to traditional hydrochloric acid but with significantly reduced health and environmental risks[4].
Strengths: Reduces acid consumption and associated risks without compromising effectiveness, environmentally friendly. Weaknesses: Initial implementation may require modifications to existing systems and processes.
Innovative HCl Safety Technologies
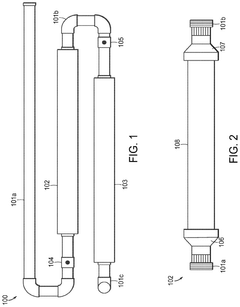
Mixing device
PatentWO2013121295A2
Innovation
- A mixing device that produces fluidic vortices within a chamber with strategically placed apertures to stabilize hypochlorous acid by controlling proton concentration and pH through the use of buffering agents like acetic acid, allowing for its production and storage for extended periods without the need for onsite generation.
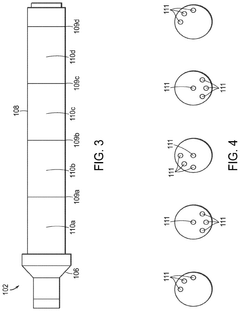
Compositions of hypochlorous acid and methods of manufacture thereof
PatentActiveUS12115185B2
Innovation
- An air-free mixing method producing stable HOCl by combining a compound that generates protons (H+) with one that generates hypochlorite anions (OCl-) in water, without using chlorine gas or electrolysis, maintaining a controlled pH and using buffering agents to stabilize the product.
Regulatory Framework for HCl Handling
The regulatory framework for handling hydrochloric acid (HCl) is a critical component in minimizing risks associated with exposure. Governments and international organizations have established comprehensive guidelines and regulations to ensure the safe handling, storage, and use of this hazardous substance.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. This system includes specific criteria for classifying HCl and requirements for labeling and safety data sheets, ensuring consistent information across borders.
In the United States, the Occupational Safety and Health Administration (OSHA) has set stringent standards for HCl handling in the workplace. The OSHA Permissible Exposure Limit (PEL) for HCl is 5 parts per million (ppm) as a ceiling limit, meaning this concentration should never be exceeded during any part of the workday. Additionally, OSHA mandates the use of personal protective equipment (PPE) and engineering controls to minimize worker exposure.
The Environmental Protection Agency (EPA) regulates HCl under various environmental laws, including the Clean Air Act and the Emergency Planning and Community Right-to-Know Act. These regulations aim to prevent and mitigate environmental releases and ensure proper reporting of HCl storage and usage.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of HCl. REACH requires manufacturers and importers to register substances and provide safety information, including exposure scenarios and risk management measures.
Many countries have adopted specific regulations for the transportation of HCl. For instance, the U.S. Department of Transportation classifies HCl as a hazardous material and prescribes specific packaging, labeling, and documentation requirements for its transport.
Industry-specific regulations also play a crucial role. For example, in the semiconductor industry, where HCl is widely used, organizations like SEMI have developed guidelines for the safe handling of hazardous gases and chemicals, including HCl.
To ensure compliance and minimize risks, companies handling HCl must implement robust management systems. This includes regular risk assessments, employee training programs, emergency response plans, and continuous monitoring of exposure levels. Many organizations adopt voluntary standards, such as ISO 45001 for occupational health and safety management, to complement regulatory requirements and demonstrate their commitment to safety.
As regulations continue to evolve, staying informed about changes and emerging best practices is essential for organizations working with HCl. Regular audits, participation in industry forums, and collaboration with regulatory bodies can help ensure ongoing compliance and safety in HCl handling operations.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. This system includes specific criteria for classifying HCl and requirements for labeling and safety data sheets, ensuring consistent information across borders.
In the United States, the Occupational Safety and Health Administration (OSHA) has set stringent standards for HCl handling in the workplace. The OSHA Permissible Exposure Limit (PEL) for HCl is 5 parts per million (ppm) as a ceiling limit, meaning this concentration should never be exceeded during any part of the workday. Additionally, OSHA mandates the use of personal protective equipment (PPE) and engineering controls to minimize worker exposure.
The Environmental Protection Agency (EPA) regulates HCl under various environmental laws, including the Clean Air Act and the Emergency Planning and Community Right-to-Know Act. These regulations aim to prevent and mitigate environmental releases and ensure proper reporting of HCl storage and usage.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of HCl. REACH requires manufacturers and importers to register substances and provide safety information, including exposure scenarios and risk management measures.
Many countries have adopted specific regulations for the transportation of HCl. For instance, the U.S. Department of Transportation classifies HCl as a hazardous material and prescribes specific packaging, labeling, and documentation requirements for its transport.
Industry-specific regulations also play a crucial role. For example, in the semiconductor industry, where HCl is widely used, organizations like SEMI have developed guidelines for the safe handling of hazardous gases and chemicals, including HCl.
To ensure compliance and minimize risks, companies handling HCl must implement robust management systems. This includes regular risk assessments, employee training programs, emergency response plans, and continuous monitoring of exposure levels. Many organizations adopt voluntary standards, such as ISO 45001 for occupational health and safety management, to complement regulatory requirements and demonstrate their commitment to safety.
As regulations continue to evolve, staying informed about changes and emerging best practices is essential for organizations working with HCl. Regular audits, participation in industry forums, and collaboration with regulatory bodies can help ensure ongoing compliance and safety in HCl handling operations.
Environmental Impact of HCl Use
The environmental impact of hydrochloric acid (HCl) use is a critical consideration in industrial processes and waste management. HCl, a strong and corrosive acid, can have significant effects on ecosystems if not properly handled and disposed of. When released into the environment, HCl can cause soil acidification, leading to changes in soil chemistry and potentially affecting plant growth and microbial communities. This acidification can also result in the mobilization of heavy metals, increasing their bioavailability and potential toxicity to organisms.
In aquatic environments, HCl discharge can dramatically alter pH levels, causing stress or mortality to fish and other aquatic life. Even small changes in pH can disrupt the delicate balance of aquatic ecosystems, affecting species composition and biodiversity. The acid can also react with minerals in water bodies, potentially releasing harmful substances and altering water chemistry.
Atmospheric emissions of HCl, often associated with industrial processes or waste incineration, contribute to acid rain formation. This phenomenon can have far-reaching consequences, affecting forests, lakes, and buildings far from the original source of emission. Acid rain can damage vegetation, leach nutrients from soils, and acidify water bodies, impacting entire ecosystems.
The production and transportation of HCl also carry environmental risks. Accidental spills during transport or storage can lead to localized environmental damage, requiring immediate containment and remediation efforts. Furthermore, the manufacturing process of HCl itself can be energy-intensive and may involve the release of other pollutants, contributing to overall environmental footprint.
To mitigate these environmental impacts, industries using HCl must implement strict handling and disposal protocols. This includes proper storage facilities, containment measures, and treatment of HCl-containing waste before release. Advanced technologies such as scrubbers and neutralization systems can significantly reduce atmospheric emissions and wastewater acidity. Additionally, adopting closed-loop systems and recycling processes can minimize the amount of HCl released into the environment.
Regulatory frameworks play a crucial role in managing HCl's environmental impact. Many countries have implemented stringent regulations governing the use, storage, and disposal of HCl and other hazardous chemicals. Compliance with these regulations, along with voluntary industry initiatives, is essential for minimizing the environmental footprint of HCl use.
In aquatic environments, HCl discharge can dramatically alter pH levels, causing stress or mortality to fish and other aquatic life. Even small changes in pH can disrupt the delicate balance of aquatic ecosystems, affecting species composition and biodiversity. The acid can also react with minerals in water bodies, potentially releasing harmful substances and altering water chemistry.
Atmospheric emissions of HCl, often associated with industrial processes or waste incineration, contribute to acid rain formation. This phenomenon can have far-reaching consequences, affecting forests, lakes, and buildings far from the original source of emission. Acid rain can damage vegetation, leach nutrients from soils, and acidify water bodies, impacting entire ecosystems.
The production and transportation of HCl also carry environmental risks. Accidental spills during transport or storage can lead to localized environmental damage, requiring immediate containment and remediation efforts. Furthermore, the manufacturing process of HCl itself can be energy-intensive and may involve the release of other pollutants, contributing to overall environmental footprint.
To mitigate these environmental impacts, industries using HCl must implement strict handling and disposal protocols. This includes proper storage facilities, containment measures, and treatment of HCl-containing waste before release. Advanced technologies such as scrubbers and neutralization systems can significantly reduce atmospheric emissions and wastewater acidity. Additionally, adopting closed-loop systems and recycling processes can minimize the amount of HCl released into the environment.
Regulatory frameworks play a crucial role in managing HCl's environmental impact. Many countries have implemented stringent regulations governing the use, storage, and disposal of HCl and other hazardous chemicals. Compliance with these regulations, along with voluntary industry initiatives, is essential for minimizing the environmental footprint of HCl use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!