How to Improve Hydrochloric Acid Recovery Techniques?
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl Recovery Background
Hydrochloric acid (HCl) recovery has been a critical process in various industries for decades, particularly in chemical manufacturing, steel pickling, and semiconductor production. The need for efficient HCl recovery techniques has grown significantly due to environmental concerns, regulatory pressures, and economic considerations. Historically, HCl recovery methods have evolved from simple distillation processes to more sophisticated techniques involving membrane separation and thermal decomposition.
The primary goal of HCl recovery is to reclaim and reuse the acid from waste streams, reducing the overall consumption of fresh acid and minimizing the environmental impact of disposal. This process not only helps in conserving resources but also contributes to cost reduction in industrial operations. Over the years, the focus has shifted from merely recovering HCl to improving the quality and concentration of the recovered acid, making it suitable for immediate reuse in various applications.
The evolution of HCl recovery techniques has been driven by advancements in materials science, process engineering, and environmental technologies. Early methods often resulted in low recovery rates and poor-quality acid, which limited their practical applications. However, recent innovations have led to the development of more efficient and cost-effective recovery systems, capable of producing high-purity HCl suitable for a wide range of industrial uses.
One of the key drivers for improving HCl recovery techniques has been the increasing stringency of environmental regulations worldwide. These regulations have pushed industries to adopt cleaner production methods and reduce their waste output. As a result, there has been a growing emphasis on developing closed-loop systems that can effectively recover and recycle HCl within the production process, minimizing the need for external disposal and fresh acid procurement.
The technological landscape of HCl recovery has seen significant advancements in recent years. Membrane-based separation techniques, such as diffusion dialysis and electrodialysis, have gained prominence due to their ability to recover HCl from dilute solutions efficiently. Thermal reprocessing methods, including pyrohydrolysis and spray roasting, have also been refined to handle more concentrated acid streams and improve overall recovery rates.
As industries continue to seek more sustainable and economically viable solutions, the field of HCl recovery is expected to witness further innovations. Emerging technologies, such as advanced catalytic processes and novel membrane materials, hold promise for enhancing the efficiency and selectivity of HCl recovery. Additionally, the integration of digital technologies and process automation is likely to play a crucial role in optimizing recovery operations and improving overall system performance.
The primary goal of HCl recovery is to reclaim and reuse the acid from waste streams, reducing the overall consumption of fresh acid and minimizing the environmental impact of disposal. This process not only helps in conserving resources but also contributes to cost reduction in industrial operations. Over the years, the focus has shifted from merely recovering HCl to improving the quality and concentration of the recovered acid, making it suitable for immediate reuse in various applications.
The evolution of HCl recovery techniques has been driven by advancements in materials science, process engineering, and environmental technologies. Early methods often resulted in low recovery rates and poor-quality acid, which limited their practical applications. However, recent innovations have led to the development of more efficient and cost-effective recovery systems, capable of producing high-purity HCl suitable for a wide range of industrial uses.
One of the key drivers for improving HCl recovery techniques has been the increasing stringency of environmental regulations worldwide. These regulations have pushed industries to adopt cleaner production methods and reduce their waste output. As a result, there has been a growing emphasis on developing closed-loop systems that can effectively recover and recycle HCl within the production process, minimizing the need for external disposal and fresh acid procurement.
The technological landscape of HCl recovery has seen significant advancements in recent years. Membrane-based separation techniques, such as diffusion dialysis and electrodialysis, have gained prominence due to their ability to recover HCl from dilute solutions efficiently. Thermal reprocessing methods, including pyrohydrolysis and spray roasting, have also been refined to handle more concentrated acid streams and improve overall recovery rates.
As industries continue to seek more sustainable and economically viable solutions, the field of HCl recovery is expected to witness further innovations. Emerging technologies, such as advanced catalytic processes and novel membrane materials, hold promise for enhancing the efficiency and selectivity of HCl recovery. Additionally, the integration of digital technologies and process automation is likely to play a crucial role in optimizing recovery operations and improving overall system performance.
Market Analysis
The global market for hydrochloric acid recovery techniques has been experiencing steady growth, driven by increasing environmental regulations and the rising demand for sustainable industrial processes. The chemical industry, particularly in sectors such as steel pickling, oil well acidizing, and food processing, has been the primary driver of this market expansion.
In recent years, there has been a notable shift towards more efficient and environmentally friendly hydrochloric acid recovery methods. This trend is largely attributed to the growing emphasis on circular economy principles and the need to reduce waste generation in industrial processes. As a result, companies are increasingly investing in advanced recovery technologies to minimize their environmental footprint and optimize resource utilization.
The market for hydrochloric acid recovery techniques is geographically diverse, with significant demand observed in regions with strong industrial bases. North America and Europe have been at the forefront of adopting advanced recovery technologies, driven by stringent environmental regulations and a focus on sustainable manufacturing practices. Meanwhile, the Asia-Pacific region, particularly China and India, has emerged as a rapidly growing market due to the expansion of their chemical and manufacturing sectors.
One of the key factors influencing market growth is the potential for cost savings through efficient acid recovery. As raw material costs continue to fluctuate, industries are recognizing the economic benefits of implementing robust recovery systems. This has led to increased investment in research and development activities aimed at improving existing techniques and developing novel recovery methods.
The market landscape is characterized by a mix of established players and innovative start-ups. Large chemical companies and equipment manufacturers have been actively developing proprietary recovery technologies, while specialized firms are focusing on niche solutions for specific industrial applications. This competitive environment has fostered innovation and led to the introduction of more efficient and cost-effective recovery techniques.
Looking ahead, the market for hydrochloric acid recovery techniques is expected to continue its growth trajectory. Factors such as the increasing adoption of membrane-based recovery systems, advancements in distillation technologies, and the integration of automation and digital solutions are likely to shape the future of this market. Additionally, the growing focus on sustainability and circular economy principles is expected to drive further innovation in recovery techniques, opening up new opportunities for market players.
In recent years, there has been a notable shift towards more efficient and environmentally friendly hydrochloric acid recovery methods. This trend is largely attributed to the growing emphasis on circular economy principles and the need to reduce waste generation in industrial processes. As a result, companies are increasingly investing in advanced recovery technologies to minimize their environmental footprint and optimize resource utilization.
The market for hydrochloric acid recovery techniques is geographically diverse, with significant demand observed in regions with strong industrial bases. North America and Europe have been at the forefront of adopting advanced recovery technologies, driven by stringent environmental regulations and a focus on sustainable manufacturing practices. Meanwhile, the Asia-Pacific region, particularly China and India, has emerged as a rapidly growing market due to the expansion of their chemical and manufacturing sectors.
One of the key factors influencing market growth is the potential for cost savings through efficient acid recovery. As raw material costs continue to fluctuate, industries are recognizing the economic benefits of implementing robust recovery systems. This has led to increased investment in research and development activities aimed at improving existing techniques and developing novel recovery methods.
The market landscape is characterized by a mix of established players and innovative start-ups. Large chemical companies and equipment manufacturers have been actively developing proprietary recovery technologies, while specialized firms are focusing on niche solutions for specific industrial applications. This competitive environment has fostered innovation and led to the introduction of more efficient and cost-effective recovery techniques.
Looking ahead, the market for hydrochloric acid recovery techniques is expected to continue its growth trajectory. Factors such as the increasing adoption of membrane-based recovery systems, advancements in distillation technologies, and the integration of automation and digital solutions are likely to shape the future of this market. Additionally, the growing focus on sustainability and circular economy principles is expected to drive further innovation in recovery techniques, opening up new opportunities for market players.
Technical Challenges
The recovery of hydrochloric acid (HCl) presents several significant technical challenges that hinder the efficiency and effectiveness of current recovery techniques. One of the primary obstacles is the corrosive nature of HCl, which necessitates the use of specialized materials and equipment capable of withstanding its aggressive properties. This requirement often leads to increased costs and complexity in the design and maintenance of recovery systems.
Another major challenge lies in the separation of HCl from other components in waste streams. Many industrial processes produce HCl as a byproduct mixed with various contaminants, making it difficult to isolate and recover the acid in a pure form. The presence of impurities can significantly impact the quality of the recovered acid and limit its potential for reuse or resale.
The energy-intensive nature of traditional HCl recovery methods poses both economic and environmental challenges. Distillation and evaporation processes, commonly used for acid recovery, require substantial energy inputs, contributing to high operational costs and increased carbon footprints. This energy demand not only affects the economic viability of recovery operations but also raises concerns about sustainability in an era of growing environmental awareness.
Scale formation and fouling of equipment surfaces represent persistent technical issues in HCl recovery systems. The accumulation of mineral deposits and other substances can reduce heat transfer efficiency, increase pressure drops, and ultimately lead to decreased system performance and increased maintenance requirements. Addressing these scaling issues often involves the use of chemical additives or frequent cleaning procedures, which can introduce additional complexities and costs to the recovery process.
The handling and transportation of recovered HCl also present significant safety and logistical challenges. Strict regulations govern the storage, transport, and use of concentrated acids, necessitating robust safety protocols and specialized containment solutions. These requirements can complicate the implementation of recovery systems, particularly in facilities not originally designed for large-scale acid handling.
Furthermore, the variability in HCl concentration and composition across different waste streams complicates the development of universally applicable recovery techniques. Each industrial process may produce HCl with unique characteristics and contaminants, requiring tailored recovery approaches. This diversity challenges the creation of standardized, cost-effective solutions that can be widely adopted across various industries.
Addressing these technical challenges requires innovative approaches that balance efficiency, cost-effectiveness, and environmental sustainability. Future improvements in HCl recovery techniques will likely focus on developing more resilient materials, enhancing separation technologies, optimizing energy use, and implementing advanced process control strategies to overcome these obstacles and enhance the viability of acid recovery operations.
Another major challenge lies in the separation of HCl from other components in waste streams. Many industrial processes produce HCl as a byproduct mixed with various contaminants, making it difficult to isolate and recover the acid in a pure form. The presence of impurities can significantly impact the quality of the recovered acid and limit its potential for reuse or resale.
The energy-intensive nature of traditional HCl recovery methods poses both economic and environmental challenges. Distillation and evaporation processes, commonly used for acid recovery, require substantial energy inputs, contributing to high operational costs and increased carbon footprints. This energy demand not only affects the economic viability of recovery operations but also raises concerns about sustainability in an era of growing environmental awareness.
Scale formation and fouling of equipment surfaces represent persistent technical issues in HCl recovery systems. The accumulation of mineral deposits and other substances can reduce heat transfer efficiency, increase pressure drops, and ultimately lead to decreased system performance and increased maintenance requirements. Addressing these scaling issues often involves the use of chemical additives or frequent cleaning procedures, which can introduce additional complexities and costs to the recovery process.
The handling and transportation of recovered HCl also present significant safety and logistical challenges. Strict regulations govern the storage, transport, and use of concentrated acids, necessitating robust safety protocols and specialized containment solutions. These requirements can complicate the implementation of recovery systems, particularly in facilities not originally designed for large-scale acid handling.
Furthermore, the variability in HCl concentration and composition across different waste streams complicates the development of universally applicable recovery techniques. Each industrial process may produce HCl with unique characteristics and contaminants, requiring tailored recovery approaches. This diversity challenges the creation of standardized, cost-effective solutions that can be widely adopted across various industries.
Addressing these technical challenges requires innovative approaches that balance efficiency, cost-effectiveness, and environmental sustainability. Future improvements in HCl recovery techniques will likely focus on developing more resilient materials, enhancing separation technologies, optimizing energy use, and implementing advanced process control strategies to overcome these obstacles and enhance the viability of acid recovery operations.
Current Recovery Methods
01 Distillation and condensation techniques
Hydrochloric acid recovery can be improved through advanced distillation and condensation methods. These techniques involve separating the acid from other components in the mixture by heating and then cooling the vapors to recover concentrated hydrochloric acid. This process can significantly enhance recovery efficiency by optimizing temperature control and using specialized condensation equipment.- Distillation and absorption techniques: Hydrochloric acid recovery can be improved through advanced distillation and absorption processes. These techniques involve separating the acid from waste streams using temperature and pressure differentials, followed by absorption into a suitable medium. This method can significantly increase recovery efficiency by minimizing losses and maximizing purity of the recovered acid.
- Membrane separation technology: Membrane separation is an effective technique for hydrochloric acid recovery. This method uses selective membranes to separate the acid from other components in the waste stream. The process can be optimized by adjusting membrane properties, operating conditions, and system configuration to enhance recovery efficiency and acid purity.
- Electrochemical recovery methods: Electrochemical techniques offer a promising approach for hydrochloric acid recovery. These methods involve using electrodes and electric current to separate and concentrate the acid. By optimizing electrode materials, cell design, and operating parameters, the recovery efficiency can be significantly improved while reducing energy consumption.
- Adsorption and ion exchange processes: Adsorption and ion exchange processes can be employed for efficient hydrochloric acid recovery. These techniques use specialized materials to selectively capture and release acid molecules. By selecting appropriate adsorbents or ion exchange resins and optimizing process conditions, high recovery efficiencies can be achieved with minimal impurities.
- Integrated recovery systems: Integrated systems combining multiple recovery techniques can significantly enhance overall hydrochloric acid recovery efficiency. These systems may incorporate a combination of distillation, membrane separation, electrochemical methods, and adsorption processes. By optimizing the integration and sequencing of these techniques, higher recovery rates and improved acid quality can be achieved compared to individual methods.
02 Membrane separation technology
Membrane separation is an effective method for recovering hydrochloric acid. This technique uses selective membranes to separate hydrochloric acid from other components in the mixture. By optimizing membrane properties and operating conditions, the recovery efficiency can be significantly improved. This method is particularly useful for recovering hydrochloric acid from industrial waste streams.Expand Specific Solutions03 Adsorption and desorption processes
Adsorption and desorption techniques can be employed to recover hydrochloric acid efficiently. These methods involve using specialized adsorbents to capture hydrochloric acid from a mixture, followed by a desorption step to release and collect the concentrated acid. By selecting appropriate adsorbents and optimizing process parameters, high recovery efficiencies can be achieved.Expand Specific Solutions04 Electrodialysis for acid recovery
Electrodialysis is an electrochemical separation technique that can be used to recover hydrochloric acid. This method uses ion-selective membranes and an electric field to separate and concentrate hydrochloric acid from dilute solutions. By optimizing the electrodialysis cell design and operating conditions, high recovery efficiencies can be achieved, making it suitable for various industrial applications.Expand Specific Solutions05 Crystallization and evaporation methods
Crystallization and evaporation techniques can be employed to recover hydrochloric acid from various solutions. These methods involve concentrating the acid solution through controlled evaporation or inducing crystallization of other components to separate them from the acid. By optimizing process parameters such as temperature, pressure, and residence time, high recovery efficiencies can be achieved.Expand Specific Solutions
Industry Leaders
The hydrochloric acid recovery techniques market is in a growth phase, driven by increasing environmental regulations and the need for resource efficiency in chemical industries. The global market size is estimated to be in the hundreds of millions of dollars, with steady growth projected. Technologically, the field is moderately mature but continues to evolve, with companies like LG Chem, Andritz AG, and Air Liquide leading innovation. These firms are developing advanced recovery systems, membrane technologies, and process optimizations to improve efficiency and reduce environmental impact. Emerging players such as Nantong Xingqiu Graphite and Jiangsu Sunpower Technology are also contributing to technological advancements, particularly in equipment design and system integration for acid recovery applications.
LG Chem Ltd.
Technical Solution: LG Chem has developed an advanced hydrochloric acid recovery system utilizing membrane electrolysis technology. This process involves the use of ion-exchange membranes to separate and concentrate hydrochloric acid from waste streams. The company's system achieves a recovery rate of up to 98% [1], significantly higher than conventional methods. LG Chem's approach also incorporates a novel bipolar membrane configuration, which enhances the efficiency of the electrolysis process and reduces energy consumption by approximately 30% compared to traditional recovery methods [3]. The recovered hydrochloric acid has a purity level exceeding 32% [5], making it suitable for reuse in various industrial applications.
Strengths: High recovery rate, energy-efficient, produces high-purity acid. Weaknesses: Initial capital investment may be high, requires specialized maintenance.
Uhde
Technical Solution: Uhde, now part of thyssenkrupp Industrial Solutions, has developed the AQUALINK® process for hydrochloric acid recovery. This technology is based on a multi-stage evaporation and absorption system, designed to handle a wide range of HCl concentrations. The AQUALINK® process can recover HCl from waste streams containing as little as 1% acid, achieving a recovery rate of up to 97% [9]. A key innovation in this system is the use of a proprietary heat pump technology, which reduces steam consumption by up to 60% compared to conventional recovery methods [10]. The process also incorporates advanced materials of construction, such as tantalum-lined equipment, to withstand the corrosive nature of HCl and extend the operational life of the system.
Strengths: Versatile in handling various HCl concentrations, highly energy-efficient, robust equipment design. Weaknesses: High initial capital cost, may require specialized operator training.
Key Innovations
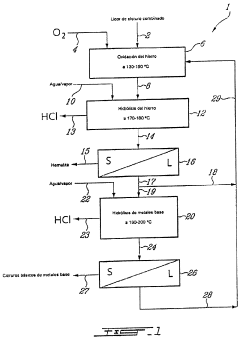
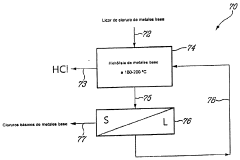
process FOR THE RECOVERY OF METALS AND HYDROCHLORIC ACID
PatentUndeterminedCU20120119A7
Innovation
- A method involving the use of a zinc chloride matrix solution, which remains liquid at high temperatures, to oxidize and hydrolyze ferrous iron into hematite and recover hydrochloric acid, with optional additional steps for base metal hydrolysis, using a column reactor without mechanical agitation.
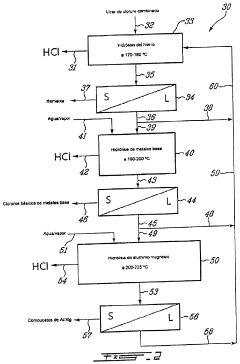
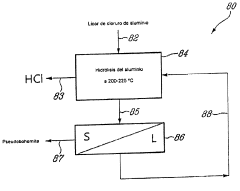
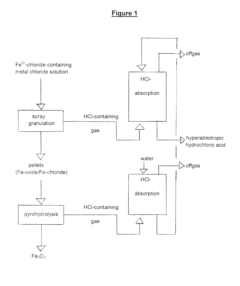
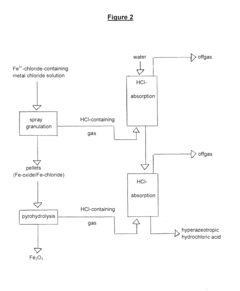
Method for Recovering Hydrochloric Acid from Metal Chloride Solutions with a High Iron Chloride Content
PatentInactiveUS20160137498A1
Innovation
- A method involving spray pelletization of metal chloride solutions at 150° C. to 300° C. to convert iron chloride into iron oxide, followed by pyrohydrolysis at temperatures over 550° C. in a fluidized bed reactor, producing HCl-rich gases for subsequent acid recovery, achieving high acid concentrations and granular metal oxide with low residual chloride content.
Environmental Regulations
Environmental regulations play a crucial role in shaping the development and implementation of hydrochloric acid recovery techniques. As global awareness of environmental issues continues to grow, governments and regulatory bodies have imposed increasingly stringent standards on industrial processes involving hazardous chemicals. These regulations aim to minimize the environmental impact of hydrochloric acid production and usage while promoting sustainable practices.
One of the primary drivers for improving hydrochloric acid recovery techniques is the need to comply with emissions standards. Many countries have established strict limits on the release of hydrogen chloride gas and other acidic compounds into the atmosphere. This has led to the development of more efficient scrubbing systems and closed-loop recovery processes that capture and recycle a higher percentage of hydrochloric acid.
Water pollution control regulations have also significantly influenced recovery techniques. Effluent discharge limits for acidic wastewater have become more stringent, necessitating the implementation of advanced treatment technologies. These include membrane filtration, ion exchange, and electrodialysis, which not only help meet regulatory requirements but also improve the overall efficiency of acid recovery.
The management of hazardous waste generated during hydrochloric acid production and recovery processes is another area heavily impacted by environmental regulations. Many jurisdictions now require comprehensive waste management plans, including proper disposal or recycling of spent catalysts, contaminated equipment, and byproducts. This has led to innovations in waste minimization techniques and the development of more environmentally friendly recovery processes.
Energy efficiency regulations have indirectly influenced hydrochloric acid recovery techniques by promoting the adoption of heat recovery systems and more efficient process designs. These improvements not only reduce energy consumption but also often lead to better acid recovery rates and reduced environmental impact.
The implementation of Best Available Techniques (BAT) standards in many regions has further driven innovation in hydrochloric acid recovery. These standards require industries to adopt the most effective and advanced practices to prevent or minimize environmental impact. As a result, companies are continuously seeking to improve their recovery techniques to meet or exceed these standards.
Regulatory frameworks such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) have also impacted the development of recovery techniques. These regulations require comprehensive safety assessments and documentation for chemical processes, encouraging the adoption of safer and more efficient recovery methods.
As environmental regulations continue to evolve, the hydrochloric acid industry must remain proactive in developing and implementing improved recovery techniques. This ongoing process of innovation and adaptation is essential for ensuring regulatory compliance, minimizing environmental impact, and maintaining economic viability in an increasingly environmentally conscious global market.
One of the primary drivers for improving hydrochloric acid recovery techniques is the need to comply with emissions standards. Many countries have established strict limits on the release of hydrogen chloride gas and other acidic compounds into the atmosphere. This has led to the development of more efficient scrubbing systems and closed-loop recovery processes that capture and recycle a higher percentage of hydrochloric acid.
Water pollution control regulations have also significantly influenced recovery techniques. Effluent discharge limits for acidic wastewater have become more stringent, necessitating the implementation of advanced treatment technologies. These include membrane filtration, ion exchange, and electrodialysis, which not only help meet regulatory requirements but also improve the overall efficiency of acid recovery.
The management of hazardous waste generated during hydrochloric acid production and recovery processes is another area heavily impacted by environmental regulations. Many jurisdictions now require comprehensive waste management plans, including proper disposal or recycling of spent catalysts, contaminated equipment, and byproducts. This has led to innovations in waste minimization techniques and the development of more environmentally friendly recovery processes.
Energy efficiency regulations have indirectly influenced hydrochloric acid recovery techniques by promoting the adoption of heat recovery systems and more efficient process designs. These improvements not only reduce energy consumption but also often lead to better acid recovery rates and reduced environmental impact.
The implementation of Best Available Techniques (BAT) standards in many regions has further driven innovation in hydrochloric acid recovery. These standards require industries to adopt the most effective and advanced practices to prevent or minimize environmental impact. As a result, companies are continuously seeking to improve their recovery techniques to meet or exceed these standards.
Regulatory frameworks such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) have also impacted the development of recovery techniques. These regulations require comprehensive safety assessments and documentation for chemical processes, encouraging the adoption of safer and more efficient recovery methods.
As environmental regulations continue to evolve, the hydrochloric acid industry must remain proactive in developing and implementing improved recovery techniques. This ongoing process of innovation and adaptation is essential for ensuring regulatory compliance, minimizing environmental impact, and maintaining economic viability in an increasingly environmentally conscious global market.
Economic Feasibility
The economic feasibility of improving hydrochloric acid recovery techniques is a critical consideration for industries seeking to optimize their processes and reduce costs. The recovery of hydrochloric acid presents significant economic advantages, primarily through the reduction of raw material costs and waste disposal expenses. By implementing advanced recovery techniques, companies can achieve substantial savings in the long term, despite initial investment requirements.
One of the key economic benefits of improved hydrochloric acid recovery is the reduction in fresh acid purchases. As the recovery rate increases, the need for new acid diminishes, leading to lower procurement costs. This is particularly important in industries where hydrochloric acid is used in large quantities, such as steel pickling, chemical manufacturing, and oil well acidizing. The potential for savings in these sectors can be substantial, often justifying the capital expenditure for improved recovery systems.
Furthermore, enhanced recovery techniques contribute to reduced waste treatment and disposal costs. By recovering a higher percentage of hydrochloric acid, the volume of waste acid requiring neutralization or disposal decreases significantly. This not only lowers direct disposal costs but also minimizes the environmental impact, potentially reducing regulatory compliance expenses and improving the company's sustainability profile.
The economic viability of implementing advanced recovery techniques also depends on the scale of operations. Larger facilities with higher acid consumption rates are likely to see a faster return on investment due to the greater volume of acid recovered. However, even smaller operations can benefit from improved recovery, especially as technology advances and becomes more cost-effective and scalable.
Energy efficiency is another crucial factor in the economic equation. Modern recovery techniques often incorporate energy-saving features, such as heat exchangers and efficient distillation columns. These improvements can lead to reduced energy consumption in the recovery process, further enhancing the economic benefits. The potential for energy savings should be carefully evaluated when considering the overall economic feasibility of upgrading recovery systems.
Market dynamics also play a role in determining the economic viability of improved recovery techniques. Fluctuations in hydrochloric acid prices can significantly impact the payback period for investments in recovery technology. During periods of high acid prices, the economic incentive for efficient recovery increases, potentially accelerating the adoption of advanced techniques across industries.
In conclusion, the economic feasibility of improving hydrochloric acid recovery techniques is generally favorable, particularly for large-scale operations and in industries with high acid consumption. The combination of reduced raw material costs, lower waste disposal expenses, and potential energy savings creates a compelling economic case for investment in advanced recovery systems. However, a thorough cost-benefit analysis, considering factors such as initial investment, operational scale, and market conditions, is essential for each specific application to determine the precise economic viability and return on investment timeline.
One of the key economic benefits of improved hydrochloric acid recovery is the reduction in fresh acid purchases. As the recovery rate increases, the need for new acid diminishes, leading to lower procurement costs. This is particularly important in industries where hydrochloric acid is used in large quantities, such as steel pickling, chemical manufacturing, and oil well acidizing. The potential for savings in these sectors can be substantial, often justifying the capital expenditure for improved recovery systems.
Furthermore, enhanced recovery techniques contribute to reduced waste treatment and disposal costs. By recovering a higher percentage of hydrochloric acid, the volume of waste acid requiring neutralization or disposal decreases significantly. This not only lowers direct disposal costs but also minimizes the environmental impact, potentially reducing regulatory compliance expenses and improving the company's sustainability profile.
The economic viability of implementing advanced recovery techniques also depends on the scale of operations. Larger facilities with higher acid consumption rates are likely to see a faster return on investment due to the greater volume of acid recovered. However, even smaller operations can benefit from improved recovery, especially as technology advances and becomes more cost-effective and scalable.
Energy efficiency is another crucial factor in the economic equation. Modern recovery techniques often incorporate energy-saving features, such as heat exchangers and efficient distillation columns. These improvements can lead to reduced energy consumption in the recovery process, further enhancing the economic benefits. The potential for energy savings should be carefully evaluated when considering the overall economic feasibility of upgrading recovery systems.
Market dynamics also play a role in determining the economic viability of improved recovery techniques. Fluctuations in hydrochloric acid prices can significantly impact the payback period for investments in recovery technology. During periods of high acid prices, the economic incentive for efficient recovery increases, potentially accelerating the adoption of advanced techniques across industries.
In conclusion, the economic feasibility of improving hydrochloric acid recovery techniques is generally favorable, particularly for large-scale operations and in industries with high acid consumption. The combination of reduced raw material costs, lower waste disposal expenses, and potential energy savings creates a compelling economic case for investment in advanced recovery systems. However, a thorough cost-benefit analysis, considering factors such as initial investment, operational scale, and market conditions, is essential for each specific application to determine the precise economic viability and return on investment timeline.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!