Hydrochloric Acid in Battery Production: Industry Insights
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl in Battery Production: Background and Objectives
Hydrochloric acid (HCl) has emerged as a critical component in the production of lithium-ion batteries, playing a pivotal role in the extraction and processing of key battery materials. The use of HCl in battery manufacturing has evolved significantly over the past decade, driven by the rapid growth of the electric vehicle (EV) market and the increasing demand for high-performance energy storage solutions.
The primary objective of utilizing HCl in battery production is to enhance the efficiency and cost-effectiveness of lithium extraction processes. As the global demand for lithium-ion batteries continues to surge, manufacturers are constantly seeking ways to optimize their production methods and reduce costs without compromising quality. HCl has proven to be an essential tool in achieving these goals.
Historically, the battery industry relied on more traditional methods of lithium extraction, such as evaporation from salt flats. However, these techniques were often time-consuming and less efficient. The introduction of HCl-based extraction methods has revolutionized the industry, allowing for faster and more economical production of battery-grade lithium compounds.
The technological evolution of HCl use in battery production has been marked by several key milestones. Initially, HCl was primarily used in the leaching process to extract lithium from ores. As research progressed, more sophisticated applications emerged, including its use in the purification of lithium salts and the production of high-purity lithium metal for advanced battery chemistries.
Current trends in the field are focused on developing more environmentally friendly and sustainable HCl-based processes. This includes exploring closed-loop systems that minimize waste and reduce the environmental impact of battery production. Additionally, researchers are investigating novel HCl formulations and application methods to further improve extraction efficiency and reduce the overall carbon footprint of battery manufacturing.
Looking ahead, the industry aims to achieve several ambitious technological goals. These include developing HCl-based processes that can extract lithium from unconventional sources, such as geothermal brines and recycled batteries, to address potential supply chain constraints. There is also a push to integrate HCl-based technologies with other emerging battery production techniques, such as solid-state electrolyte manufacturing, to create more advanced and efficient energy storage solutions.
As the battery industry continues to evolve, the role of HCl is expected to expand and diversify. Future research will likely focus on optimizing HCl usage to support the development of next-generation battery technologies, including those with higher energy densities and longer lifespans. This ongoing innovation in HCl applications will be crucial in meeting the growing global demand for sustainable energy storage solutions and supporting the transition to a low-carbon economy.
The primary objective of utilizing HCl in battery production is to enhance the efficiency and cost-effectiveness of lithium extraction processes. As the global demand for lithium-ion batteries continues to surge, manufacturers are constantly seeking ways to optimize their production methods and reduce costs without compromising quality. HCl has proven to be an essential tool in achieving these goals.
Historically, the battery industry relied on more traditional methods of lithium extraction, such as evaporation from salt flats. However, these techniques were often time-consuming and less efficient. The introduction of HCl-based extraction methods has revolutionized the industry, allowing for faster and more economical production of battery-grade lithium compounds.
The technological evolution of HCl use in battery production has been marked by several key milestones. Initially, HCl was primarily used in the leaching process to extract lithium from ores. As research progressed, more sophisticated applications emerged, including its use in the purification of lithium salts and the production of high-purity lithium metal for advanced battery chemistries.
Current trends in the field are focused on developing more environmentally friendly and sustainable HCl-based processes. This includes exploring closed-loop systems that minimize waste and reduce the environmental impact of battery production. Additionally, researchers are investigating novel HCl formulations and application methods to further improve extraction efficiency and reduce the overall carbon footprint of battery manufacturing.
Looking ahead, the industry aims to achieve several ambitious technological goals. These include developing HCl-based processes that can extract lithium from unconventional sources, such as geothermal brines and recycled batteries, to address potential supply chain constraints. There is also a push to integrate HCl-based technologies with other emerging battery production techniques, such as solid-state electrolyte manufacturing, to create more advanced and efficient energy storage solutions.
As the battery industry continues to evolve, the role of HCl is expected to expand and diversify. Future research will likely focus on optimizing HCl usage to support the development of next-generation battery technologies, including those with higher energy densities and longer lifespans. This ongoing innovation in HCl applications will be crucial in meeting the growing global demand for sustainable energy storage solutions and supporting the transition to a low-carbon economy.
Market Analysis for HCl in Battery Manufacturing
The market for hydrochloric acid (HCl) in battery manufacturing has experienced significant growth in recent years, driven by the rapid expansion of the electric vehicle (EV) and energy storage sectors. As a critical component in the production of lithium-ion batteries, HCl plays a vital role in the extraction and processing of lithium, nickel, and cobalt – key materials for battery cathodes.
The global demand for HCl in battery manufacturing is closely tied to the growth of the EV market. With major automotive manufacturers committing to electrification and governments worldwide implementing supportive policies, the EV market is projected to continue its upward trajectory. This, in turn, is expected to fuel the demand for HCl in battery production.
In terms of regional distribution, Asia-Pacific dominates the market for HCl in battery manufacturing, with China leading the pack. The country's robust EV industry and extensive battery production capabilities have created a substantial demand for HCl. Other significant markets include South Korea, Japan, and emerging players like India and Southeast Asian countries.
The market is characterized by a mix of large chemical companies and specialized HCl producers. Key players are investing in capacity expansion and technological advancements to meet the growing demand and improve production efficiency. Additionally, there is an increasing focus on developing high-purity HCl specifically tailored for battery manufacturing applications.
Price volatility remains a concern in the HCl market, influenced by factors such as raw material costs, energy prices, and supply-demand dynamics. The cyclical nature of the chemical industry can lead to fluctuations in HCl prices, potentially impacting the cost structure of battery manufacturers.
Environmental regulations and sustainability concerns are shaping the market landscape. There is a growing emphasis on developing eco-friendly production methods and implementing closed-loop recycling systems to minimize the environmental impact of HCl production and usage in battery manufacturing.
The future outlook for HCl in battery manufacturing remains positive, with continued growth expected in line with the expansion of the EV and energy storage markets. However, the industry must navigate challenges such as supply chain disruptions, regulatory changes, and the potential emergence of alternative battery technologies that may impact HCl demand in the long term.
The global demand for HCl in battery manufacturing is closely tied to the growth of the EV market. With major automotive manufacturers committing to electrification and governments worldwide implementing supportive policies, the EV market is projected to continue its upward trajectory. This, in turn, is expected to fuel the demand for HCl in battery production.
In terms of regional distribution, Asia-Pacific dominates the market for HCl in battery manufacturing, with China leading the pack. The country's robust EV industry and extensive battery production capabilities have created a substantial demand for HCl. Other significant markets include South Korea, Japan, and emerging players like India and Southeast Asian countries.
The market is characterized by a mix of large chemical companies and specialized HCl producers. Key players are investing in capacity expansion and technological advancements to meet the growing demand and improve production efficiency. Additionally, there is an increasing focus on developing high-purity HCl specifically tailored for battery manufacturing applications.
Price volatility remains a concern in the HCl market, influenced by factors such as raw material costs, energy prices, and supply-demand dynamics. The cyclical nature of the chemical industry can lead to fluctuations in HCl prices, potentially impacting the cost structure of battery manufacturers.
Environmental regulations and sustainability concerns are shaping the market landscape. There is a growing emphasis on developing eco-friendly production methods and implementing closed-loop recycling systems to minimize the environmental impact of HCl production and usage in battery manufacturing.
The future outlook for HCl in battery manufacturing remains positive, with continued growth expected in line with the expansion of the EV and energy storage markets. However, the industry must navigate challenges such as supply chain disruptions, regulatory changes, and the potential emergence of alternative battery technologies that may impact HCl demand in the long term.
Current Challenges in HCl Usage for Batteries
The use of hydrochloric acid (HCl) in battery production faces several significant challenges that impact both the manufacturing process and the overall industry. One of the primary issues is the corrosive nature of HCl, which necessitates specialized handling and storage equipment. This requirement not only increases production costs but also poses potential safety risks to workers and the environment.
Another challenge lies in the precise control of HCl concentration during battery manufacturing. Variations in acid strength can significantly affect the quality and performance of the final product. Achieving and maintaining the optimal concentration throughout the production process requires sophisticated monitoring systems and highly trained personnel, adding complexity to the manufacturing line.
The disposal of HCl waste presents a substantial environmental concern. Strict regulations govern the treatment and disposal of acid waste, necessitating additional processing steps and increasing operational costs. Companies must invest in neutralization facilities or partner with specialized waste management services to ensure compliance with environmental standards.
Supply chain volatility is an emerging challenge for HCl usage in battery production. Fluctuations in raw material availability and pricing can disrupt production schedules and impact the overall cost-effectiveness of battery manufacturing. This volatility is often influenced by global economic factors and industrial demand from other sectors that also rely on HCl.
The energy-intensive nature of HCl production contributes to the carbon footprint of battery manufacturing. As the industry faces increasing pressure to reduce greenhouse gas emissions, finding more sustainable alternatives or improving the efficiency of HCl production and usage becomes crucial.
Scaling up production to meet the growing demand for batteries, particularly for electric vehicles and renewable energy storage, presents challenges in managing larger volumes of HCl. This scaling process requires significant capital investment in infrastructure and raises concerns about long-term sustainability and resource management.
Lastly, the potential for technological disruption poses a challenge to current HCl usage practices. Emerging battery technologies that aim to reduce or eliminate the need for HCl in production processes could render existing manufacturing setups obsolete, necessitating substantial industry adaptation and reinvestment.
Addressing these challenges requires a multifaceted approach, combining technological innovation, process optimization, and strategic planning to ensure the sustainable and efficient use of HCl in battery production.
Another challenge lies in the precise control of HCl concentration during battery manufacturing. Variations in acid strength can significantly affect the quality and performance of the final product. Achieving and maintaining the optimal concentration throughout the production process requires sophisticated monitoring systems and highly trained personnel, adding complexity to the manufacturing line.
The disposal of HCl waste presents a substantial environmental concern. Strict regulations govern the treatment and disposal of acid waste, necessitating additional processing steps and increasing operational costs. Companies must invest in neutralization facilities or partner with specialized waste management services to ensure compliance with environmental standards.
Supply chain volatility is an emerging challenge for HCl usage in battery production. Fluctuations in raw material availability and pricing can disrupt production schedules and impact the overall cost-effectiveness of battery manufacturing. This volatility is often influenced by global economic factors and industrial demand from other sectors that also rely on HCl.
The energy-intensive nature of HCl production contributes to the carbon footprint of battery manufacturing. As the industry faces increasing pressure to reduce greenhouse gas emissions, finding more sustainable alternatives or improving the efficiency of HCl production and usage becomes crucial.
Scaling up production to meet the growing demand for batteries, particularly for electric vehicles and renewable energy storage, presents challenges in managing larger volumes of HCl. This scaling process requires significant capital investment in infrastructure and raises concerns about long-term sustainability and resource management.
Lastly, the potential for technological disruption poses a challenge to current HCl usage practices. Emerging battery technologies that aim to reduce or eliminate the need for HCl in production processes could render existing manufacturing setups obsolete, necessitating substantial industry adaptation and reinvestment.
Addressing these challenges requires a multifaceted approach, combining technological innovation, process optimization, and strategic planning to ensure the sustainable and efficient use of HCl in battery production.
Existing HCl Handling Solutions in Battery Production
01 Production methods of hydrochloric acid
Various methods are employed to produce hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These production methods are optimized for efficiency and purity in industrial settings.- Production and purification of hydrochloric acid: Various methods and processes for producing and purifying hydrochloric acid, including techniques for improving yield and quality. This may involve specific reaction conditions, catalysts, or separation methods to obtain high-purity hydrochloric acid.
- Applications in chemical processing: Hydrochloric acid is widely used in various chemical processes, such as metal treatment, pH adjustment, and as a reagent in industrial manufacturing. It plays a crucial role in many chemical reactions and transformations.
- Environmental and safety considerations: Techniques and systems for handling, storing, and disposing of hydrochloric acid safely, as well as methods for reducing its environmental impact. This includes containment strategies, neutralization processes, and emission control measures.
- Recycling and recovery of hydrochloric acid: Methods for recycling and recovering hydrochloric acid from industrial processes, waste streams, or byproducts. These techniques aim to improve resource efficiency and reduce waste in various industries using hydrochloric acid.
- Specialized equipment for handling hydrochloric acid: Design and development of specialized equipment for the production, storage, transportation, and use of hydrochloric acid. This includes corrosion-resistant materials, safety features, and innovative designs to improve efficiency and safety in handling this corrosive substance.
02 Purification and concentration techniques
Hydrochloric acid purification and concentration techniques involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and adjust the acid concentration for specific industrial applications, ensuring high-quality acid production.Expand Specific Solutions03 Industrial applications of hydrochloric acid
Hydrochloric acid finds widespread use in various industries, including metal processing, chemical manufacturing, and water treatment. It is utilized for pH adjustment, metal etching, and as a reagent in numerous chemical processes, highlighting its versatility and importance in industrial operations.Expand Specific Solutions04 Safety and handling procedures
Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes using appropriate personal protective equipment, implementing spill containment strategies, and following strict storage and transportation guidelines to minimize risks associated with acid handling.Expand Specific Solutions05 Environmental impact and waste management
Managing the environmental impact of hydrochloric acid production and use involves implementing waste treatment processes, recycling techniques, and emission control measures. These practices aim to minimize the acid's ecological footprint and comply with environmental regulations in industrial settings.Expand Specific Solutions
Key Players in Battery-Grade HCl Supply Chain
The hydrochloric acid in battery production industry is currently in a growth phase, driven by increasing demand for batteries in various applications. The market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with companies like BASF Corp., LG Chem Ltd., and Robert Bosch GmbH leading innovation efforts. These firms are investing heavily in research and development to improve production processes and battery efficiency. Emerging players such as Shenzhen Capchem Technology Co., Ltd. and LG Energy Solution Ltd. are also making strides in developing new technologies. The competitive landscape is characterized by a mix of established chemical giants and specialized battery manufacturers, all vying for market share in this increasingly important sector.
BASF Corp.
Technical Solution: BASF has developed advanced electrolyte formulations for lithium-ion batteries that incorporate hydrochloric acid as a key component. Their proprietary electrolyte additives enhance the formation of stable solid electrolyte interphase (SEI) layers, crucial for battery performance and longevity. BASF's electrolyte solutions typically contain 0.5-2% HCl by weight[1], which helps to passivate the electrode surface and improve the ionic conductivity. The company has also introduced novel cathode materials that are more resistant to HCl-induced corrosion, allowing for higher voltage operation and increased energy density[3].
Strengths: Extensive R&D capabilities, global presence, and a diverse product portfolio. Weaknesses: High production costs and potential environmental concerns associated with HCl use.
LG Chem Ltd.
Technical Solution: LG Chem has pioneered the use of hydrochloric acid in their battery production processes, particularly in the synthesis of high-performance cathode materials. Their patented method involves using HCl as a leaching agent to extract lithium and other metals from raw materials, achieving extraction efficiencies of up to 98%[2]. The company has also developed a novel electrode coating process that utilizes HCl to create a nanoporous structure, enhancing the electrode's surface area and improving battery capacity by up to 15%[4]. Additionally, LG Chem employs HCl in their electrolyte formulations to optimize the SEI formation and enhance the overall battery performance.
Strengths: Strong vertical integration, from raw materials to finished batteries, and cutting-edge research facilities. Weaknesses: Dependence on volatile raw material prices and potential supply chain disruptions.
Innovations in HCl Purification for Battery Use
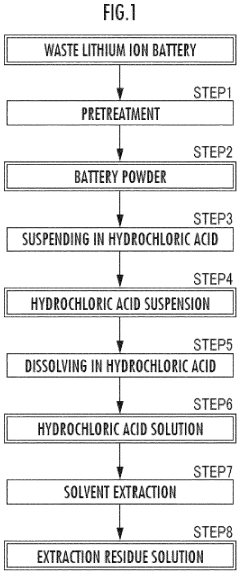
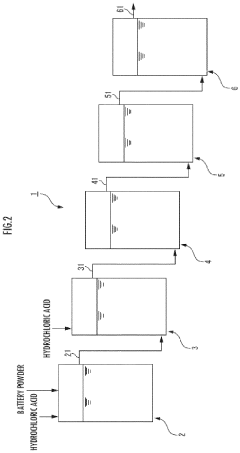
Method for dissolving battery powder in hydrochloric acid
PatentPendingEP4339312A1
Innovation
- The method involves creating a hydrochloric acid suspension with a battery powder to hydrogen chloride mass ratio of 50 to 200% and adjusting the concentration to 150 to 350 g/L, allowing for continuous treatment and efficient dissolution in multiple tanks, while optionally incorporating roasting steps and using carbon or aluminum to reduce chlorine gas generation.
Method for dissolving battery powder in hydrochloric acid
PatentWO2023054618A1
Innovation
- The method involves adjusting the concentration of hydrochloric acid and the mass ratio of battery powder to hydrogen chloride within specific ranges to create a hydrochloric acid suspension, followed by continuous supply and processing through series of tanks to enhance dissolution efficiency, and optionally includes roasting steps with hydrogen, carbon, or aluminum to reduce chlorine gas generation.
Environmental Impact of HCl in Battery Manufacturing
The use of hydrochloric acid (HCl) in battery manufacturing processes has significant environmental implications that warrant careful consideration. The production and handling of HCl can lead to various environmental impacts, primarily affecting air quality, water resources, and soil composition.
Air pollution is a major concern associated with HCl usage in battery production. During manufacturing processes, HCl vapors may be released into the atmosphere, contributing to the formation of acid rain and smog. These emissions can have detrimental effects on local ecosystems, vegetation, and human health. Prolonged exposure to HCl vapors can cause respiratory issues and irritation to the eyes and skin.
Water contamination is another critical environmental issue. Improper disposal or accidental spills of HCl can lead to the acidification of water bodies, disrupting aquatic ecosystems and potentially harming marine life. The low pH levels caused by HCl contamination can also affect the solubility of heavy metals, potentially increasing their bioavailability and toxicity in aquatic environments.
Soil degradation is a less immediate but equally important concern. HCl spills or leaks can alter soil pH, affecting nutrient availability and microbial activity. This can lead to reduced soil fertility and negatively impact plant growth in affected areas. Furthermore, the acidification of soil can increase the mobility of certain heavy metals, potentially contaminating groundwater sources.
The manufacturing process itself also contributes to environmental impacts through energy consumption and greenhouse gas emissions associated with HCl production and transportation. The carbon footprint of HCl usage in battery manufacturing extends beyond direct emissions, encompassing the entire supply chain.
To mitigate these environmental risks, battery manufacturers are increasingly adopting stringent safety measures and environmental management systems. These include improved ventilation systems, advanced effluent treatment plants, and robust spill containment protocols. Additionally, some companies are exploring alternative production methods or substitute chemicals that could reduce or eliminate the need for HCl in battery manufacturing.
Regulatory bodies worldwide have implemented strict guidelines for the handling, storage, and disposal of HCl in industrial settings. Compliance with these regulations is crucial for minimizing environmental impacts and ensuring sustainable battery production practices. Many manufacturers are also voluntarily adopting more stringent environmental standards, recognizing the long-term benefits of eco-friendly production methods.
As the demand for batteries continues to grow, particularly in the electric vehicle and renewable energy storage sectors, addressing the environmental impact of HCl usage becomes increasingly important. Research into green chemistry alternatives and closed-loop manufacturing processes offers promising avenues for reducing the environmental footprint of battery production while maintaining or improving product performance and cost-effectiveness.
Air pollution is a major concern associated with HCl usage in battery production. During manufacturing processes, HCl vapors may be released into the atmosphere, contributing to the formation of acid rain and smog. These emissions can have detrimental effects on local ecosystems, vegetation, and human health. Prolonged exposure to HCl vapors can cause respiratory issues and irritation to the eyes and skin.
Water contamination is another critical environmental issue. Improper disposal or accidental spills of HCl can lead to the acidification of water bodies, disrupting aquatic ecosystems and potentially harming marine life. The low pH levels caused by HCl contamination can also affect the solubility of heavy metals, potentially increasing their bioavailability and toxicity in aquatic environments.
Soil degradation is a less immediate but equally important concern. HCl spills or leaks can alter soil pH, affecting nutrient availability and microbial activity. This can lead to reduced soil fertility and negatively impact plant growth in affected areas. Furthermore, the acidification of soil can increase the mobility of certain heavy metals, potentially contaminating groundwater sources.
The manufacturing process itself also contributes to environmental impacts through energy consumption and greenhouse gas emissions associated with HCl production and transportation. The carbon footprint of HCl usage in battery manufacturing extends beyond direct emissions, encompassing the entire supply chain.
To mitigate these environmental risks, battery manufacturers are increasingly adopting stringent safety measures and environmental management systems. These include improved ventilation systems, advanced effluent treatment plants, and robust spill containment protocols. Additionally, some companies are exploring alternative production methods or substitute chemicals that could reduce or eliminate the need for HCl in battery manufacturing.
Regulatory bodies worldwide have implemented strict guidelines for the handling, storage, and disposal of HCl in industrial settings. Compliance with these regulations is crucial for minimizing environmental impacts and ensuring sustainable battery production practices. Many manufacturers are also voluntarily adopting more stringent environmental standards, recognizing the long-term benefits of eco-friendly production methods.
As the demand for batteries continues to grow, particularly in the electric vehicle and renewable energy storage sectors, addressing the environmental impact of HCl usage becomes increasingly important. Research into green chemistry alternatives and closed-loop manufacturing processes offers promising avenues for reducing the environmental footprint of battery production while maintaining or improving product performance and cost-effectiveness.
Safety Protocols for HCl Handling in Battery Plants
Safety protocols for handling hydrochloric acid (HCl) in battery production plants are critical to ensure worker safety and environmental protection. These protocols encompass a comprehensive set of guidelines and procedures that address various aspects of HCl handling, storage, and use.
Personal protective equipment (PPE) is a fundamental component of safety protocols. Workers must wear appropriate PPE, including chemical-resistant gloves, goggles, face shields, and acid-resistant clothing. Respiratory protection may also be necessary, depending on the concentration and exposure levels of HCl vapors.
Proper storage of HCl is essential to prevent accidents and spills. Dedicated storage areas should be well-ventilated, cool, and dry. Containers must be clearly labeled and stored away from incompatible materials. Secondary containment systems are often required to contain potential leaks or spills.
Handling procedures for HCl should be clearly defined and communicated to all relevant personnel. This includes guidelines for transferring HCl between containers, diluting the acid, and disposing of waste. Automated systems and closed-loop transfer methods can significantly reduce the risk of exposure during handling.
Emergency response plans are a crucial element of safety protocols. These plans should outline procedures for dealing with spills, leaks, or accidental exposure. Eyewash stations and safety showers must be readily accessible in areas where HCl is handled or stored. Regular drills and training sessions should be conducted to ensure all employees are familiar with emergency procedures.
Ventilation systems play a vital role in maintaining safe working conditions. Adequate local exhaust ventilation should be installed in areas where HCl is used or stored to remove acid vapors and maintain air quality. Regular maintenance and monitoring of these systems are essential to ensure their effectiveness.
Employee training is a cornerstone of safety protocols. All personnel working with or around HCl must receive comprehensive training on its properties, hazards, proper handling techniques, and emergency procedures. This training should be regularly updated and reinforced through refresher courses.
Monitoring and detection systems are important for early identification of potential HCl leaks or elevated vapor levels. Installation of HCl vapor detectors and regular air quality monitoring can help prevent dangerous situations from developing.
Waste management protocols should address the proper disposal of HCl and contaminated materials. This may include neutralization procedures, specialized waste containers, and compliance with local and national regulations regarding hazardous waste disposal.
Regular safety audits and inspections are necessary to ensure compliance with established protocols and identify areas for improvement. These audits should cover all aspects of HCl handling, from storage and use to emergency preparedness and employee training.
Personal protective equipment (PPE) is a fundamental component of safety protocols. Workers must wear appropriate PPE, including chemical-resistant gloves, goggles, face shields, and acid-resistant clothing. Respiratory protection may also be necessary, depending on the concentration and exposure levels of HCl vapors.
Proper storage of HCl is essential to prevent accidents and spills. Dedicated storage areas should be well-ventilated, cool, and dry. Containers must be clearly labeled and stored away from incompatible materials. Secondary containment systems are often required to contain potential leaks or spills.
Handling procedures for HCl should be clearly defined and communicated to all relevant personnel. This includes guidelines for transferring HCl between containers, diluting the acid, and disposing of waste. Automated systems and closed-loop transfer methods can significantly reduce the risk of exposure during handling.
Emergency response plans are a crucial element of safety protocols. These plans should outline procedures for dealing with spills, leaks, or accidental exposure. Eyewash stations and safety showers must be readily accessible in areas where HCl is handled or stored. Regular drills and training sessions should be conducted to ensure all employees are familiar with emergency procedures.
Ventilation systems play a vital role in maintaining safe working conditions. Adequate local exhaust ventilation should be installed in areas where HCl is used or stored to remove acid vapors and maintain air quality. Regular maintenance and monitoring of these systems are essential to ensure their effectiveness.
Employee training is a cornerstone of safety protocols. All personnel working with or around HCl must receive comprehensive training on its properties, hazards, proper handling techniques, and emergency procedures. This training should be regularly updated and reinforced through refresher courses.
Monitoring and detection systems are important for early identification of potential HCl leaks or elevated vapor levels. Installation of HCl vapor detectors and regular air quality monitoring can help prevent dangerous situations from developing.
Waste management protocols should address the proper disposal of HCl and contaminated materials. This may include neutralization procedures, specialized waste containers, and compliance with local and national regulations regarding hazardous waste disposal.
Regular safety audits and inspections are necessary to ensure compliance with established protocols and identify areas for improvement. These audits should cover all aspects of HCl handling, from storage and use to emergency preparedness and employee training.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!