Innovative Uses of Hydrochloric Acid in Metallurgy
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl in Metallurgy: Background and Objectives
Hydrochloric acid (HCl) has been a cornerstone in metallurgical processes for decades, playing a crucial role in various stages of metal extraction and processing. The evolution of HCl usage in metallurgy can be traced back to the early 20th century when industrial-scale production of hydrochloric acid became feasible. Since then, its applications have expanded significantly, revolutionizing the metallurgical industry.
The primary objective of exploring innovative uses of HCl in metallurgy is to enhance efficiency, reduce environmental impact, and improve the quality of metal products. As global demand for metals continues to rise, coupled with stricter environmental regulations, the need for advanced HCl-based processes has become increasingly important. This has led to a surge in research and development efforts aimed at optimizing existing applications and discovering novel uses for HCl in metallurgical operations.
One of the key trends in HCl utilization is its application in hydrometallurgy, where it serves as a leaching agent for extracting valuable metals from ores. This process has gained prominence due to its ability to handle low-grade ores and complex mineral compositions, which are becoming more common as high-grade deposits are depleted. The development of selective leaching techniques using HCl has opened up new possibilities for recovering metals from previously uneconomical sources.
Another significant area of innovation lies in the use of HCl for metal surface treatment and preparation. Advanced pickling processes employing HCl have been developed to remove oxides and impurities from metal surfaces, improving the quality of finished products and enhancing their corrosion resistance. These processes are particularly important in the steel industry, where surface quality directly impacts the performance of downstream products.
The recycling and recovery of metals from industrial waste and end-of-life products represent another frontier for HCl application in metallurgy. As the circular economy concept gains traction, HCl-based processes are being developed to extract valuable metals from electronic waste, spent catalysts, and other secondary sources. This not only addresses environmental concerns but also helps conserve primary mineral resources.
Looking ahead, the metallurgical industry aims to further refine HCl-based processes to achieve greater selectivity, reduce acid consumption, and minimize waste generation. Research is ongoing to develop novel HCl regeneration techniques, which could significantly reduce the environmental footprint of metallurgical operations. Additionally, the integration of HCl processes with other emerging technologies, such as advanced separation methods and process intensification, holds promise for creating more sustainable and efficient metallurgical practices.
The primary objective of exploring innovative uses of HCl in metallurgy is to enhance efficiency, reduce environmental impact, and improve the quality of metal products. As global demand for metals continues to rise, coupled with stricter environmental regulations, the need for advanced HCl-based processes has become increasingly important. This has led to a surge in research and development efforts aimed at optimizing existing applications and discovering novel uses for HCl in metallurgical operations.
One of the key trends in HCl utilization is its application in hydrometallurgy, where it serves as a leaching agent for extracting valuable metals from ores. This process has gained prominence due to its ability to handle low-grade ores and complex mineral compositions, which are becoming more common as high-grade deposits are depleted. The development of selective leaching techniques using HCl has opened up new possibilities for recovering metals from previously uneconomical sources.
Another significant area of innovation lies in the use of HCl for metal surface treatment and preparation. Advanced pickling processes employing HCl have been developed to remove oxides and impurities from metal surfaces, improving the quality of finished products and enhancing their corrosion resistance. These processes are particularly important in the steel industry, where surface quality directly impacts the performance of downstream products.
The recycling and recovery of metals from industrial waste and end-of-life products represent another frontier for HCl application in metallurgy. As the circular economy concept gains traction, HCl-based processes are being developed to extract valuable metals from electronic waste, spent catalysts, and other secondary sources. This not only addresses environmental concerns but also helps conserve primary mineral resources.
Looking ahead, the metallurgical industry aims to further refine HCl-based processes to achieve greater selectivity, reduce acid consumption, and minimize waste generation. Research is ongoing to develop novel HCl regeneration techniques, which could significantly reduce the environmental footprint of metallurgical operations. Additionally, the integration of HCl processes with other emerging technologies, such as advanced separation methods and process intensification, holds promise for creating more sustainable and efficient metallurgical practices.
Market Analysis for HCl in Metal Processing
The market for hydrochloric acid (HCl) in metal processing has shown significant growth and potential in recent years. This versatile chemical plays a crucial role in various metallurgical processes, including metal pickling, surface treatment, and ore processing. The global demand for HCl in the metallurgy sector is primarily driven by the expanding steel and aluminum industries, particularly in emerging economies.
In the steel industry, HCl is extensively used for pickling, a process that removes impurities and oxide scales from steel surfaces. This application accounts for a substantial portion of HCl consumption in metal processing. The growing automotive and construction sectors, major consumers of steel products, are indirectly boosting the demand for HCl in metal processing.
The aluminum industry also contributes significantly to the HCl market in metallurgy. HCl is used in the production of high-purity aluminum chloride, a key component in the electrolytic production of aluminum. As the demand for lightweight materials in automotive and aerospace industries increases, the consumption of HCl in aluminum processing is expected to rise.
Geographically, Asia-Pacific dominates the market for HCl in metal processing, with China being the largest consumer. The rapid industrialization and urbanization in this region have led to increased metal production and processing activities. North America and Europe follow, with steady demand from their well-established metallurgical industries.
The market is characterized by a mix of large multinational chemical companies and regional players. Key market participants are focusing on expanding their production capacities and developing innovative applications to gain a competitive edge. The increasing emphasis on sustainable practices in the metallurgy sector is driving research into more environmentally friendly HCl-based processes.
Despite the positive outlook, the market faces challenges such as stringent environmental regulations and the volatility of raw material prices. The corrosive nature of HCl also necessitates significant investments in handling and storage infrastructure, which can be a barrier for smaller players in the industry.
Looking ahead, the market for HCl in metal processing is expected to continue its growth trajectory. Emerging applications, such as the use of HCl in rare earth metal extraction and recycling processes, are opening new avenues for market expansion. Additionally, the ongoing research into more efficient and sustainable HCl-based metal processing techniques is likely to drive innovation and create new market opportunities in the coming years.
In the steel industry, HCl is extensively used for pickling, a process that removes impurities and oxide scales from steel surfaces. This application accounts for a substantial portion of HCl consumption in metal processing. The growing automotive and construction sectors, major consumers of steel products, are indirectly boosting the demand for HCl in metal processing.
The aluminum industry also contributes significantly to the HCl market in metallurgy. HCl is used in the production of high-purity aluminum chloride, a key component in the electrolytic production of aluminum. As the demand for lightweight materials in automotive and aerospace industries increases, the consumption of HCl in aluminum processing is expected to rise.
Geographically, Asia-Pacific dominates the market for HCl in metal processing, with China being the largest consumer. The rapid industrialization and urbanization in this region have led to increased metal production and processing activities. North America and Europe follow, with steady demand from their well-established metallurgical industries.
The market is characterized by a mix of large multinational chemical companies and regional players. Key market participants are focusing on expanding their production capacities and developing innovative applications to gain a competitive edge. The increasing emphasis on sustainable practices in the metallurgy sector is driving research into more environmentally friendly HCl-based processes.
Despite the positive outlook, the market faces challenges such as stringent environmental regulations and the volatility of raw material prices. The corrosive nature of HCl also necessitates significant investments in handling and storage infrastructure, which can be a barrier for smaller players in the industry.
Looking ahead, the market for HCl in metal processing is expected to continue its growth trajectory. Emerging applications, such as the use of HCl in rare earth metal extraction and recycling processes, are opening new avenues for market expansion. Additionally, the ongoing research into more efficient and sustainable HCl-based metal processing techniques is likely to drive innovation and create new market opportunities in the coming years.
Current Challenges in HCl Metallurgical Applications
The application of hydrochloric acid (HCl) in metallurgy has been a cornerstone of many industrial processes, yet it faces several significant challenges in modern metallurgical applications. One of the primary issues is the corrosive nature of HCl, which can lead to equipment degradation and increased maintenance costs. This corrosivity not only affects the longevity of processing equipment but also raises safety concerns for workers handling the acid.
Another challenge lies in the environmental impact of HCl usage. Stringent regulations on emissions and waste disposal have put pressure on industries to find more sustainable practices. The release of chlorine gas, a by-product of some HCl-based processes, is particularly problematic due to its toxicity and potential for atmospheric pollution.
The recovery and recycling of HCl present additional hurdles. While recycling is crucial for both economic and environmental reasons, the processes involved can be complex and energy-intensive. The presence of impurities in recycled HCl can also affect the quality of subsequent metallurgical processes, necessitating additional purification steps.
In the realm of leaching processes, where HCl is extensively used, there are ongoing challenges in optimizing acid consumption and improving metal recovery rates. The selective leaching of target metals while minimizing the dissolution of unwanted elements remains a key area of focus for researchers and industry professionals.
The transportation and storage of HCl pose logistical challenges due to its hazardous nature. Special containment systems and safety protocols are required, which can increase operational costs and complexity. Moreover, the volatility of HCl can lead to concentration changes during storage, affecting process consistency.
As the metallurgical industry moves towards more advanced materials and alloys, there is a growing need for HCl-based processes that can handle these complex compositions. Developing tailored solutions for new materials while maintaining efficiency and cost-effectiveness is an ongoing challenge.
The integration of HCl processes with emerging technologies, such as automation and real-time monitoring systems, presents both opportunities and challenges. While these technologies promise improved process control and safety, their implementation requires significant investment and expertise.
Lastly, the fluctuating global supply and pricing of HCl can impact the economic viability of certain metallurgical processes. Industries must navigate these market dynamics while striving to maintain competitive and sustainable operations.
Another challenge lies in the environmental impact of HCl usage. Stringent regulations on emissions and waste disposal have put pressure on industries to find more sustainable practices. The release of chlorine gas, a by-product of some HCl-based processes, is particularly problematic due to its toxicity and potential for atmospheric pollution.
The recovery and recycling of HCl present additional hurdles. While recycling is crucial for both economic and environmental reasons, the processes involved can be complex and energy-intensive. The presence of impurities in recycled HCl can also affect the quality of subsequent metallurgical processes, necessitating additional purification steps.
In the realm of leaching processes, where HCl is extensively used, there are ongoing challenges in optimizing acid consumption and improving metal recovery rates. The selective leaching of target metals while minimizing the dissolution of unwanted elements remains a key area of focus for researchers and industry professionals.
The transportation and storage of HCl pose logistical challenges due to its hazardous nature. Special containment systems and safety protocols are required, which can increase operational costs and complexity. Moreover, the volatility of HCl can lead to concentration changes during storage, affecting process consistency.
As the metallurgical industry moves towards more advanced materials and alloys, there is a growing need for HCl-based processes that can handle these complex compositions. Developing tailored solutions for new materials while maintaining efficiency and cost-effectiveness is an ongoing challenge.
The integration of HCl processes with emerging technologies, such as automation and real-time monitoring systems, presents both opportunities and challenges. While these technologies promise improved process control and safety, their implementation requires significant investment and expertise.
Lastly, the fluctuating global supply and pricing of HCl can impact the economic viability of certain metallurgical processes. Industries must navigate these market dynamics while striving to maintain competitive and sustainable operations.
Existing HCl Metallurgical Processes
01 Production methods of hydrochloric acid
Various methods are employed to produce hydrochloric acid, including the reaction of chlorine with hydrogen, the chlorination of organic compounds, and as a byproduct in chemical processes. These production methods aim to optimize yield, purity, and efficiency while minimizing environmental impact.- Production methods of hydrochloric acid: Various methods are employed for the production of hydrochloric acid, including chemical reactions and industrial processes. These methods may involve the use of specific catalysts, reactants, or equipment to efficiently produce hydrochloric acid at different concentrations and purities.
- Applications in chemical processing: Hydrochloric acid is widely used in chemical processing industries for various applications such as pH adjustment, metal treatment, and as a reagent in chemical reactions. Its strong acidic properties make it suitable for diverse industrial processes and manufacturing operations.
- Purification and treatment of hydrochloric acid: Techniques for purifying and treating hydrochloric acid are essential for removing impurities and achieving desired concentrations. These processes may involve distillation, filtration, or other separation methods to produce high-quality hydrochloric acid for specific applications.
- Safety and handling of hydrochloric acid: Due to its corrosive nature, special safety measures and handling procedures are required for hydrochloric acid. This includes the use of appropriate containment systems, personal protective equipment, and storage solutions to minimize risks associated with its use and transportation.
- Environmental considerations and waste management: Environmental aspects of hydrochloric acid use and production are important, including waste management and emission control. Techniques for neutralizing, recycling, or safely disposing of hydrochloric acid waste are developed to minimize environmental impact and comply with regulations.
02 Purification and concentration of hydrochloric acid
Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and increase the acid concentration for various industrial applications, ensuring high-quality products for specific uses.Expand Specific Solutions03 Applications of hydrochloric acid in chemical processing
Hydrochloric acid is widely used in various chemical processes, including metal treatment, pH regulation, and as a reagent in organic synthesis. Its versatility makes it essential in industries such as metallurgy, pharmaceuticals, and food processing, where it plays a crucial role in numerous reactions and treatments.Expand Specific Solutions04 Safety and handling of hydrochloric acid
Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes the use of appropriate personal protective equipment, specialized storage containers, and neutralization techniques for spills. Implementing these practices helps prevent accidents and ensures worker safety in industrial settings.Expand Specific Solutions05 Environmental considerations and waste management
Managing the environmental impact of hydrochloric acid production and use involves developing sustainable practices for waste treatment and disposal. This includes neutralization processes, recycling methods, and the implementation of closed-loop systems to minimize emissions and reduce the ecological footprint of industrial operations involving hydrochloric acid.Expand Specific Solutions
Key Industry Players in HCl-based Metallurgy
The innovative uses of hydrochloric acid in metallurgy represent a mature yet evolving field, with the market in a growth phase. The global market size for hydrochloric acid in metallurgical applications is substantial, driven by increasing demand in various industrial sectors. Companies like Vale SA, POSCO Holdings, and LG Chem Ltd. are at the forefront of technological advancements, leveraging their extensive research capabilities. The competitive landscape is characterized by a mix of established players and innovative startups, such as Fluid Energy Group Ltd. and Australian BioRefining Pty Ltd., which are developing novel applications and more environmentally friendly processes. Academic institutions like Central South University and Montanuniversität Leoben are contributing significantly to research and development in this field, fostering industry-academia collaborations and driving technological progress.
Vale SA
Technical Solution: Vale SA has developed an innovative process for using hydrochloric acid in the extraction of nickel from laterite ores. Their method involves high-pressure acid leaching (HPAL) with HCl, followed by a proprietary solvent extraction process. This technique allows for more efficient nickel recovery compared to traditional sulfuric acid leaching, with extraction rates reaching up to 95% [1]. The process also enables the recovery of valuable by-products such as cobalt and scandium. Vale's approach includes a closed-loop system for acid regeneration, significantly reducing environmental impact and operational costs [3].
Strengths: Higher metal recovery rates, valuable by-product extraction, and reduced environmental impact. Weaknesses: High initial capital investment and potential corrosion issues with HCl handling equipment.
Andritz AG
Technical Solution: Andritz AG has pioneered the use of hydrochloric acid in the pickling process for stainless steel production. Their advanced HCl regeneration technology allows for the continuous reuse of acid in the pickling line, significantly reducing waste and improving efficiency. The system employs a fluidized bed reactor that can regenerate up to 99% of the spent acid [2]. Andritz's process also incorporates sophisticated control systems that optimize acid concentration and temperature, resulting in superior surface quality of the finished steel products. Additionally, their technology includes a heat recovery system that captures and reuses thermal energy from the regeneration process, further enhancing overall efficiency [4].
Strengths: High acid regeneration rate, improved product quality, and energy efficiency. Weaknesses: Complex system requiring specialized maintenance and potential safety concerns due to HCl handling.
Innovative HCl Technologies in Metal Refining
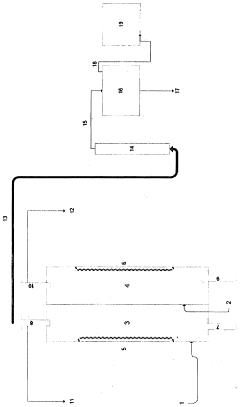
Method of metal recovery
PatentWO2007122720A1
Innovation
- A method involving the use of hydrochloric acid to dissolve metals, followed by adsorption onto cellulose dispersed in an organic solvent, and subsequent desorption using water or hydrochloric acid, allowing for efficient recovery of metals like chromium, nickel, manganese, copper, and lanthanides without expensive reagents or high energy consumption.
Process and apparatus for generating or recovering hydrochloric acid from metal salt solutions
PatentActiveHK1210233A
Innovation
- The process uses an anion exchange membrane to separate chloride ions from metal ions, allowing for efficient production of high-purity HCl.
- The method combines electrolysis with catalytic gas-phase reaction to convert chlorine and hydrogen into HCl, avoiding issues with direct chlorine production.
- The process can treat solutions with high metal chloride concentrations and acidity, making it versatile for various industrial waste streams.
Environmental Impact of HCl in Metallurgy
The use of hydrochloric acid (HCl) in metallurgy, while innovative and effective, raises significant environmental concerns. The primary environmental impacts stem from the acid's corrosive nature and the potential for harmful emissions and waste products. When used in metal extraction and processing, HCl can lead to the release of acidic fumes and vapors into the atmosphere. These emissions can contribute to air pollution, potentially causing respiratory issues for workers and nearby communities if not properly controlled.
Water pollution is another critical environmental concern. Improper handling or disposal of HCl and its byproducts can result in the contamination of water sources. Acidic runoff from metallurgical processes can lower the pH of water bodies, adversely affecting aquatic ecosystems and biodiversity. This can lead to the death of fish and other aquatic organisms, as well as long-term ecological imbalances in affected areas.
Soil contamination is also a potential risk associated with HCl use in metallurgy. Accidental spills or improper storage can lead to soil acidification, which can have detrimental effects on plant life and soil microorganisms. This, in turn, can impact local flora and fauna, potentially leading to reduced biodiversity and ecosystem disruption.
The production and transportation of HCl for metallurgical purposes also contribute to the overall environmental footprint. The manufacturing process of HCl itself requires energy and resources, contributing to greenhouse gas emissions and resource depletion. Additionally, the transportation of large quantities of this corrosive substance poses risks of accidental spills during transit, which could have severe environmental consequences.
However, it's important to note that the metallurgical industry has made significant strides in mitigating these environmental impacts. Advanced emission control technologies, such as scrubbers and filtration systems, are increasingly being employed to reduce air pollution from HCl use. Closed-loop systems and improved waste management practices are helping to minimize water pollution and soil contamination risks.
Furthermore, research into alternative, more environmentally friendly processes is ongoing. This includes the development of less hazardous acid alternatives and the exploration of non-acid based metallurgical techniques. These efforts aim to reduce the reliance on HCl and other strong acids in metallurgy, thereby minimizing the associated environmental risks.
In conclusion, while the innovative use of HCl in metallurgy offers significant benefits in terms of efficiency and effectiveness, it also presents substantial environmental challenges. Balancing these technological advantages with environmental protection requires ongoing research, stringent regulatory oversight, and the implementation of best practices in industrial processes. The future of HCl use in metallurgy will likely involve a combination of improved handling techniques, advanced pollution control measures, and the gradual transition to more environmentally sustainable alternatives.
Water pollution is another critical environmental concern. Improper handling or disposal of HCl and its byproducts can result in the contamination of water sources. Acidic runoff from metallurgical processes can lower the pH of water bodies, adversely affecting aquatic ecosystems and biodiversity. This can lead to the death of fish and other aquatic organisms, as well as long-term ecological imbalances in affected areas.
Soil contamination is also a potential risk associated with HCl use in metallurgy. Accidental spills or improper storage can lead to soil acidification, which can have detrimental effects on plant life and soil microorganisms. This, in turn, can impact local flora and fauna, potentially leading to reduced biodiversity and ecosystem disruption.
The production and transportation of HCl for metallurgical purposes also contribute to the overall environmental footprint. The manufacturing process of HCl itself requires energy and resources, contributing to greenhouse gas emissions and resource depletion. Additionally, the transportation of large quantities of this corrosive substance poses risks of accidental spills during transit, which could have severe environmental consequences.
However, it's important to note that the metallurgical industry has made significant strides in mitigating these environmental impacts. Advanced emission control technologies, such as scrubbers and filtration systems, are increasingly being employed to reduce air pollution from HCl use. Closed-loop systems and improved waste management practices are helping to minimize water pollution and soil contamination risks.
Furthermore, research into alternative, more environmentally friendly processes is ongoing. This includes the development of less hazardous acid alternatives and the exploration of non-acid based metallurgical techniques. These efforts aim to reduce the reliance on HCl and other strong acids in metallurgy, thereby minimizing the associated environmental risks.
In conclusion, while the innovative use of HCl in metallurgy offers significant benefits in terms of efficiency and effectiveness, it also presents substantial environmental challenges. Balancing these technological advantages with environmental protection requires ongoing research, stringent regulatory oversight, and the implementation of best practices in industrial processes. The future of HCl use in metallurgy will likely involve a combination of improved handling techniques, advanced pollution control measures, and the gradual transition to more environmentally sustainable alternatives.
Safety Protocols for HCl Handling in Metal Industry
The safe handling of hydrochloric acid (HCl) in the metal industry is paramount due to its corrosive nature and potential health hazards. Comprehensive safety protocols must be implemented to protect workers and maintain operational integrity. Personal protective equipment (PPE) is the first line of defense, including chemical-resistant suits, gloves, boots, and face shields or goggles. Respiratory protection, such as acid gas respirators, is essential when working with HCl vapors.
Proper storage and containment systems are crucial. HCl should be stored in corrosion-resistant tanks or containers, preferably made of polyethylene or other suitable materials. These storage areas must be well-ventilated, temperature-controlled, and equipped with secondary containment to prevent spills from spreading. Regular inspections of storage facilities and transfer equipment are necessary to detect and address potential leaks or damage.
Emergency response procedures must be clearly defined and regularly practiced. This includes spill containment and neutralization protocols, as well as evacuation plans. Eyewash stations and safety showers should be readily accessible in all areas where HCl is handled or stored. Training programs for employees must cover the proper use of PPE, handling procedures, and emergency response techniques.
Ventilation systems play a critical role in maintaining safe working conditions. Local exhaust ventilation should be installed at points of HCl use to capture and remove acid vapors. General ventilation systems must be designed to ensure adequate air exchange rates throughout the facility.
Monitoring and detection systems are essential for early warning of potential leaks or exposure. This includes the use of HCl vapor detectors and pH monitors in key areas. Regular air quality testing and employee health monitoring programs should be implemented to track potential long-term exposure effects.
Waste management and disposal procedures for HCl and related materials must comply with environmental regulations. This includes proper neutralization techniques and the use of certified disposal services for hazardous waste.
Documentation and record-keeping are vital components of safety protocols. This includes maintaining up-to-date safety data sheets (SDS), detailed handling procedures, and incident reports. Regular safety audits and reviews should be conducted to ensure compliance with established protocols and identify areas for improvement.
Collaboration with local emergency services is essential for effective response to major incidents. This includes providing facility layouts, chemical inventories, and coordinating joint training exercises. By implementing these comprehensive safety protocols, the metal industry can minimize risks associated with HCl handling and ensure a safer working environment for all employees.
Proper storage and containment systems are crucial. HCl should be stored in corrosion-resistant tanks or containers, preferably made of polyethylene or other suitable materials. These storage areas must be well-ventilated, temperature-controlled, and equipped with secondary containment to prevent spills from spreading. Regular inspections of storage facilities and transfer equipment are necessary to detect and address potential leaks or damage.
Emergency response procedures must be clearly defined and regularly practiced. This includes spill containment and neutralization protocols, as well as evacuation plans. Eyewash stations and safety showers should be readily accessible in all areas where HCl is handled or stored. Training programs for employees must cover the proper use of PPE, handling procedures, and emergency response techniques.
Ventilation systems play a critical role in maintaining safe working conditions. Local exhaust ventilation should be installed at points of HCl use to capture and remove acid vapors. General ventilation systems must be designed to ensure adequate air exchange rates throughout the facility.
Monitoring and detection systems are essential for early warning of potential leaks or exposure. This includes the use of HCl vapor detectors and pH monitors in key areas. Regular air quality testing and employee health monitoring programs should be implemented to track potential long-term exposure effects.
Waste management and disposal procedures for HCl and related materials must comply with environmental regulations. This includes proper neutralization techniques and the use of certified disposal services for hazardous waste.
Documentation and record-keeping are vital components of safety protocols. This includes maintaining up-to-date safety data sheets (SDS), detailed handling procedures, and incident reports. Regular safety audits and reviews should be conducted to ensure compliance with established protocols and identify areas for improvement.
Collaboration with local emergency services is essential for effective response to major incidents. This includes providing facility layouts, chemical inventories, and coordinating joint training exercises. By implementing these comprehensive safety protocols, the metal industry can minimize risks associated with HCl handling and ensure a safer working environment for all employees.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!