Analyzing Magnesium Nitrate’s Effect on Nutrient Stratification
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 and Nutrient Stratification Background
Magnesium nitrate (Mg(NO3)2) plays a crucial role in nutrient stratification within agricultural systems. This phenomenon, characterized by the uneven distribution of nutrients in soil or water, has significant implications for crop growth and ecosystem health. The study of Mg(NO3)2's effect on nutrient stratification has gained prominence due to its potential to influence fertilizer efficiency and environmental sustainability.
Nutrient stratification occurs when certain elements accumulate in specific layers of soil or water bodies, creating zones of varying nutrient concentrations. This process can be influenced by various factors, including soil properties, water movement, and the chemical characteristics of the nutrients themselves. Magnesium nitrate, being highly soluble and mobile, has the potential to significantly impact this stratification process.
The historical context of this research dates back to the mid-20th century when agricultural intensification led to increased use of chemical fertilizers. As concerns about environmental impacts and resource efficiency grew, scientists began to focus on understanding the behavior of nutrients in soil and water systems. The study of Mg(NO3)2 in relation to nutrient stratification emerged as a critical area of investigation due to its dual role as a source of both magnesium and nitrogen.
In recent years, the importance of this research has been amplified by global challenges such as climate change and food security. The need for sustainable agricultural practices that optimize nutrient use while minimizing environmental impact has driven further exploration into the mechanisms of nutrient stratification and the role of compounds like Mg(NO3)2.
The technological evolution in this field has been marked by advancements in analytical techniques, including high-resolution soil sampling methods, spectroscopic analysis, and computer modeling. These tools have enabled researchers to gain deeper insights into the complex interactions between Mg(NO3)2 and other nutrients in various environmental conditions.
Current research objectives in this area focus on several key aspects: understanding the kinetics of Mg(NO3)2 movement in different soil types, quantifying its impact on the distribution of other essential nutrients, and developing strategies to manage nutrient stratification for optimal crop nutrition and environmental protection.
As we delve deeper into the analysis of Mg(NO3)2's effect on nutrient stratification, it is essential to consider the broader implications for agricultural practices, ecosystem management, and the development of next-generation fertilizers. This research not only contributes to our fundamental understanding of soil chemistry but also has practical applications in precision agriculture and sustainable resource management.
Nutrient stratification occurs when certain elements accumulate in specific layers of soil or water bodies, creating zones of varying nutrient concentrations. This process can be influenced by various factors, including soil properties, water movement, and the chemical characteristics of the nutrients themselves. Magnesium nitrate, being highly soluble and mobile, has the potential to significantly impact this stratification process.
The historical context of this research dates back to the mid-20th century when agricultural intensification led to increased use of chemical fertilizers. As concerns about environmental impacts and resource efficiency grew, scientists began to focus on understanding the behavior of nutrients in soil and water systems. The study of Mg(NO3)2 in relation to nutrient stratification emerged as a critical area of investigation due to its dual role as a source of both magnesium and nitrogen.
In recent years, the importance of this research has been amplified by global challenges such as climate change and food security. The need for sustainable agricultural practices that optimize nutrient use while minimizing environmental impact has driven further exploration into the mechanisms of nutrient stratification and the role of compounds like Mg(NO3)2.
The technological evolution in this field has been marked by advancements in analytical techniques, including high-resolution soil sampling methods, spectroscopic analysis, and computer modeling. These tools have enabled researchers to gain deeper insights into the complex interactions between Mg(NO3)2 and other nutrients in various environmental conditions.
Current research objectives in this area focus on several key aspects: understanding the kinetics of Mg(NO3)2 movement in different soil types, quantifying its impact on the distribution of other essential nutrients, and developing strategies to manage nutrient stratification for optimal crop nutrition and environmental protection.
As we delve deeper into the analysis of Mg(NO3)2's effect on nutrient stratification, it is essential to consider the broader implications for agricultural practices, ecosystem management, and the development of next-generation fertilizers. This research not only contributes to our fundamental understanding of soil chemistry but also has practical applications in precision agriculture and sustainable resource management.
Market Demand Analysis
The market demand for solutions addressing nutrient stratification in agriculture has been steadily growing, driven by the increasing need for sustainable and efficient farming practices. Magnesium nitrate, as a potential tool for managing nutrient distribution in soil, has garnered significant attention from both farmers and agricultural researchers.
The global fertilizer market, which includes specialty products like magnesium nitrate, is projected to reach substantial growth in the coming years. This growth is primarily fueled by the rising global population and the subsequent demand for increased food production. Farmers are increasingly seeking innovative solutions to maximize crop yields while minimizing environmental impact, creating a favorable market environment for products that can effectively manage nutrient stratification.
In regions with intensive agriculture, such as North America, Europe, and parts of Asia, the demand for magnesium nitrate and similar products is particularly strong. These areas often face challenges related to soil degradation and nutrient imbalances due to long-term intensive farming practices. The ability of magnesium nitrate to potentially address these issues positions it as a valuable tool in the agricultural market.
The horticulture sector, including greenhouse and hydroponic farming, represents another significant market segment for magnesium nitrate. These controlled environment agriculture systems require precise nutrient management, making products that can effectively control nutrient distribution highly desirable.
Environmental regulations and sustainability initiatives are also driving market demand for solutions that can reduce nutrient runoff and improve fertilizer efficiency. As governments worldwide implement stricter policies on agricultural practices, farmers are increasingly looking for products that can help them comply with these regulations while maintaining productivity.
The organic farming sector, which has been experiencing rapid growth, presents another potential market for magnesium nitrate, particularly if it can be produced or sourced in compliance with organic standards. Organic farmers often struggle with nutrient management and could benefit from solutions that address nutrient stratification without relying on synthetic chemicals.
Market analysis indicates that there is a growing trend towards precision agriculture and smart farming techniques. Products that can be integrated into these high-tech farming systems, potentially including magnesium nitrate-based solutions, are likely to see increased demand as farmers seek to optimize their operations through data-driven decision-making.
In conclusion, the market demand for solutions addressing nutrient stratification, including the potential use of magnesium nitrate, is robust and diverse. The agricultural sector's ongoing challenges with soil health, productivity, and sustainability create a favorable environment for innovative products in this space. As research continues to explore the effects of magnesium nitrate on nutrient stratification, the market is likely to respond positively to solutions that can demonstrate clear benefits in terms of crop yield, soil health, and environmental sustainability.
The global fertilizer market, which includes specialty products like magnesium nitrate, is projected to reach substantial growth in the coming years. This growth is primarily fueled by the rising global population and the subsequent demand for increased food production. Farmers are increasingly seeking innovative solutions to maximize crop yields while minimizing environmental impact, creating a favorable market environment for products that can effectively manage nutrient stratification.
In regions with intensive agriculture, such as North America, Europe, and parts of Asia, the demand for magnesium nitrate and similar products is particularly strong. These areas often face challenges related to soil degradation and nutrient imbalances due to long-term intensive farming practices. The ability of magnesium nitrate to potentially address these issues positions it as a valuable tool in the agricultural market.
The horticulture sector, including greenhouse and hydroponic farming, represents another significant market segment for magnesium nitrate. These controlled environment agriculture systems require precise nutrient management, making products that can effectively control nutrient distribution highly desirable.
Environmental regulations and sustainability initiatives are also driving market demand for solutions that can reduce nutrient runoff and improve fertilizer efficiency. As governments worldwide implement stricter policies on agricultural practices, farmers are increasingly looking for products that can help them comply with these regulations while maintaining productivity.
The organic farming sector, which has been experiencing rapid growth, presents another potential market for magnesium nitrate, particularly if it can be produced or sourced in compliance with organic standards. Organic farmers often struggle with nutrient management and could benefit from solutions that address nutrient stratification without relying on synthetic chemicals.
Market analysis indicates that there is a growing trend towards precision agriculture and smart farming techniques. Products that can be integrated into these high-tech farming systems, potentially including magnesium nitrate-based solutions, are likely to see increased demand as farmers seek to optimize their operations through data-driven decision-making.
In conclusion, the market demand for solutions addressing nutrient stratification, including the potential use of magnesium nitrate, is robust and diverse. The agricultural sector's ongoing challenges with soil health, productivity, and sustainability create a favorable environment for innovative products in this space. As research continues to explore the effects of magnesium nitrate on nutrient stratification, the market is likely to respond positively to solutions that can demonstrate clear benefits in terms of crop yield, soil health, and environmental sustainability.
Current Challenges in Nutrient Management
Nutrient management in agriculture faces several critical challenges that hinder optimal crop production and environmental sustainability. One of the primary issues is nutrient stratification, particularly in no-till systems, where nutrients accumulate in the upper soil layers. This phenomenon can lead to reduced nutrient availability for plant roots, especially during dry periods when the topsoil moisture is limited.
The use of magnesium nitrate and its effect on nutrient stratification adds another layer of complexity to this challenge. While magnesium nitrate can provide essential nutrients, its application may exacerbate stratification issues if not managed properly. Farmers and agronomists struggle to find the right balance between supplying adequate nutrients and preventing excessive accumulation in specific soil layers.
Another significant challenge is the variability in nutrient uptake efficiency across different crop types and growth stages. This variability makes it difficult to develop universal nutrient management strategies, requiring more tailored approaches for each crop and field condition. The interaction between magnesium nitrate and other nutrients in the soil further complicates this issue, as it can affect the availability and uptake of other essential elements.
Environmental concerns also play a crucial role in current nutrient management challenges. Excessive application of nutrients, including magnesium nitrate, can lead to nutrient runoff and leaching, contributing to water pollution and eutrophication of water bodies. Balancing the need for optimal crop nutrition with environmental stewardship remains a significant challenge for the agricultural sector.
Climate change introduces additional uncertainties in nutrient management. Changing precipitation patterns and temperature regimes affect soil moisture levels and microbial activity, which in turn influence nutrient availability and plant uptake. These shifts make it increasingly difficult to predict and manage nutrient requirements effectively, especially when considering the long-term effects of magnesium nitrate application on soil health and nutrient dynamics.
The lack of real-time, cost-effective monitoring tools for nutrient levels and stratification patterns in the field presents another hurdle. While soil testing provides valuable information, it often represents a snapshot in time and may not capture the dynamic nature of nutrient movement and availability, particularly in relation to magnesium nitrate's influence on stratification.
Lastly, the economic aspects of nutrient management pose significant challenges. Farmers must balance the costs of inputs, including magnesium nitrate, with potential yield benefits and environmental considerations. The volatility of fertilizer prices and crop markets adds to the complexity of making sound nutrient management decisions that are both economically viable and agronomically effective.
The use of magnesium nitrate and its effect on nutrient stratification adds another layer of complexity to this challenge. While magnesium nitrate can provide essential nutrients, its application may exacerbate stratification issues if not managed properly. Farmers and agronomists struggle to find the right balance between supplying adequate nutrients and preventing excessive accumulation in specific soil layers.
Another significant challenge is the variability in nutrient uptake efficiency across different crop types and growth stages. This variability makes it difficult to develop universal nutrient management strategies, requiring more tailored approaches for each crop and field condition. The interaction between magnesium nitrate and other nutrients in the soil further complicates this issue, as it can affect the availability and uptake of other essential elements.
Environmental concerns also play a crucial role in current nutrient management challenges. Excessive application of nutrients, including magnesium nitrate, can lead to nutrient runoff and leaching, contributing to water pollution and eutrophication of water bodies. Balancing the need for optimal crop nutrition with environmental stewardship remains a significant challenge for the agricultural sector.
Climate change introduces additional uncertainties in nutrient management. Changing precipitation patterns and temperature regimes affect soil moisture levels and microbial activity, which in turn influence nutrient availability and plant uptake. These shifts make it increasingly difficult to predict and manage nutrient requirements effectively, especially when considering the long-term effects of magnesium nitrate application on soil health and nutrient dynamics.
The lack of real-time, cost-effective monitoring tools for nutrient levels and stratification patterns in the field presents another hurdle. While soil testing provides valuable information, it often represents a snapshot in time and may not capture the dynamic nature of nutrient movement and availability, particularly in relation to magnesium nitrate's influence on stratification.
Lastly, the economic aspects of nutrient management pose significant challenges. Farmers must balance the costs of inputs, including magnesium nitrate, with potential yield benefits and environmental considerations. The volatility of fertilizer prices and crop markets adds to the complexity of making sound nutrient management decisions that are both economically viable and agronomically effective.
Existing Mg(NO3)2 Application Methods
01 Magnesium nitrate in nutrient solutions
Magnesium nitrate is used as a key component in nutrient solutions for hydroponic and soil-based cultivation systems. It provides both magnesium and nitrogen, essential elements for plant growth and development. The stratification of magnesium nitrate in these solutions can affect nutrient availability and uptake by plants.- Magnesium nitrate in nutrient solutions: Magnesium nitrate is used in nutrient solutions for hydroponic and soil-based cultivation systems. It provides both magnesium and nitrogen, essential nutrients for plant growth. The stratification of magnesium nitrate in these solutions can affect nutrient availability and uptake by plants.
- Controlled release of magnesium nitrate: Techniques for controlling the release of magnesium nitrate in fertilizers and nutrient solutions. This includes encapsulation methods, slow-release formulations, and the use of carrier materials to regulate the dissolution and distribution of magnesium nitrate in the growing medium.
- Nutrient stratification prevention methods: Methods to prevent or minimize nutrient stratification in solutions containing magnesium nitrate. This includes mixing techniques, circulation systems, and the use of stabilizing agents to maintain a homogeneous distribution of nutrients throughout the solution or growing medium.
- Magnesium nitrate in foliar applications: The use of magnesium nitrate in foliar sprays and its effects on nutrient stratification in plant tissues. This includes formulations designed for optimal leaf absorption and translocation of magnesium and nitrogen within the plant.
- Monitoring and adjusting nutrient stratification: Systems and methods for monitoring and adjusting nutrient stratification in solutions containing magnesium nitrate. This includes sensors, analytical techniques, and automated systems for maintaining optimal nutrient balance and preventing excessive stratification in hydroponic and fertigation systems.
02 Controlled release of magnesium nitrate
Techniques for controlling the release of magnesium nitrate in fertilizers and nutrient solutions have been developed. These methods aim to prevent rapid leaching and ensure a steady supply of nutrients to plants over time. Controlled release can help mitigate nutrient stratification issues in various growing media.Expand Specific Solutions03 Nutrient stratification prevention methods
Various methods have been invented to prevent or reduce nutrient stratification in growing systems using magnesium nitrate. These include specialized mixing techniques, the use of stabilizing agents, and innovative delivery systems that maintain a more uniform distribution of nutrients throughout the growing medium.Expand Specific Solutions04 Magnesium nitrate in foliar applications
Foliar application of magnesium nitrate solutions has been explored as a method to bypass soil stratification issues. This technique allows for direct nutrient absorption through plant leaves, ensuring more uniform distribution of magnesium and nitrogen within the plant tissues.Expand Specific Solutions05 Monitoring and adjusting nutrient stratification
Advanced systems and methods for monitoring and adjusting nutrient stratification in growing media have been developed. These include sensor-based technologies, automated nutrient delivery systems, and data-driven approaches to optimize the distribution and availability of magnesium nitrate and other nutrients throughout the root zone.Expand Specific Solutions
Key Players in Agrochemical Industry
The competitive landscape for analyzing magnesium nitrate's effect on nutrient stratification is in an early development stage, with a growing market as agricultural technology advances. The market size is expanding due to increased focus on precision agriculture and soil health management. Technologically, it's still evolving, with varying levels of maturity among key players. Companies like Yara International ASA and Syngenta Participations AG are at the forefront, leveraging their extensive agricultural research capabilities. Academic institutions such as Zhejiang University and China Agricultural University are contributing significant research. Emerging players like Shanxi Jiaocheng Hongxing Chemicals Co. Ltd. are also entering the market, indicating growing interest and potential for innovation in this field.
Pioneer Hi-Bred International, Inc.
Technical Solution: Pioneer Hi-Bred International has developed a multi-faceted approach to managing nutrient stratification, including the use of magnesium nitrate. Their strategy combines advanced crop genetics with precision nutrient management. The company has bred crop varieties with enhanced root systems that can more effectively access nutrients throughout the soil profile, reducing the impact of stratification[8]. In conjunction with these improved genetics, Pioneer has developed a decision support system that integrates soil test data, crop nutrient requirements, and environmental factors to optimize magnesium nitrate application. This system uses machine learning algorithms to continuously improve recommendations based on field performance data[9].
Strengths: Integration of crop genetics and nutrient management strategies. Extensive field testing and data collection capabilities. Weaknesses: Reliance on farmers adopting proprietary seed varieties. Potential for increased seed costs.
Yara International ASA
Technical Solution: Yara International ASA has developed a precision farming approach to address nutrient stratification, including the effects of magnesium nitrate. Their solution involves using sensor technology and data analytics to create detailed soil nutrient maps[1]. This allows for targeted application of magnesium nitrate and other nutrients, precisely where they are needed. The company has also developed slow-release fertilizer formulations containing magnesium nitrate, which help to maintain consistent nutrient levels throughout the soil profile over time[2]. Additionally, Yara has implemented machine learning algorithms to predict nutrient movement in soil, enabling proactive management of stratification issues[3].
Strengths: Global presence and extensive research capabilities. Advanced technology integration for precision agriculture. Weaknesses: Reliance on farmers adopting new technologies. Potential high initial costs for implementation.
Core Research on Mg(NO3)2 Effects
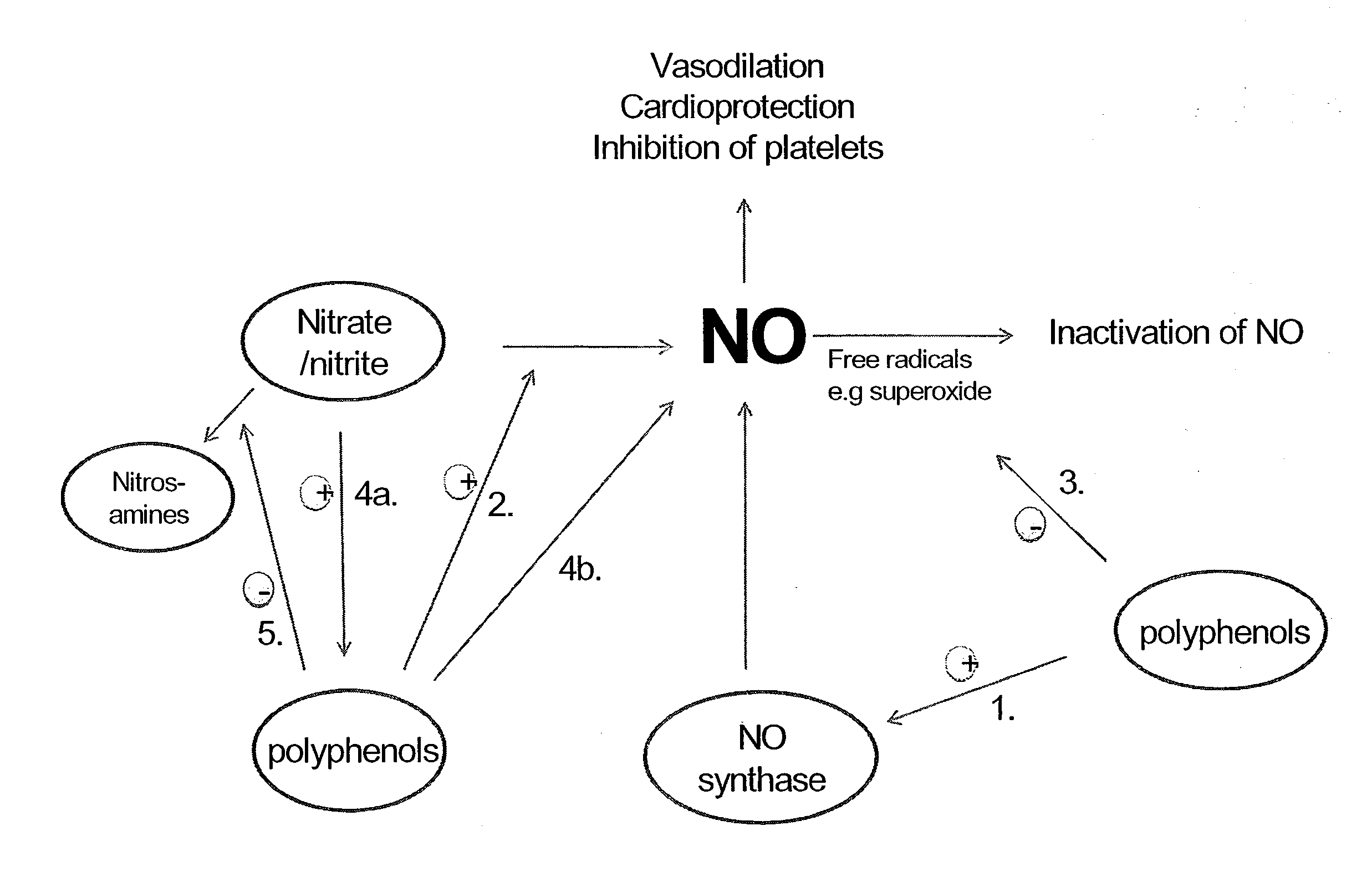
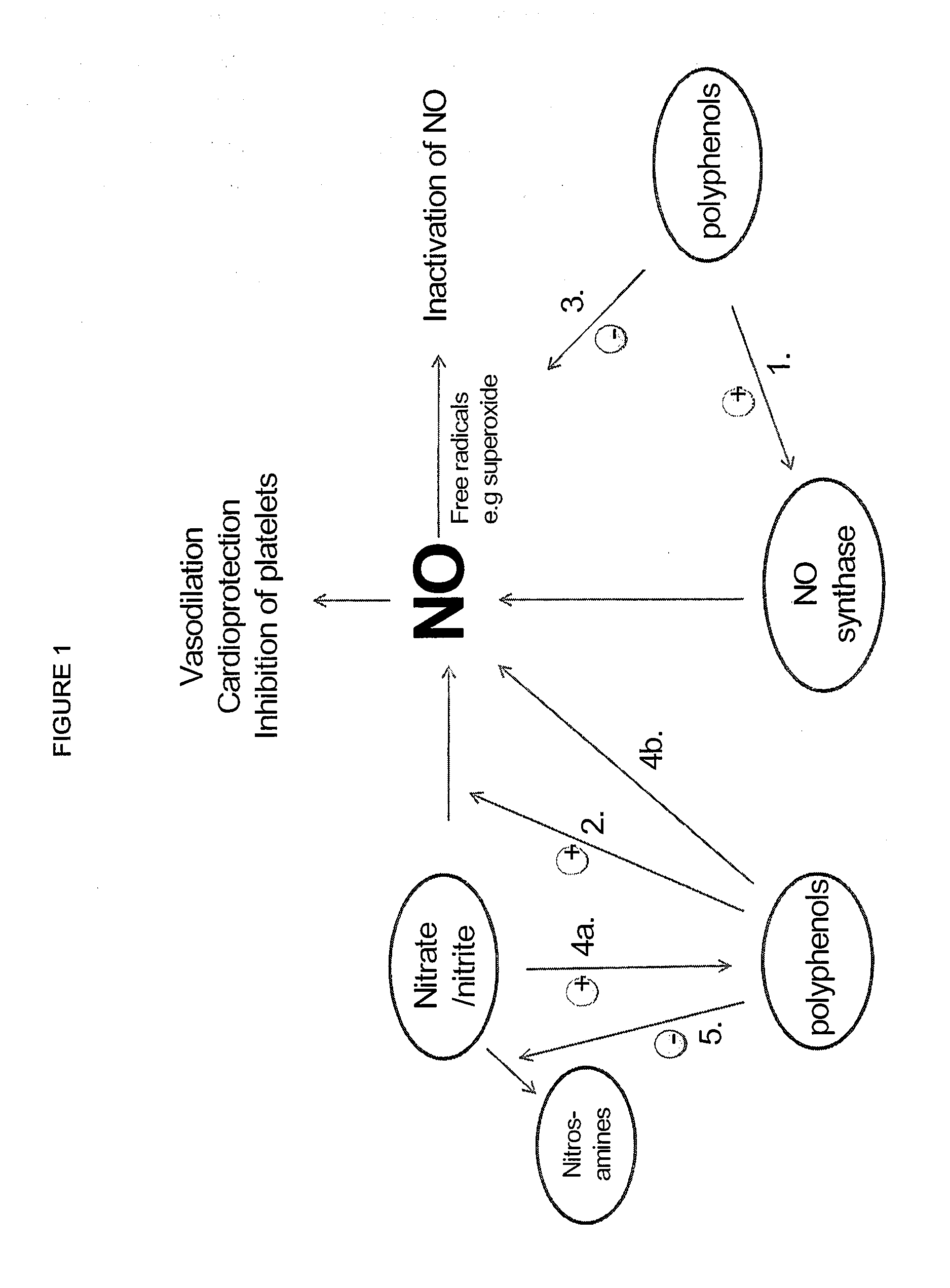
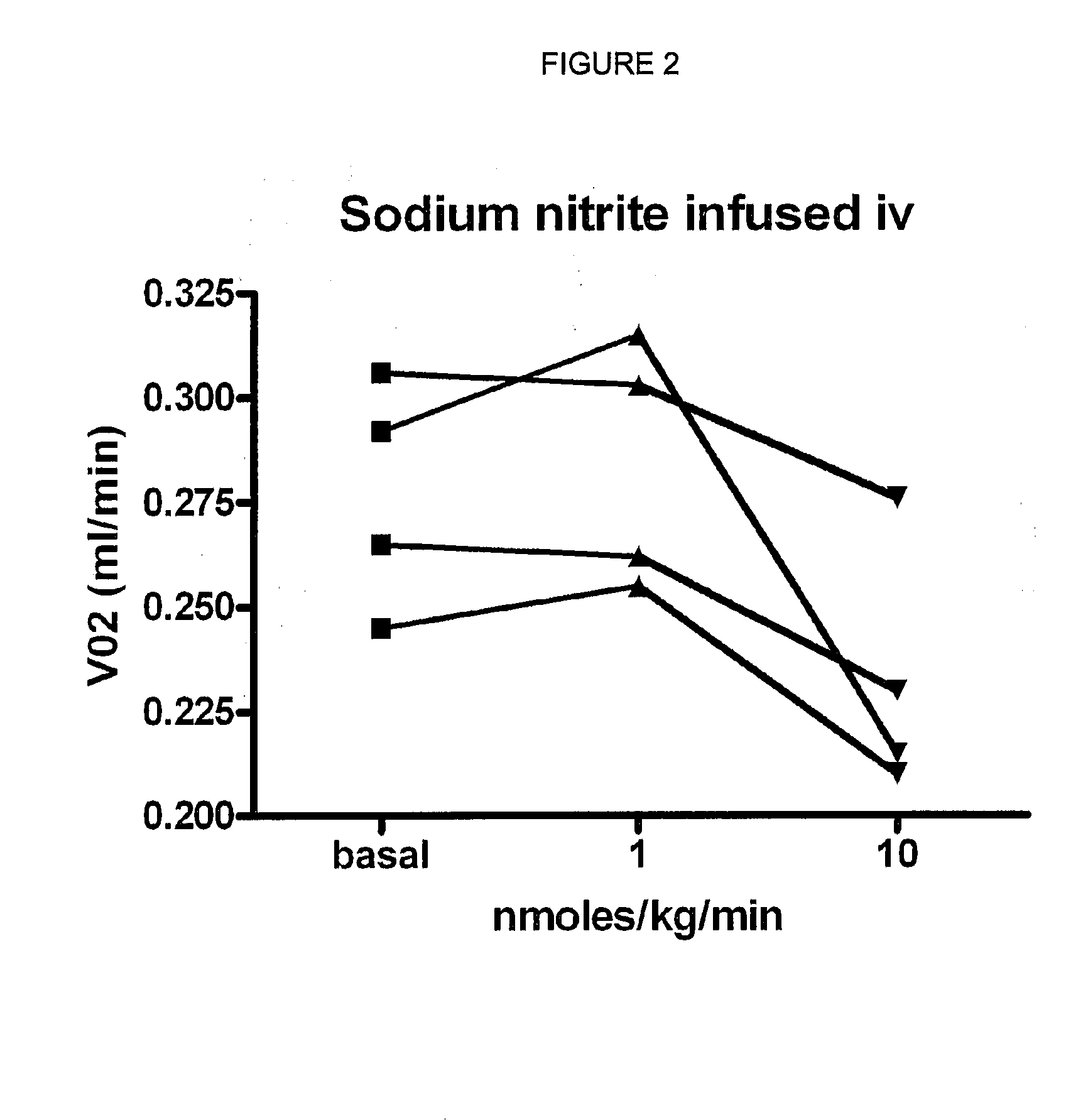
Use of nitrites and nitrates and compositions containing these
PatentActiveUS20100092441A1
Innovation
- Administering inorganic nitrite and nitrate in specific doses, either orally or parenterally, to decrease oxygen consumption without inducing hypotension, and combining them with polyphenols to enhance bioavailability and reduce harmful compound formation.
Environmental Impact Assessment
The environmental impact assessment of magnesium nitrate's effect on nutrient stratification is a critical aspect of understanding the broader ecological implications of this agricultural practice. Magnesium nitrate, when used as a fertilizer, can significantly alter soil chemistry and nutrient distribution, leading to both positive and negative environmental consequences.
One of the primary concerns is the potential for increased nutrient leaching. As magnesium nitrate is highly soluble, it can rapidly move through the soil profile, potentially reaching groundwater and surface water bodies. This movement can contribute to eutrophication in aquatic ecosystems, leading to algal blooms and oxygen depletion. The severity of this impact depends on factors such as soil type, precipitation patterns, and application rates.
The use of magnesium nitrate can also affect soil pH levels. While it is generally considered less acidifying than some other nitrogen fertilizers, prolonged use may still lead to soil acidification in certain conditions. This change in soil pH can influence the availability of other nutrients and impact soil microbial communities, potentially altering ecosystem functions and biodiversity in the rhizosphere.
Another consideration is the effect on soil structure and water retention capacity. The addition of magnesium can improve soil aggregation in some cases, enhancing water infiltration and reducing erosion risk. However, excessive application may lead to soil dispersion, particularly in soils with high sodium content, potentially degrading soil structure and increasing erosion susceptibility.
The impact on plant biodiversity is another important factor. While magnesium nitrate can enhance the growth of target crops, it may also favor certain plant species over others, potentially altering the composition of natural plant communities in adjacent areas. This shift can have cascading effects on local ecosystems, affecting insect populations and other wildlife that depend on specific plant species.
Atmospheric emissions are also a concern, particularly in terms of nitrous oxide (N2O) production. N2O is a potent greenhouse gas, and the application of nitrogen-based fertilizers like magnesium nitrate can increase its emission from soil. The magnitude of these emissions depends on various factors, including soil moisture, temperature, and microbial activity.
Lastly, the production and transportation of magnesium nitrate fertilizers have their own environmental footprints. The manufacturing process requires energy and resources, contributing to greenhouse gas emissions and resource depletion. Additionally, the transportation of these fertilizers from production facilities to agricultural areas adds to the overall environmental impact through fuel consumption and emissions.
One of the primary concerns is the potential for increased nutrient leaching. As magnesium nitrate is highly soluble, it can rapidly move through the soil profile, potentially reaching groundwater and surface water bodies. This movement can contribute to eutrophication in aquatic ecosystems, leading to algal blooms and oxygen depletion. The severity of this impact depends on factors such as soil type, precipitation patterns, and application rates.
The use of magnesium nitrate can also affect soil pH levels. While it is generally considered less acidifying than some other nitrogen fertilizers, prolonged use may still lead to soil acidification in certain conditions. This change in soil pH can influence the availability of other nutrients and impact soil microbial communities, potentially altering ecosystem functions and biodiversity in the rhizosphere.
Another consideration is the effect on soil structure and water retention capacity. The addition of magnesium can improve soil aggregation in some cases, enhancing water infiltration and reducing erosion risk. However, excessive application may lead to soil dispersion, particularly in soils with high sodium content, potentially degrading soil structure and increasing erosion susceptibility.
The impact on plant biodiversity is another important factor. While magnesium nitrate can enhance the growth of target crops, it may also favor certain plant species over others, potentially altering the composition of natural plant communities in adjacent areas. This shift can have cascading effects on local ecosystems, affecting insect populations and other wildlife that depend on specific plant species.
Atmospheric emissions are also a concern, particularly in terms of nitrous oxide (N2O) production. N2O is a potent greenhouse gas, and the application of nitrogen-based fertilizers like magnesium nitrate can increase its emission from soil. The magnitude of these emissions depends on various factors, including soil moisture, temperature, and microbial activity.
Lastly, the production and transportation of magnesium nitrate fertilizers have their own environmental footprints. The manufacturing process requires energy and resources, contributing to greenhouse gas emissions and resource depletion. Additionally, the transportation of these fertilizers from production facilities to agricultural areas adds to the overall environmental impact through fuel consumption and emissions.
Regulatory Framework for Fertilizer Use
The regulatory framework for fertilizer use plays a crucial role in managing the application of magnesium nitrate and its potential effects on nutrient stratification. In many countries, fertilizer regulations are designed to ensure agricultural productivity while minimizing environmental impacts and protecting public health.
At the federal level, agencies such as the Environmental Protection Agency (EPA) and the Department of Agriculture often oversee fertilizer regulations. These agencies establish guidelines for fertilizer composition, labeling requirements, and application rates. For magnesium nitrate specifically, regulations may address its classification as a fertilizer or soil amendment, as well as any restrictions on its use due to its potential impact on soil chemistry and nutrient availability.
State and local governments may impose additional regulations tailored to regional agricultural practices and environmental concerns. These regulations can include specific guidelines for the timing and method of fertilizer application, particularly in areas prone to nutrient runoff or leaching. Some jurisdictions may require soil testing before fertilizer application to prevent over-fertilization and reduce the risk of nutrient stratification.
International standards and agreements also influence regulatory frameworks for fertilizer use. Organizations like the Food and Agriculture Organization (FAO) of the United Nations provide guidelines and best practices for sustainable fertilizer management, which many countries incorporate into their national policies.
Regulations often address the environmental impacts of fertilizer use, including potential effects on water quality. In the case of magnesium nitrate, its high solubility may lead to concerns about nitrate leaching into groundwater. As a result, regulations may limit application rates or require specific management practices in vulnerable areas.
The regulatory framework also encompasses fertilizer quality control and safety standards. Manufacturers and distributors of magnesium nitrate and other fertilizers must comply with regulations regarding product purity, contaminant levels, and storage conditions. These measures help ensure that the fertilizers used by farmers are safe and effective.
Compliance and enforcement mechanisms are integral parts of the regulatory framework. This may include regular inspections, reporting requirements, and penalties for non-compliance. Farmers and agricultural businesses using magnesium nitrate must stay informed about applicable regulations and maintain proper documentation of their fertilizer management practices.
As research on nutrient stratification and the effects of specific fertilizers like magnesium nitrate continues to evolve, regulatory frameworks are likely to adapt. Policymakers may update regulations based on new scientific findings, potentially leading to more targeted approaches for managing nutrient stratification and optimizing fertilizer use efficiency.
At the federal level, agencies such as the Environmental Protection Agency (EPA) and the Department of Agriculture often oversee fertilizer regulations. These agencies establish guidelines for fertilizer composition, labeling requirements, and application rates. For magnesium nitrate specifically, regulations may address its classification as a fertilizer or soil amendment, as well as any restrictions on its use due to its potential impact on soil chemistry and nutrient availability.
State and local governments may impose additional regulations tailored to regional agricultural practices and environmental concerns. These regulations can include specific guidelines for the timing and method of fertilizer application, particularly in areas prone to nutrient runoff or leaching. Some jurisdictions may require soil testing before fertilizer application to prevent over-fertilization and reduce the risk of nutrient stratification.
International standards and agreements also influence regulatory frameworks for fertilizer use. Organizations like the Food and Agriculture Organization (FAO) of the United Nations provide guidelines and best practices for sustainable fertilizer management, which many countries incorporate into their national policies.
Regulations often address the environmental impacts of fertilizer use, including potential effects on water quality. In the case of magnesium nitrate, its high solubility may lead to concerns about nitrate leaching into groundwater. As a result, regulations may limit application rates or require specific management practices in vulnerable areas.
The regulatory framework also encompasses fertilizer quality control and safety standards. Manufacturers and distributors of magnesium nitrate and other fertilizers must comply with regulations regarding product purity, contaminant levels, and storage conditions. These measures help ensure that the fertilizers used by farmers are safe and effective.
Compliance and enforcement mechanisms are integral parts of the regulatory framework. This may include regular inspections, reporting requirements, and penalties for non-compliance. Farmers and agricultural businesses using magnesium nitrate must stay informed about applicable regulations and maintain proper documentation of their fertilizer management practices.
As research on nutrient stratification and the effects of specific fertilizers like magnesium nitrate continues to evolve, regulatory frameworks are likely to adapt. Policymakers may update regulations based on new scientific findings, potentially leading to more targeted approaches for managing nutrient stratification and optimizing fertilizer use efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!