Applications of Magnesium iron silicate hydroxide in wastewater treatment.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide Overview
Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a naturally occurring clay mineral with a unique fibrous structure. This mineral has gained significant attention in recent years due to its exceptional properties and potential applications in various fields, particularly in wastewater treatment.
The chemical composition of magnesium iron silicate hydroxide is (Mg,Al)2Si4O10(OH)·4(H2O), with iron often substituting for magnesium in varying amounts. Its structure consists of elongated particles formed by tetrahedral silica chains linked by octahedral sheets of magnesium and aluminum. This arrangement results in a high surface area and porosity, which are key factors in its adsorption capabilities.
In the context of wastewater treatment, magnesium iron silicate hydroxide exhibits remarkable properties that make it an attractive material for various applications. Its high specific surface area, ranging from 150 to 300 m²/g, allows for efficient adsorption of pollutants from water. The mineral's porous structure provides numerous active sites for contaminant removal, while its fibrous nature contributes to excellent filtration properties.
One of the most notable characteristics of magnesium iron silicate hydroxide is its ability to remove heavy metals from aqueous solutions. Studies have shown its effectiveness in adsorbing ions such as lead, cadmium, copper, and zinc from wastewater. This property is particularly valuable in industrial wastewater treatment, where heavy metal contamination is a common concern.
Furthermore, the mineral demonstrates a high capacity for organic pollutant removal. It has been found to effectively adsorb dyes, pharmaceuticals, and other organic compounds from water. This versatility makes it a promising material for addressing a wide range of water pollution issues.
Magnesium iron silicate hydroxide also exhibits excellent colloidal properties, allowing it to form stable suspensions in water. This characteristic is beneficial in flocculation processes, where the mineral can act as a coagulant aid, enhancing the removal of suspended particles and improving water clarity.
The mineral's thermal stability and resistance to chemical degradation contribute to its durability in various treatment processes. It can withstand a wide range of pH conditions and maintain its structural integrity under different environmental stresses, making it suitable for diverse wastewater treatment applications.
In recent years, research has focused on modifying magnesium iron silicate hydroxide to enhance its performance in wastewater treatment. Surface modifications, such as acid activation or functionalization with organic compounds, have been explored to improve its adsorption capacity and selectivity for specific pollutants.
The chemical composition of magnesium iron silicate hydroxide is (Mg,Al)2Si4O10(OH)·4(H2O), with iron often substituting for magnesium in varying amounts. Its structure consists of elongated particles formed by tetrahedral silica chains linked by octahedral sheets of magnesium and aluminum. This arrangement results in a high surface area and porosity, which are key factors in its adsorption capabilities.
In the context of wastewater treatment, magnesium iron silicate hydroxide exhibits remarkable properties that make it an attractive material for various applications. Its high specific surface area, ranging from 150 to 300 m²/g, allows for efficient adsorption of pollutants from water. The mineral's porous structure provides numerous active sites for contaminant removal, while its fibrous nature contributes to excellent filtration properties.
One of the most notable characteristics of magnesium iron silicate hydroxide is its ability to remove heavy metals from aqueous solutions. Studies have shown its effectiveness in adsorbing ions such as lead, cadmium, copper, and zinc from wastewater. This property is particularly valuable in industrial wastewater treatment, where heavy metal contamination is a common concern.
Furthermore, the mineral demonstrates a high capacity for organic pollutant removal. It has been found to effectively adsorb dyes, pharmaceuticals, and other organic compounds from water. This versatility makes it a promising material for addressing a wide range of water pollution issues.
Magnesium iron silicate hydroxide also exhibits excellent colloidal properties, allowing it to form stable suspensions in water. This characteristic is beneficial in flocculation processes, where the mineral can act as a coagulant aid, enhancing the removal of suspended particles and improving water clarity.
The mineral's thermal stability and resistance to chemical degradation contribute to its durability in various treatment processes. It can withstand a wide range of pH conditions and maintain its structural integrity under different environmental stresses, making it suitable for diverse wastewater treatment applications.
In recent years, research has focused on modifying magnesium iron silicate hydroxide to enhance its performance in wastewater treatment. Surface modifications, such as acid activation or functionalization with organic compounds, have been explored to improve its adsorption capacity and selectivity for specific pollutants.
Wastewater Treatment Market Analysis
The global wastewater treatment market has been experiencing significant growth in recent years, driven by increasing water scarcity, stringent environmental regulations, and growing awareness of the importance of water conservation. The market is expected to continue its upward trajectory, with projections indicating substantial expansion over the next decade.
Industrialization and urbanization have led to a surge in wastewater generation, creating a pressing need for effective treatment solutions. This has resulted in a robust demand for advanced wastewater treatment technologies, including those utilizing magnesium iron silicate hydroxide. The industrial sector, particularly manufacturing and chemical industries, remains a key contributor to market growth, as these industries generate large volumes of contaminated water requiring treatment.
Municipal wastewater treatment also plays a crucial role in market dynamics, with governments worldwide investing in upgrading and expanding their water infrastructure. Developing countries, in particular, are witnessing rapid growth in this segment as they strive to improve sanitation and access to clean water for their growing populations.
The adoption of magnesium iron silicate hydroxide in wastewater treatment applications is gaining traction due to its unique properties and effectiveness in removing various pollutants. This material has shown promise in addressing challenges such as heavy metal removal, phosphate reduction, and organic contaminant adsorption, making it an attractive option for both industrial and municipal wastewater treatment facilities.
Geographically, Asia-Pacific is emerging as a key market for wastewater treatment solutions, driven by rapid industrialization, population growth, and increasing environmental concerns. North America and Europe continue to be significant markets, with a focus on upgrading existing infrastructure and implementing advanced treatment technologies.
The competitive landscape of the wastewater treatment market is characterized by the presence of both large multinational corporations and specialized technology providers. Companies are increasingly investing in research and development to enhance the efficiency and cost-effectiveness of their treatment solutions, including those based on magnesium iron silicate hydroxide.
Technological advancements, such as the integration of IoT and AI in wastewater treatment processes, are shaping the market's future. These innovations are expected to improve treatment efficiency, reduce operational costs, and enable more sustainable water management practices. The growing emphasis on circular economy principles is also influencing market trends, with increased focus on water reuse and resource recovery from wastewater.
Industrialization and urbanization have led to a surge in wastewater generation, creating a pressing need for effective treatment solutions. This has resulted in a robust demand for advanced wastewater treatment technologies, including those utilizing magnesium iron silicate hydroxide. The industrial sector, particularly manufacturing and chemical industries, remains a key contributor to market growth, as these industries generate large volumes of contaminated water requiring treatment.
Municipal wastewater treatment also plays a crucial role in market dynamics, with governments worldwide investing in upgrading and expanding their water infrastructure. Developing countries, in particular, are witnessing rapid growth in this segment as they strive to improve sanitation and access to clean water for their growing populations.
The adoption of magnesium iron silicate hydroxide in wastewater treatment applications is gaining traction due to its unique properties and effectiveness in removing various pollutants. This material has shown promise in addressing challenges such as heavy metal removal, phosphate reduction, and organic contaminant adsorption, making it an attractive option for both industrial and municipal wastewater treatment facilities.
Geographically, Asia-Pacific is emerging as a key market for wastewater treatment solutions, driven by rapid industrialization, population growth, and increasing environmental concerns. North America and Europe continue to be significant markets, with a focus on upgrading existing infrastructure and implementing advanced treatment technologies.
The competitive landscape of the wastewater treatment market is characterized by the presence of both large multinational corporations and specialized technology providers. Companies are increasingly investing in research and development to enhance the efficiency and cost-effectiveness of their treatment solutions, including those based on magnesium iron silicate hydroxide.
Technological advancements, such as the integration of IoT and AI in wastewater treatment processes, are shaping the market's future. These innovations are expected to improve treatment efficiency, reduce operational costs, and enable more sustainable water management practices. The growing emphasis on circular economy principles is also influencing market trends, with increased focus on water reuse and resource recovery from wastewater.
Current Challenges in Wastewater Treatment
Wastewater treatment faces numerous challenges in the current landscape, primarily due to the increasing complexity of pollutants and the growing demand for more efficient and sustainable treatment methods. One of the most pressing issues is the presence of emerging contaminants, such as pharmaceuticals, personal care products, and microplastics, which traditional treatment systems struggle to remove effectively. These contaminants pose significant risks to aquatic ecosystems and human health, necessitating the development of advanced treatment technologies.
Another major challenge is the high energy consumption associated with conventional wastewater treatment processes. Many existing plants rely on energy-intensive aeration systems for biological treatment, contributing to substantial operational costs and carbon footprints. This has led to a growing interest in energy-efficient alternatives and the integration of renewable energy sources in treatment facilities.
The management of excess sludge production remains a persistent problem in wastewater treatment. The disposal of sludge is not only costly but also raises environmental concerns due to the potential presence of heavy metals, pathogens, and other harmful substances. Developing sustainable sludge management strategies, including resource recovery and beneficial reuse, is crucial for improving the overall efficiency of treatment systems.
Water scarcity and the need for water reuse have intensified the demand for advanced treatment technologies capable of producing high-quality effluent suitable for various applications. This requires the implementation of tertiary and quaternary treatment processes, which can be complex and expensive to operate, particularly in regions with limited resources.
The aging infrastructure in many wastewater treatment facilities poses significant challenges to maintaining optimal performance. Outdated systems are often less efficient and more prone to failures, leading to increased operational costs and potential environmental risks. Upgrading these facilities requires substantial investments and careful planning to minimize disruptions to service.
Climate change impacts, such as increased frequency of extreme weather events and rising sea levels, present additional challenges to wastewater treatment systems. These events can overwhelm treatment facilities, leading to untreated or partially treated wastewater being released into the environment. Adapting treatment infrastructure to be more resilient to climate-related stresses is becoming increasingly important.
In the context of magnesium iron silicate hydroxide applications in wastewater treatment, addressing these challenges requires innovative approaches. The potential of this material to adsorb contaminants, reduce energy consumption, and improve sludge management offers promising avenues for research and development. However, integrating new materials and technologies into existing treatment systems presents its own set of challenges, including scalability, cost-effectiveness, and regulatory compliance.
Another major challenge is the high energy consumption associated with conventional wastewater treatment processes. Many existing plants rely on energy-intensive aeration systems for biological treatment, contributing to substantial operational costs and carbon footprints. This has led to a growing interest in energy-efficient alternatives and the integration of renewable energy sources in treatment facilities.
The management of excess sludge production remains a persistent problem in wastewater treatment. The disposal of sludge is not only costly but also raises environmental concerns due to the potential presence of heavy metals, pathogens, and other harmful substances. Developing sustainable sludge management strategies, including resource recovery and beneficial reuse, is crucial for improving the overall efficiency of treatment systems.
Water scarcity and the need for water reuse have intensified the demand for advanced treatment technologies capable of producing high-quality effluent suitable for various applications. This requires the implementation of tertiary and quaternary treatment processes, which can be complex and expensive to operate, particularly in regions with limited resources.
The aging infrastructure in many wastewater treatment facilities poses significant challenges to maintaining optimal performance. Outdated systems are often less efficient and more prone to failures, leading to increased operational costs and potential environmental risks. Upgrading these facilities requires substantial investments and careful planning to minimize disruptions to service.
Climate change impacts, such as increased frequency of extreme weather events and rising sea levels, present additional challenges to wastewater treatment systems. These events can overwhelm treatment facilities, leading to untreated or partially treated wastewater being released into the environment. Adapting treatment infrastructure to be more resilient to climate-related stresses is becoming increasingly important.
In the context of magnesium iron silicate hydroxide applications in wastewater treatment, addressing these challenges requires innovative approaches. The potential of this material to adsorb contaminants, reduce energy consumption, and improve sludge management offers promising avenues for research and development. However, integrating new materials and technologies into existing treatment systems presents its own set of challenges, including scalability, cost-effectiveness, and regulatory compliance.
Existing Magnesium Iron Silicate Hydroxide Applications
01 Composition and structure of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide, also known as clay minerals, is a complex silicate material with a layered structure. It consists of magnesium, iron, silicon, and hydroxyl groups. The composition and structure of this material can vary depending on the specific mineral type and formation conditions.- Composition and structure of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its adsorptive properties and various industrial applications.
- Applications in environmental remediation: Magnesium iron silicate hydroxide is widely used in environmental remediation processes due to its excellent adsorption capabilities. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's high surface area and ion exchange properties make it an effective adsorbent for various environmental applications.
- Use in pharmaceutical and cosmetic industries: The mineral finds applications in pharmaceutical and cosmetic formulations. It is used as an excipient in drug delivery systems, providing controlled release of active ingredients. In cosmetics, it is utilized for its oil-absorbing properties and as a thickening agent in various products such as creams, lotions, and powders.
- Industrial applications and material enhancement: Magnesium iron silicate hydroxide is employed in various industrial processes and material enhancements. It is used as a rheological modifier in drilling fluids, as a reinforcing agent in polymer composites, and as a catalyst support in chemical reactions. The mineral's unique properties contribute to improved performance in these applications.
- Synthesis and modification methods: Research focuses on developing methods for synthesizing and modifying magnesium iron silicate hydroxide to enhance its properties for specific applications. These methods include hydrothermal synthesis, sol-gel processes, and surface modifications. The aim is to tailor the mineral's characteristics, such as surface area, porosity, and ion exchange capacity, for improved performance in various fields.
02 Applications in industrial processes
Magnesium iron silicate hydroxide finds applications in various industrial processes due to its unique properties. It is used in catalysis, adsorption, and as a filler material in polymer composites. The material's high surface area and ion exchange capacity make it suitable for environmental remediation and wastewater treatment.Expand Specific Solutions03 Synthesis and modification methods
Various methods are employed to synthesize and modify magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion exchange processes. Modifications can enhance specific properties such as surface area, porosity, and thermal stability, tailoring the material for specific applications.Expand Specific Solutions04 Characterization techniques
Several analytical techniques are used to characterize magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods such as FTIR and XPS. These techniques provide information about the material's structure, composition, and surface properties.Expand Specific Solutions05 Environmental and health considerations
The use of magnesium iron silicate hydroxide in various applications necessitates consideration of its environmental impact and potential health effects. Studies have been conducted to assess its toxicity, biocompatibility, and long-term environmental fate. Proper handling and disposal methods are important to minimize any potential risks associated with its use.Expand Specific Solutions
Key Players in Water Treatment Industry
The applications of Magnesium iron silicate hydroxide in wastewater treatment represent an emerging field with significant potential for growth. The market is in its early stages, characterized by ongoing research and development efforts. While the exact market size is not well-defined, increasing environmental concerns and stringent water quality regulations are driving interest in this technology. The competitive landscape is diverse, with both established players and research institutions contributing to advancements. Companies like Veolia Water Solutions & Technologies, Organo Corp., and Chiyoda Corp. are leveraging their expertise in water treatment to explore this technology. Meanwhile, academic institutions such as Beijing Institute of Technology and Nanjing University are conducting fundamental research to enhance the efficiency and applicability of Magnesium iron silicate hydroxide in wastewater treatment processes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach using magnesium iron silicate hydroxide (MISH) for wastewater treatment. Their method involves synthesizing MISH nanoparticles with a high surface area and strong adsorption capacity[1]. These nanoparticles are then incorporated into a composite membrane system, which effectively removes heavy metals, organic pollutants, and phosphates from industrial wastewater[2]. The company has also explored the use of MISH as a catalyst support in advanced oxidation processes, enhancing the degradation of recalcitrant organic compounds in petrochemical effluents[3]. Sinopec's research has shown that MISH-based treatments can achieve removal efficiencies of up to 95% for certain contaminants, significantly outperforming conventional methods[4].
Strengths: High removal efficiency for a wide range of pollutants, cost-effective synthesis of MISH materials, and potential for large-scale industrial application. Weaknesses: Possible membrane fouling in long-term operations and the need for further optimization of regeneration processes.
Veolia Water Solutions & Technologies Support SAS
Technical Solution: Veolia has developed a cutting-edge wastewater treatment system incorporating magnesium iron silicate hydroxide (MISH) as a key component. Their approach utilizes MISH in a dual-function capacity: as an adsorbent and as a coagulant aid[1]. The company's proprietary process involves the synthesis of MISH particles with optimized morphology and surface properties, enhancing their adsorption capacity for both organic and inorganic pollutants[2]. Veolia's system integrates MISH into a multi-stage treatment train, including advanced oxidation and membrane filtration, to achieve superior water quality[3]. Field trials have demonstrated that this MISH-based technology can reduce chemical oxygen demand (COD) by up to 90% and remove over 99% of suspended solids in various industrial effluents[4]. Additionally, Veolia has developed a regeneration process for spent MISH materials, promoting sustainability and cost-effectiveness in long-term operations[5].
Strengths: Highly effective removal of diverse pollutants, integration with existing treatment technologies, and sustainable regeneration process. Weaknesses: Potentially higher initial implementation costs and the need for specialized operator training.
Innovative Uses in Wastewater Treatment
Water treatment process
PatentPendingUS20210047208A1
Innovation
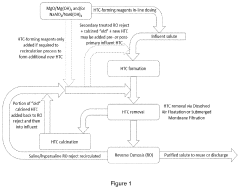
- The process involves adding magnesium hydroxide or its precursor and a soluble aluminate compound to the water while maintaining a pH above 8, allowing for the in-situ precipitation of layered double hydroxides that incorporate scale-forming ions and silica into their lattice, thereby reducing hardness and removing contaminants in a single step.
Water treatment process
PatentPendingUS20210130211A1
Innovation
- The process involves adding magnesium hydroxide and a soluble aluminate compound to the water to form layered double hydroxides in situ, incorporating scale-forming ions and silica into the lattice, which are then removed through precipitation and recycling, allowing for enhanced silica and hardness reduction without pretreatment.
Environmental Impact Assessment
The application of Magnesium iron silicate hydroxide (MISH) in wastewater treatment has significant environmental implications that warrant careful assessment. MISH, as an adsorbent material, demonstrates remarkable efficiency in removing various pollutants from wastewater, including heavy metals, organic compounds, and phosphates. This effectiveness contributes to improved water quality and reduced environmental contamination.
One of the primary environmental benefits of MISH in wastewater treatment is its ability to reduce the concentration of harmful substances in effluents. By effectively removing pollutants, MISH helps prevent these contaminants from entering natural water bodies, thereby protecting aquatic ecosystems and reducing the risk of bioaccumulation in the food chain. This positive impact extends to both freshwater and marine environments, potentially mitigating the effects of water pollution on biodiversity.
The use of MISH also presents advantages in terms of resource conservation. As a naturally occurring mineral, its extraction and processing generally have a lower environmental footprint compared to synthetic adsorbents. Additionally, the high adsorption capacity of MISH means that smaller quantities are required to achieve the same treatment results, potentially reducing the overall material consumption in wastewater treatment processes.
However, the environmental impact assessment must also consider potential drawbacks. The mining and processing of MISH, while less intensive than some alternatives, still involves energy consumption and potential habitat disruption. The long-term fate of spent MISH adsorbents after use in wastewater treatment also requires consideration, as improper disposal could lead to secondary environmental issues.
Another aspect to evaluate is the energy efficiency of MISH-based treatment systems. While the material itself is passive, the overall treatment process may require energy for pumping, mixing, and separation. Comparative studies with other treatment technologies are necessary to determine if MISH applications offer net energy savings and reduced carbon footprints.
The potential for MISH to be regenerated and reused in multiple treatment cycles is an important factor in its environmental assessment. If effective regeneration methods can be developed, it would significantly extend the lifecycle of the material, reducing waste and the need for continuous raw material extraction. This aspect could greatly enhance the overall sustainability of MISH-based wastewater treatment systems.
Lastly, the environmental impact assessment should consider the broader implications of widespread MISH adoption in wastewater treatment. This includes evaluating the potential for reducing the use of chemical treatments, which often have their own environmental concerns, and the possibility of achieving higher water quality standards that could positively impact public health and ecosystem integrity.
One of the primary environmental benefits of MISH in wastewater treatment is its ability to reduce the concentration of harmful substances in effluents. By effectively removing pollutants, MISH helps prevent these contaminants from entering natural water bodies, thereby protecting aquatic ecosystems and reducing the risk of bioaccumulation in the food chain. This positive impact extends to both freshwater and marine environments, potentially mitigating the effects of water pollution on biodiversity.
The use of MISH also presents advantages in terms of resource conservation. As a naturally occurring mineral, its extraction and processing generally have a lower environmental footprint compared to synthetic adsorbents. Additionally, the high adsorption capacity of MISH means that smaller quantities are required to achieve the same treatment results, potentially reducing the overall material consumption in wastewater treatment processes.
However, the environmental impact assessment must also consider potential drawbacks. The mining and processing of MISH, while less intensive than some alternatives, still involves energy consumption and potential habitat disruption. The long-term fate of spent MISH adsorbents after use in wastewater treatment also requires consideration, as improper disposal could lead to secondary environmental issues.
Another aspect to evaluate is the energy efficiency of MISH-based treatment systems. While the material itself is passive, the overall treatment process may require energy for pumping, mixing, and separation. Comparative studies with other treatment technologies are necessary to determine if MISH applications offer net energy savings and reduced carbon footprints.
The potential for MISH to be regenerated and reused in multiple treatment cycles is an important factor in its environmental assessment. If effective regeneration methods can be developed, it would significantly extend the lifecycle of the material, reducing waste and the need for continuous raw material extraction. This aspect could greatly enhance the overall sustainability of MISH-based wastewater treatment systems.
Lastly, the environmental impact assessment should consider the broader implications of widespread MISH adoption in wastewater treatment. This includes evaluating the potential for reducing the use of chemical treatments, which often have their own environmental concerns, and the possibility of achieving higher water quality standards that could positively impact public health and ecosystem integrity.
Regulatory Framework for Water Treatment
The regulatory framework for water treatment plays a crucial role in ensuring the safe and effective use of technologies like magnesium iron silicate hydroxide (MISH) in wastewater treatment. This framework encompasses a complex web of laws, regulations, and standards at various levels of governance.
At the international level, organizations such as the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) provide guidelines and recommendations for water quality and treatment standards. These serve as a foundation for many national and regional regulatory bodies in developing their own frameworks.
In the United States, the Environmental Protection Agency (EPA) is the primary federal agency responsible for regulating water treatment. The Clean Water Act (CWA) and the Safe Drinking Water Act (SDWA) form the cornerstone of the U.S. regulatory framework. These acts establish water quality standards, effluent limitations, and treatment requirements for various pollutants.
The European Union has implemented the Water Framework Directive (WFD) and the Urban Waste Water Treatment Directive, which set comprehensive standards for water quality and treatment across member states. These directives require the implementation of best available techniques (BAT) in wastewater treatment processes.
Many countries have their own regulatory bodies and legislation governing water treatment. For instance, in China, the Ministry of Ecology and Environment oversees water quality regulations, while in India, the Central Pollution Control Board plays a similar role.
Specific to the application of MISH in wastewater treatment, regulatory frameworks typically address several key aspects. These include the permissible levels of contaminants in treated water, the safety and efficacy of treatment methods, and the disposal of waste products generated during the treatment process.
Regulatory bodies often require extensive testing and validation of new treatment technologies before approval for widespread use. This process may involve pilot studies, environmental impact assessments, and toxicological evaluations to ensure that the use of MISH does not introduce new risks to human health or the environment.
Furthermore, regulations often mandate regular monitoring and reporting of water quality parameters to ensure ongoing compliance. This may include testing for specific contaminants that MISH is designed to remove, as well as monitoring for any potential byproducts of the treatment process.
As environmental concerns and scientific understanding evolve, regulatory frameworks for water treatment continue to adapt. Emerging contaminants, such as pharmaceuticals and microplastics, are increasingly becoming subjects of regulatory scrutiny, potentially influencing the development and application of technologies like MISH in wastewater treatment.
At the international level, organizations such as the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) provide guidelines and recommendations for water quality and treatment standards. These serve as a foundation for many national and regional regulatory bodies in developing their own frameworks.
In the United States, the Environmental Protection Agency (EPA) is the primary federal agency responsible for regulating water treatment. The Clean Water Act (CWA) and the Safe Drinking Water Act (SDWA) form the cornerstone of the U.S. regulatory framework. These acts establish water quality standards, effluent limitations, and treatment requirements for various pollutants.
The European Union has implemented the Water Framework Directive (WFD) and the Urban Waste Water Treatment Directive, which set comprehensive standards for water quality and treatment across member states. These directives require the implementation of best available techniques (BAT) in wastewater treatment processes.
Many countries have their own regulatory bodies and legislation governing water treatment. For instance, in China, the Ministry of Ecology and Environment oversees water quality regulations, while in India, the Central Pollution Control Board plays a similar role.
Specific to the application of MISH in wastewater treatment, regulatory frameworks typically address several key aspects. These include the permissible levels of contaminants in treated water, the safety and efficacy of treatment methods, and the disposal of waste products generated during the treatment process.
Regulatory bodies often require extensive testing and validation of new treatment technologies before approval for widespread use. This process may involve pilot studies, environmental impact assessments, and toxicological evaluations to ensure that the use of MISH does not introduce new risks to human health or the environment.
Furthermore, regulations often mandate regular monitoring and reporting of water quality parameters to ensure ongoing compliance. This may include testing for specific contaminants that MISH is designed to remove, as well as monitoring for any potential byproducts of the treatment process.
As environmental concerns and scientific understanding evolve, regulatory frameworks for water treatment continue to adapt. Emerging contaminants, such as pharmaceuticals and microplastics, are increasingly becoming subjects of regulatory scrutiny, potentially influencing the development and application of technologies like MISH in wastewater treatment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!