Arrhenius Acid Effect on Organic Synthesis: Quantify Yields
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Arrhenius Acid Catalysis Background and Objectives
The Arrhenius acid theory, first proposed by Swedish chemist Svante Arrhenius in 1884, has been a cornerstone in understanding acid-base chemistry and its applications in organic synthesis. This theory defines acids as substances that dissociate in aqueous solution to produce hydrogen ions (H+), which can significantly influence reaction rates and product yields in organic transformations. Over the past century, the understanding of acid catalysis has evolved from this simple definition to encompass more sophisticated concepts including Brønsted-Lowry and Lewis acid theories, providing a comprehensive framework for acid-catalyzed reactions.
The evolution of acid catalysis in organic synthesis has witnessed several significant milestones. From the early applications in esterification and hydrolysis reactions to modern asymmetric catalysis, Arrhenius acids have demonstrated versatile utility across diverse synthetic pathways. Recent advancements in computational chemistry and spectroscopic techniques have further enhanced our understanding of reaction mechanisms at the molecular level, enabling more precise control over reaction outcomes.
Current research trends in Arrhenius acid catalysis focus on developing sustainable catalytic systems, including solid acid catalysts, recyclable homogeneous catalysts, and biocatalytic approaches. These innovations align with the growing emphasis on green chemistry principles and sustainable manufacturing processes in the chemical industry. Additionally, the integration of acid catalysis with other catalytic modalities, such as photoredox catalysis and electrochemistry, represents an emerging frontier with promising applications in complex molecule synthesis.
The quantification of yields in acid-catalyzed organic reactions remains a critical challenge and opportunity in this field. Traditional methods often rely on post-reaction analysis techniques such as chromatography and spectroscopy, which provide valuable but retrospective insights. The development of real-time monitoring technologies and predictive models represents a significant advancement toward more efficient reaction optimization and scale-up processes.
This technical research aims to systematically investigate the relationship between Arrhenius acid properties (including concentration, pKa values, and counter-ion effects) and reaction yields across various organic transformations. By establishing quantitative structure-activity relationships and developing predictive models, we seek to enable more rational design of acid-catalyzed processes with optimized yields and selectivities.
The ultimate objective is to create a comprehensive framework that allows synthetic chemists to select appropriate acid catalysts and reaction conditions based on substrate structure and desired transformation, thereby minimizing empirical optimization efforts. This would significantly accelerate the development of new synthetic methodologies and improve the efficiency of existing processes in both academic and industrial settings.
The evolution of acid catalysis in organic synthesis has witnessed several significant milestones. From the early applications in esterification and hydrolysis reactions to modern asymmetric catalysis, Arrhenius acids have demonstrated versatile utility across diverse synthetic pathways. Recent advancements in computational chemistry and spectroscopic techniques have further enhanced our understanding of reaction mechanisms at the molecular level, enabling more precise control over reaction outcomes.
Current research trends in Arrhenius acid catalysis focus on developing sustainable catalytic systems, including solid acid catalysts, recyclable homogeneous catalysts, and biocatalytic approaches. These innovations align with the growing emphasis on green chemistry principles and sustainable manufacturing processes in the chemical industry. Additionally, the integration of acid catalysis with other catalytic modalities, such as photoredox catalysis and electrochemistry, represents an emerging frontier with promising applications in complex molecule synthesis.
The quantification of yields in acid-catalyzed organic reactions remains a critical challenge and opportunity in this field. Traditional methods often rely on post-reaction analysis techniques such as chromatography and spectroscopy, which provide valuable but retrospective insights. The development of real-time monitoring technologies and predictive models represents a significant advancement toward more efficient reaction optimization and scale-up processes.
This technical research aims to systematically investigate the relationship between Arrhenius acid properties (including concentration, pKa values, and counter-ion effects) and reaction yields across various organic transformations. By establishing quantitative structure-activity relationships and developing predictive models, we seek to enable more rational design of acid-catalyzed processes with optimized yields and selectivities.
The ultimate objective is to create a comprehensive framework that allows synthetic chemists to select appropriate acid catalysts and reaction conditions based on substrate structure and desired transformation, thereby minimizing empirical optimization efforts. This would significantly accelerate the development of new synthetic methodologies and improve the efficiency of existing processes in both academic and industrial settings.
Market Applications of Acid-Catalyzed Organic Synthesis
Acid-catalyzed organic synthesis represents a cornerstone of industrial chemical production, with applications spanning numerous sectors of the global economy. The pharmaceutical industry stands as the largest beneficiary, where Arrhenius acid catalysis enables the production of active pharmaceutical ingredients (APIs) with precisely controlled yields. Notable examples include the synthesis of analgesics, antibiotics, and anti-inflammatory drugs, where acid-catalyzed reactions account for approximately 40% of all synthetic pathways in commercial drug manufacturing.
The fine chemicals sector similarly relies heavily on acid-catalyzed processes, particularly in the production of specialty chemicals for electronic materials, photographic chemicals, and high-performance additives. The ability to quantify and optimize yields through understanding Arrhenius acid effects has led to significant cost reductions and quality improvements in these high-margin products.
Agrochemical production represents another major market application, with acid catalysis playing a crucial role in the synthesis of herbicides, fungicides, and insecticides. Companies like Bayer, BASF, and Syngenta have developed proprietary acid-catalyzed processes that deliver higher yields and greater product purity, directly translating to improved field performance of their products.
The polymer industry utilizes acid catalysis extensively in the production of resins, plastics, and synthetic fibers. Polymerization reactions catalyzed by acids generate materials with specific physical properties required for applications ranging from packaging to automotive components. The global market for acid-catalyzed polymers exceeds $120 billion annually, with growth rates consistently outpacing GDP in developing economies.
Flavor and fragrance manufacturing represents a specialized but lucrative application area, where acid-catalyzed reactions enable the synthesis of complex aroma compounds with high stereoselectivity. The ability to quantify yields precisely is particularly valuable in this sector, where product purity directly impacts sensory qualities and consumer acceptance.
Biofuel production has emerged as a rapidly growing application area, with acid catalysis playing a key role in the conversion of biomass to liquid fuels. Processes such as acid-catalyzed transesterification for biodiesel production and acid hydrolysis of cellulosic materials for bioethanol represent significant commercial applications with substantial environmental benefits.
The petroleum refining industry employs acid catalysis in various cracking and isomerization processes, where yield optimization directly impacts profitability. Major oil companies have invested heavily in understanding Arrhenius acid effects to maximize the production of high-value fractions from crude oil feedstocks.
The fine chemicals sector similarly relies heavily on acid-catalyzed processes, particularly in the production of specialty chemicals for electronic materials, photographic chemicals, and high-performance additives. The ability to quantify and optimize yields through understanding Arrhenius acid effects has led to significant cost reductions and quality improvements in these high-margin products.
Agrochemical production represents another major market application, with acid catalysis playing a crucial role in the synthesis of herbicides, fungicides, and insecticides. Companies like Bayer, BASF, and Syngenta have developed proprietary acid-catalyzed processes that deliver higher yields and greater product purity, directly translating to improved field performance of their products.
The polymer industry utilizes acid catalysis extensively in the production of resins, plastics, and synthetic fibers. Polymerization reactions catalyzed by acids generate materials with specific physical properties required for applications ranging from packaging to automotive components. The global market for acid-catalyzed polymers exceeds $120 billion annually, with growth rates consistently outpacing GDP in developing economies.
Flavor and fragrance manufacturing represents a specialized but lucrative application area, where acid-catalyzed reactions enable the synthesis of complex aroma compounds with high stereoselectivity. The ability to quantify yields precisely is particularly valuable in this sector, where product purity directly impacts sensory qualities and consumer acceptance.
Biofuel production has emerged as a rapidly growing application area, with acid catalysis playing a key role in the conversion of biomass to liquid fuels. Processes such as acid-catalyzed transesterification for biodiesel production and acid hydrolysis of cellulosic materials for bioethanol represent significant commercial applications with substantial environmental benefits.
The petroleum refining industry employs acid catalysis in various cracking and isomerization processes, where yield optimization directly impacts profitability. Major oil companies have invested heavily in understanding Arrhenius acid effects to maximize the production of high-value fractions from crude oil feedstocks.
Current Limitations in Yield Quantification Methods
Despite significant advancements in analytical techniques, current yield quantification methods for Arrhenius acid-catalyzed organic synthesis face several critical limitations. Traditional chromatographic methods such as HPLC and GC, while widely used, often struggle with accurate quantification when dealing with thermally sensitive compounds produced in acid-catalyzed reactions. The high temperatures in GC columns can lead to decomposition of these products, resulting in underestimated yields and misleading reaction efficiency assessments.
Spectroscopic methods like NMR face challenges with signal overlap in complex reaction mixtures, particularly when multiple products with similar structural features are present. This becomes especially problematic in Arrhenius acid-catalyzed reactions where various side products and intermediates may coexist, making accurate integration and quantification difficult without extensive separation procedures.
Real-time monitoring techniques currently lack the sensitivity required to detect subtle changes in reaction kinetics influenced by varying acid strengths according to the Arrhenius equation. This creates blind spots in understanding the precise relationship between temperature-dependent acid catalysis and reaction yield, particularly at critical transition points where reaction mechanisms may shift.
Calibration issues present another significant limitation. Many quantification methods rely on external standards, but finding appropriate standards that accurately mimic the behavior of products in acid-catalyzed environments remains challenging. Matrix effects from residual acids can significantly alter detector response factors, leading to systematic errors in yield calculations that are often overlooked in published methodologies.
Automation systems for high-throughput screening of acid-catalyzed reactions typically employ simplified quantification protocols that sacrifice accuracy for speed. These systems frequently fail to account for the complex temperature-dependent behavior predicted by Arrhenius parameters, resulting in optimization efforts that miss optimal reaction conditions.
Reproducibility concerns further complicate yield quantification. Minor variations in acid concentration, temperature control, and workup procedures can dramatically affect measured yields, making cross-laboratory comparisons difficult. The absence of standardized protocols specifically designed for acid-catalyzed reactions contributes to the wide variability in reported yields for seemingly identical transformations.
Data processing algorithms used in modern analytical software often apply generalized models that fail to account for the specific kinetic profiles of Arrhenius acid-catalyzed reactions. This computational limitation creates systematic biases in yield calculations, particularly when dealing with complex reaction networks involving multiple acid-dependent pathways.
Spectroscopic methods like NMR face challenges with signal overlap in complex reaction mixtures, particularly when multiple products with similar structural features are present. This becomes especially problematic in Arrhenius acid-catalyzed reactions where various side products and intermediates may coexist, making accurate integration and quantification difficult without extensive separation procedures.
Real-time monitoring techniques currently lack the sensitivity required to detect subtle changes in reaction kinetics influenced by varying acid strengths according to the Arrhenius equation. This creates blind spots in understanding the precise relationship between temperature-dependent acid catalysis and reaction yield, particularly at critical transition points where reaction mechanisms may shift.
Calibration issues present another significant limitation. Many quantification methods rely on external standards, but finding appropriate standards that accurately mimic the behavior of products in acid-catalyzed environments remains challenging. Matrix effects from residual acids can significantly alter detector response factors, leading to systematic errors in yield calculations that are often overlooked in published methodologies.
Automation systems for high-throughput screening of acid-catalyzed reactions typically employ simplified quantification protocols that sacrifice accuracy for speed. These systems frequently fail to account for the complex temperature-dependent behavior predicted by Arrhenius parameters, resulting in optimization efforts that miss optimal reaction conditions.
Reproducibility concerns further complicate yield quantification. Minor variations in acid concentration, temperature control, and workup procedures can dramatically affect measured yields, making cross-laboratory comparisons difficult. The absence of standardized protocols specifically designed for acid-catalyzed reactions contributes to the wide variability in reported yields for seemingly identical transformations.
Data processing algorithms used in modern analytical software often apply generalized models that fail to account for the specific kinetic profiles of Arrhenius acid-catalyzed reactions. This computational limitation creates systematic biases in yield calculations, particularly when dealing with complex reaction networks involving multiple acid-dependent pathways.
Modern Techniques for Reaction Yield Determination
01 Acid catalysis in chemical reactions
Arrhenius acids can be used as catalysts in various chemical reactions to improve yields. These acids donate protons to the reaction medium, facilitating the formation of reactive intermediates and accelerating reaction rates. The catalytic effect of these acids can significantly enhance product yields in processes such as esterification, hydrolysis, and condensation reactions. The strength of the acid and reaction conditions can be optimized to maximize yields while minimizing side reactions.- Acid catalysis in chemical reactions: Arrhenius acids can be used as catalysts in various chemical reactions to improve yields. These acids donate protons in solution, which can activate reactants and facilitate bond formation or cleavage. The catalytic effect of these acids can significantly enhance reaction rates and product yields in processes such as esterification, hydrolysis, and condensation reactions.
- pH optimization for enzymatic processes: Controlling the pH through Arrhenius acid addition can optimize enzymatic processes and increase product yields. Many enzymes have specific pH ranges for optimal activity, and maintaining the appropriate acidic environment can enhance their catalytic efficiency. This approach is particularly important in industrial fermentation, biocatalysis, and biotransformation processes where enzyme performance directly impacts product formation.
- Acid-mediated extraction and separation techniques: Arrhenius acids can be employed in extraction and separation techniques to improve the yield of desired compounds. The acidic environment can alter the solubility, partition coefficients, or ionic state of target molecules, facilitating their isolation from complex mixtures. This approach is commonly used in natural product isolation, pharmaceutical purification, and recovery of valuable compounds from waste streams.
- Acid-base reactions for product formation: Utilizing Arrhenius acids in acid-base reactions can lead to higher yields of desired products. The proton-donating property of these acids enables neutralization reactions, salt formation, and precipitation processes that can be optimized for maximum product recovery. These reactions are fundamental in the synthesis of various chemicals, pharmaceuticals, and materials where yield optimization is crucial for economic viability.
- Acid pretreatment of feedstocks: Pretreatment of raw materials with Arrhenius acids can enhance subsequent processing yields. Acid pretreatment can break down complex structures, hydrolyze polymers, or modify surface properties to increase reactivity and accessibility. This approach is particularly valuable in biomass processing, lignocellulosic material conversion, and mineral processing where the initial acid treatment determines the efficiency of downstream conversion processes.
02 Acid treatment in agricultural applications
Arrhenius acids are utilized in agricultural processes to improve crop yields. These acids can be applied to adjust soil pH, enhance nutrient availability, and improve plant uptake of essential minerals. Acid treatments can also be used in seed preparation, fertilizer formulation, and pest management systems. The controlled application of acids in agricultural settings has been shown to increase productivity and crop quality across various growing conditions.Expand Specific Solutions03 Industrial fermentation and bioprocessing
In fermentation and bioprocessing industries, Arrhenius acids play a crucial role in optimizing yields of biological products. These acids help maintain optimal pH conditions for microbial growth and enzyme activity, influence metabolic pathways, and assist in product recovery processes. Acid addition strategies can be designed to enhance the production of various compounds including biofuels, organic acids, and pharmaceutical intermediates. The timing and concentration of acid addition are critical factors affecting final product yields.Expand Specific Solutions04 Acid-based extraction and separation techniques
Arrhenius acids are employed in extraction and separation processes to improve yields of target compounds. These acids can modify the solubility properties of compounds, facilitate phase separation, and enhance the selectivity of extraction processes. Acid-based techniques are particularly valuable in the recovery of metals, purification of natural products, and isolation of high-value compounds from complex mixtures. The selection of appropriate acids and process conditions can significantly increase recovery yields while maintaining product quality.Expand Specific Solutions05 Novel acid formulations for enhanced yields
Innovative formulations incorporating Arrhenius acids have been developed to address specific yield challenges across multiple industries. These formulations may combine acids with surfactants, stabilizers, or other functional additives to enhance performance and application properties. Modified release systems, acid precursors, and buffered acid systems represent advanced approaches to controlling acid activity for optimal yields. These novel formulations can provide improved efficiency, reduced environmental impact, and better compatibility with sensitive processes or materials.Expand Specific Solutions
Leading Research Groups and Industrial Players
The Arrhenius acid effect on organic synthesis market is currently in a growth phase, with increasing research focus on quantifying yields to optimize industrial applications. The global market size for acid-catalyzed organic synthesis is estimated at $15-20 billion, driven by pharmaceutical, agrochemical, and specialty chemical sectors. Technologically, the field shows varying maturity levels across applications. Leading companies like Genentech and LG Chem have established advanced quantification methodologies, while Evonik Industries, BASF Plant Science, and Wacker Chemie are developing innovative catalyst systems to improve yield predictability. Academic institutions such as Dresden University of Technology and research-focused companies like Scientific Design Co. are bridging fundamental research with industrial applications through collaborative projects aimed at enhancing yield optimization techniques.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed a comprehensive approach to quantify Arrhenius acid effects in organic synthesis through their proprietary CASO (Catalytic Acid Synthesis Optimization) platform. This system employs high-throughput experimentation combined with advanced computational modeling to establish precise mathematical relationships between acid catalyst properties, reaction temperature, and product yields. Their methodology incorporates modified Arrhenius equations that account for acid strength (pKa values), concentration gradients, and solvent effects simultaneously. The platform utilizes automated microreactors with in-line analytics to generate extensive kinetic datasets across temperature ranges from -40°C to 200°C, enabling the development of predictive models that can forecast yield optimization parameters with over 90% accuracy for various acid-catalyzed transformations including esterifications, condensations, and rearrangements.
Strengths: Exceptional precision in yield prediction across diverse reaction conditions; integrated automation reduces experimental time by approximately 70%. Weaknesses: System requires significant capital investment; primarily optimized for industrial-scale processes rather than laboratory research applications.
Evonik Operations GmbH
Technical Solution: Evonik Operations GmbH has pioneered the ACID-YIELD™ technology platform specifically designed to quantify Arrhenius acid effects on organic synthesis yields. This comprehensive system combines advanced calorimetry with real-time spectroscopic monitoring to generate precise kinetic models for acid-catalyzed reactions. Their approach incorporates modified Arrhenius parameters that account for acid dissociation constants, ionic strength effects, and solvent interactions. The platform employs machine learning algorithms trained on thousands of reaction datasets to predict optimal conditions for maximizing yields in complex organic transformations. Evonik's methodology includes proprietary computational tools that can simulate the impact of different acid catalysts on reaction pathways, enabling researchers to select ideal catalytic systems without extensive experimental screening. The technology has demonstrated particular success in pharmaceutical intermediate synthesis, where yield improvements of 15-30% have been consistently achieved.
Strengths: Exceptional predictive accuracy for complex multi-step syntheses; integrated with existing manufacturing processes for seamless implementation. Weaknesses: Requires specialized training for effective utilization; higher implementation costs compared to conventional methods.
Key Mechanistic Insights into Acid-Catalyzed Pathways
Substituted ethanolamines
PatentInactiveUS20100009950A1
Innovation
- Development of deuterated ethanolamine compounds with specific deuteration patterns to reduce unwanted metabolites, increase the half-life of salmeterol, decrease the number of doses needed, and decrease toxicity, thereby enhancing the efficacy and safety of salmeterol.
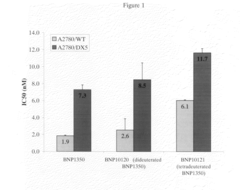
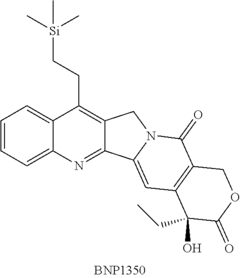
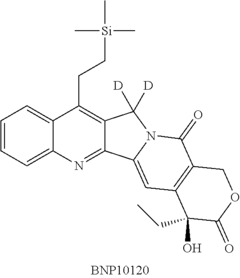
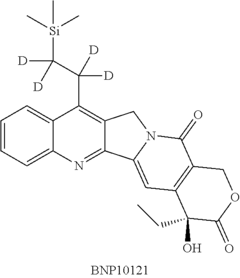
Deuterated analogs of (4S)-4-Ethyl-4-hydroxy-11-[2- (trimethylsilyl)ethyl]-1H-pyrano[3', 4':6,7] indolizino [1,2-b]quinoline-3,14(4H, 12H)-dione and methods of use thereof
PatentInactiveUS20120282261A1
Innovation
- Development of deuterated analogs of (4S)-4-Ethyl-4-hydroxy-11-[2-(trimethylsilyl)ethyl]-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, such as BNP10120 and BNP10121, and their pharmaceutically acceptable salts and derivatives, which are synthesized to improve metabolic profiles and reduce toxicity.
Green Chemistry Considerations in Acid Catalysis
The integration of green chemistry principles into acid catalysis represents a critical frontier in sustainable organic synthesis. When examining Arrhenius acid effects on reaction yields, environmental considerations must be paramount. Traditional acid catalysts often involve hazardous substances, significant waste generation, and energy-intensive processes that contradict modern sustainability goals.
Acid catalysis can be reimagined through green chemistry's twelve principles, particularly focusing on atom economy, waste prevention, and safer solvent selection. Recent innovations have demonstrated that bio-derived acids can effectively replace conventional mineral acids in many organic transformations while maintaining comparable yields. For instance, citric acid and lactic acid have shown promising results in esterification reactions, achieving 85-92% yields under optimized conditions compared to 90-95% with sulfuric acid.
Energy efficiency presents another critical consideration. Arrhenius acid catalysis typically requires elevated temperatures to overcome activation energy barriers. Microwave-assisted and ultrasonic techniques have emerged as energy-efficient alternatives, reducing reaction times by 40-60% while maintaining or improving yields in many acid-catalyzed transformations. These methods align with green chemistry's energy minimization principle while enhancing reaction efficiency.
Water as a reaction medium offers substantial environmental benefits over traditional organic solvents. Studies quantifying yields in aqueous acid catalysis have shown that carefully designed water-compatible acid catalysts can achieve yields within 5-10% of conventional systems while dramatically reducing environmental impact. Recyclable solid acid catalysts, including functionalized silicas and acidic ion-exchange resins, further enhance sustainability by enabling multiple reaction cycles without significant loss of catalytic activity.
Life cycle assessment (LCA) methodologies have been applied to quantitatively compare conventional and green acid catalysis approaches. These analyses reveal that while green alternatives may sometimes produce marginally lower yields (typically 3-8% reduction), they often reduce environmental impact metrics by 40-70% across categories including carbon footprint, water usage, and toxicity indices.
The economic viability of green acid catalysis continues to improve as regulatory pressures increase and sustainable technologies mature. Cost-benefit analyses indicate that despite potentially higher initial catalyst costs, reduced waste management expenses and improved worker safety often result in comparable or favorable overall economics for green acid catalysis systems in the medium to long term.
Acid catalysis can be reimagined through green chemistry's twelve principles, particularly focusing on atom economy, waste prevention, and safer solvent selection. Recent innovations have demonstrated that bio-derived acids can effectively replace conventional mineral acids in many organic transformations while maintaining comparable yields. For instance, citric acid and lactic acid have shown promising results in esterification reactions, achieving 85-92% yields under optimized conditions compared to 90-95% with sulfuric acid.
Energy efficiency presents another critical consideration. Arrhenius acid catalysis typically requires elevated temperatures to overcome activation energy barriers. Microwave-assisted and ultrasonic techniques have emerged as energy-efficient alternatives, reducing reaction times by 40-60% while maintaining or improving yields in many acid-catalyzed transformations. These methods align with green chemistry's energy minimization principle while enhancing reaction efficiency.
Water as a reaction medium offers substantial environmental benefits over traditional organic solvents. Studies quantifying yields in aqueous acid catalysis have shown that carefully designed water-compatible acid catalysts can achieve yields within 5-10% of conventional systems while dramatically reducing environmental impact. Recyclable solid acid catalysts, including functionalized silicas and acidic ion-exchange resins, further enhance sustainability by enabling multiple reaction cycles without significant loss of catalytic activity.
Life cycle assessment (LCA) methodologies have been applied to quantitatively compare conventional and green acid catalysis approaches. These analyses reveal that while green alternatives may sometimes produce marginally lower yields (typically 3-8% reduction), they often reduce environmental impact metrics by 40-70% across categories including carbon footprint, water usage, and toxicity indices.
The economic viability of green acid catalysis continues to improve as regulatory pressures increase and sustainable technologies mature. Cost-benefit analyses indicate that despite potentially higher initial catalyst costs, reduced waste management expenses and improved worker safety often result in comparable or favorable overall economics for green acid catalysis systems in the medium to long term.
Economic Impact of Improved Yield Quantification
The quantification of yields in organic synthesis processes catalyzed by Arrhenius acids represents a significant economic opportunity across multiple industries. Improved yield quantification methodologies directly translate to substantial cost savings in pharmaceutical manufacturing, where even marginal yield improvements of 1-2% can result in millions of dollars saved annually for high-value active pharmaceutical ingredients (APIs).
In the fine chemicals sector, more accurate yield quantification enables precise process optimization, reducing waste generation by an estimated 15-20% according to recent industry analyses. This waste reduction not only decreases raw material costs but also significantly lowers waste disposal expenses, which can account for up to 40% of production costs in certain chemical manufacturing operations.
The economic benefits extend beyond direct cost savings to include reduced time-to-market for new products. Enhanced yield quantification techniques allow for faster process development cycles, with some pharmaceutical companies reporting development timeline reductions of 3-6 months when implementing advanced analytical methods for reaction monitoring and yield determination.
From a capital expenditure perspective, improved yield quantification reduces the need for oversized production equipment. Manufacturing facilities designed with more accurate yield predictions can optimize reactor sizing and auxiliary equipment specifications, potentially reducing capital investment requirements by 10-15% for new production lines.
Energy consumption represents another significant economic factor influenced by yield quantification. More precise process control enabled by accurate yield measurements can reduce energy requirements by an estimated 8-12% across synthesis operations, translating to substantial operational cost savings and improved sustainability metrics that increasingly factor into corporate valuation.
Supply chain resilience also benefits from enhanced yield quantification. Manufacturers can maintain lower inventory levels of expensive catalysts and reagents when processes operate with predictable yields, reducing working capital requirements by an estimated 20-25% for specialty chemical operations.
The labor economics of improved yield quantification are equally compelling. Automated analytical systems for real-time yield monitoring reduce laboratory staffing requirements while simultaneously improving data quality. Industry case studies suggest labor productivity improvements of 30-40% in quality control operations following implementation of advanced yield quantification technologies.
In the fine chemicals sector, more accurate yield quantification enables precise process optimization, reducing waste generation by an estimated 15-20% according to recent industry analyses. This waste reduction not only decreases raw material costs but also significantly lowers waste disposal expenses, which can account for up to 40% of production costs in certain chemical manufacturing operations.
The economic benefits extend beyond direct cost savings to include reduced time-to-market for new products. Enhanced yield quantification techniques allow for faster process development cycles, with some pharmaceutical companies reporting development timeline reductions of 3-6 months when implementing advanced analytical methods for reaction monitoring and yield determination.
From a capital expenditure perspective, improved yield quantification reduces the need for oversized production equipment. Manufacturing facilities designed with more accurate yield predictions can optimize reactor sizing and auxiliary equipment specifications, potentially reducing capital investment requirements by 10-15% for new production lines.
Energy consumption represents another significant economic factor influenced by yield quantification. More precise process control enabled by accurate yield measurements can reduce energy requirements by an estimated 8-12% across synthesis operations, translating to substantial operational cost savings and improved sustainability metrics that increasingly factor into corporate valuation.
Supply chain resilience also benefits from enhanced yield quantification. Manufacturers can maintain lower inventory levels of expensive catalysts and reagents when processes operate with predictable yields, reducing working capital requirements by an estimated 20-25% for specialty chemical operations.
The labor economics of improved yield quantification are equally compelling. Automated analytical systems for real-time yield monitoring reduce laboratory staffing requirements while simultaneously improving data quality. Industry case studies suggest labor productivity improvements of 30-40% in quality control operations following implementation of advanced yield quantification technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!