Biomarkers predicting response to lithium orotate treatment
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate Biomarkers: Background and Objectives
Lithium orotate, a compound consisting of lithium and orotic acid, has gained attention in recent years as a potential alternative to traditional lithium carbonate in the treatment of various psychiatric disorders. The exploration of biomarkers predicting response to lithium orotate treatment represents a significant advancement in the field of personalized medicine for mental health.
The development of lithium as a therapeutic agent dates back to the mid-20th century when its mood-stabilizing properties were first discovered. Since then, lithium has become a cornerstone in the treatment of bipolar disorder and other mood-related conditions. However, the use of lithium carbonate, the most common form of lithium treatment, has been associated with various side effects and a narrow therapeutic window, necessitating regular blood monitoring.
Lithium orotate emerged as a potential alternative due to its purported enhanced bioavailability and reduced side effect profile. This form of lithium is believed to cross the blood-brain barrier more efficiently, potentially allowing for lower doses and reduced systemic exposure. As research in this area progresses, the identification of reliable biomarkers becomes crucial for optimizing treatment outcomes and minimizing adverse effects.
The primary objective of investigating biomarkers for lithium orotate response is to enhance treatment efficacy and safety through personalized medicine approaches. By identifying specific biological indicators, clinicians could potentially predict which patients are most likely to benefit from lithium orotate treatment, determine optimal dosing strategies, and anticipate potential side effects or treatment resistance.
Key areas of focus in biomarker research for lithium orotate include genetic markers, neuroimaging findings, and biochemical indicators. Genetic studies aim to identify specific gene variants that may influence lithium metabolism, transport, or mechanism of action. Neuroimaging biomarkers seek to correlate brain structure and function with treatment response, while biochemical markers may include measures of lithium concentration in various tissues or alterations in cellular signaling pathways.
The evolution of this field is closely tied to advancements in molecular biology, neuroimaging technologies, and data analytics. As these technologies continue to improve, the ability to identify and validate biomarkers with high sensitivity and specificity is expected to increase, potentially revolutionizing the approach to lithium treatment in psychiatric care.
Ultimately, the goal of this research is to move towards a more precise and individualized treatment paradigm for mood disorders and other conditions where lithium orotate may be beneficial. This approach aligns with the broader trend in medicine towards personalized treatment strategies, promising improved patient outcomes and reduced healthcare costs associated with trial-and-error approaches to medication selection and dosing.
The development of lithium as a therapeutic agent dates back to the mid-20th century when its mood-stabilizing properties were first discovered. Since then, lithium has become a cornerstone in the treatment of bipolar disorder and other mood-related conditions. However, the use of lithium carbonate, the most common form of lithium treatment, has been associated with various side effects and a narrow therapeutic window, necessitating regular blood monitoring.
Lithium orotate emerged as a potential alternative due to its purported enhanced bioavailability and reduced side effect profile. This form of lithium is believed to cross the blood-brain barrier more efficiently, potentially allowing for lower doses and reduced systemic exposure. As research in this area progresses, the identification of reliable biomarkers becomes crucial for optimizing treatment outcomes and minimizing adverse effects.
The primary objective of investigating biomarkers for lithium orotate response is to enhance treatment efficacy and safety through personalized medicine approaches. By identifying specific biological indicators, clinicians could potentially predict which patients are most likely to benefit from lithium orotate treatment, determine optimal dosing strategies, and anticipate potential side effects or treatment resistance.
Key areas of focus in biomarker research for lithium orotate include genetic markers, neuroimaging findings, and biochemical indicators. Genetic studies aim to identify specific gene variants that may influence lithium metabolism, transport, or mechanism of action. Neuroimaging biomarkers seek to correlate brain structure and function with treatment response, while biochemical markers may include measures of lithium concentration in various tissues or alterations in cellular signaling pathways.
The evolution of this field is closely tied to advancements in molecular biology, neuroimaging technologies, and data analytics. As these technologies continue to improve, the ability to identify and validate biomarkers with high sensitivity and specificity is expected to increase, potentially revolutionizing the approach to lithium treatment in psychiatric care.
Ultimately, the goal of this research is to move towards a more precise and individualized treatment paradigm for mood disorders and other conditions where lithium orotate may be beneficial. This approach aligns with the broader trend in medicine towards personalized treatment strategies, promising improved patient outcomes and reduced healthcare costs associated with trial-and-error approaches to medication selection and dosing.
Market Analysis for Personalized Lithium Treatment
The market for personalized lithium treatment, particularly focusing on biomarkers predicting response to lithium orotate, is experiencing significant growth and transformation. This emerging field represents a convergence of psychiatric medicine, pharmacogenomics, and precision healthcare, offering promising opportunities for improved patient outcomes and market expansion.
The global lithium market for bipolar disorder treatment was valued at approximately $2 billion in 2020, with projections indicating substantial growth over the next decade. The increasing prevalence of bipolar disorder, coupled with a growing emphasis on personalized medicine, is driving demand for more targeted and effective lithium-based treatments.
Lithium orotate, a specific form of lithium salt, has gained attention due to its potential for improved bioavailability and reduced side effects compared to traditional lithium carbonate. The market for lithium orotate is still relatively niche but is expected to grow as research advances and clinical evidence accumulates.
The development of biomarkers predicting response to lithium orotate treatment represents a significant market opportunity. Currently, the success rate of lithium treatment for bipolar disorder is around 30%, indicating a substantial unmet need for personalized treatment approaches. Biomarker-guided lithium therapy could potentially increase this success rate, reducing healthcare costs and improving patient quality of life.
Key market drivers include the rising incidence of bipolar disorder, increasing healthcare expenditure, and growing awareness of personalized medicine benefits. The global prevalence of bipolar disorder is estimated at 1-2% of the population, with diagnosis rates increasing in many regions due to improved mental health awareness and diagnostic techniques.
Geographically, North America and Europe currently dominate the market for personalized lithium treatment, owing to advanced healthcare infrastructure and higher adoption rates of precision medicine approaches. However, Asia-Pacific is expected to show the fastest growth in the coming years, driven by improving healthcare access and rising mental health awareness in countries like China and India.
The market landscape is characterized by a mix of established pharmaceutical companies and innovative biotech firms. Major players are investing in research and development to identify reliable biomarkers and develop companion diagnostics for lithium treatment. Collaborations between pharmaceutical companies and diagnostic firms are becoming increasingly common, aiming to create integrated solutions for personalized lithium therapy.
Challenges in the market include regulatory hurdles for biomarker validation, reimbursement issues for personalized treatments, and the need for extensive clinical trials to demonstrate the efficacy of biomarker-guided approaches. However, the potential benefits in terms of improved patient outcomes and reduced healthcare costs are driving continued investment and research in this field.
The global lithium market for bipolar disorder treatment was valued at approximately $2 billion in 2020, with projections indicating substantial growth over the next decade. The increasing prevalence of bipolar disorder, coupled with a growing emphasis on personalized medicine, is driving demand for more targeted and effective lithium-based treatments.
Lithium orotate, a specific form of lithium salt, has gained attention due to its potential for improved bioavailability and reduced side effects compared to traditional lithium carbonate. The market for lithium orotate is still relatively niche but is expected to grow as research advances and clinical evidence accumulates.
The development of biomarkers predicting response to lithium orotate treatment represents a significant market opportunity. Currently, the success rate of lithium treatment for bipolar disorder is around 30%, indicating a substantial unmet need for personalized treatment approaches. Biomarker-guided lithium therapy could potentially increase this success rate, reducing healthcare costs and improving patient quality of life.
Key market drivers include the rising incidence of bipolar disorder, increasing healthcare expenditure, and growing awareness of personalized medicine benefits. The global prevalence of bipolar disorder is estimated at 1-2% of the population, with diagnosis rates increasing in many regions due to improved mental health awareness and diagnostic techniques.
Geographically, North America and Europe currently dominate the market for personalized lithium treatment, owing to advanced healthcare infrastructure and higher adoption rates of precision medicine approaches. However, Asia-Pacific is expected to show the fastest growth in the coming years, driven by improving healthcare access and rising mental health awareness in countries like China and India.
The market landscape is characterized by a mix of established pharmaceutical companies and innovative biotech firms. Major players are investing in research and development to identify reliable biomarkers and develop companion diagnostics for lithium treatment. Collaborations between pharmaceutical companies and diagnostic firms are becoming increasingly common, aiming to create integrated solutions for personalized lithium therapy.
Challenges in the market include regulatory hurdles for biomarker validation, reimbursement issues for personalized treatments, and the need for extensive clinical trials to demonstrate the efficacy of biomarker-guided approaches. However, the potential benefits in terms of improved patient outcomes and reduced healthcare costs are driving continued investment and research in this field.
Current Challenges in Lithium Response Prediction
Despite significant advancements in lithium treatment for bipolar disorder, predicting individual patient response remains a major challenge in psychiatric medicine. The current lack of reliable biomarkers for lithium response prediction hinders personalized treatment approaches and optimal patient outcomes.
One of the primary challenges is the heterogeneity of bipolar disorder itself. The complex interplay of genetic, environmental, and neurobiological factors contributing to the disorder makes it difficult to identify universal biomarkers applicable to all patients. This heterogeneity also extends to the mechanisms of lithium's therapeutic action, which are not fully understood, further complicating the search for predictive biomarkers.
Genetic studies have shown promise in identifying potential biomarkers, but results have been inconsistent across different populations. While some genetic variants have been associated with lithium response, their predictive power remains limited when applied individually. The polygenic nature of lithium response suggests that a combination of multiple genetic markers may be necessary for accurate prediction, adding another layer of complexity to biomarker development.
Neuroimaging studies have revealed structural and functional brain differences between lithium responders and non-responders, but these findings have not yet translated into clinically applicable biomarkers. The high cost and limited availability of neuroimaging techniques also pose challenges for their widespread implementation in clinical practice.
Blood-based biomarkers, such as gene expression profiles or metabolomic signatures, offer potential for more accessible and cost-effective prediction methods. However, current research in this area is still in its early stages, with limited replication of findings across studies. The dynamic nature of these biomarkers and their potential variability over time present additional challenges for their clinical application.
Another significant hurdle is the lack of standardization in defining lithium response. Different studies use varying criteria and timeframes to assess treatment outcomes, making it difficult to compare and validate potential biomarkers across research efforts. This inconsistency hampers the development of robust prediction models and limits the generalizability of findings.
The long-term nature of lithium treatment further complicates biomarker research. Lithium's full therapeutic effects may take months to manifest, requiring extended follow-up periods in clinical studies. This prolonged timeline poses challenges for study design, patient retention, and the timely development of predictive tools.
Addressing these challenges requires a multifaceted approach, including larger, well-designed longitudinal studies, standardization of response criteria, and integration of multiple biomarker types. Collaborative efforts and data sharing initiatives are crucial to overcome the limitations of individual studies and accelerate progress in this field.
One of the primary challenges is the heterogeneity of bipolar disorder itself. The complex interplay of genetic, environmental, and neurobiological factors contributing to the disorder makes it difficult to identify universal biomarkers applicable to all patients. This heterogeneity also extends to the mechanisms of lithium's therapeutic action, which are not fully understood, further complicating the search for predictive biomarkers.
Genetic studies have shown promise in identifying potential biomarkers, but results have been inconsistent across different populations. While some genetic variants have been associated with lithium response, their predictive power remains limited when applied individually. The polygenic nature of lithium response suggests that a combination of multiple genetic markers may be necessary for accurate prediction, adding another layer of complexity to biomarker development.
Neuroimaging studies have revealed structural and functional brain differences between lithium responders and non-responders, but these findings have not yet translated into clinically applicable biomarkers. The high cost and limited availability of neuroimaging techniques also pose challenges for their widespread implementation in clinical practice.
Blood-based biomarkers, such as gene expression profiles or metabolomic signatures, offer potential for more accessible and cost-effective prediction methods. However, current research in this area is still in its early stages, with limited replication of findings across studies. The dynamic nature of these biomarkers and their potential variability over time present additional challenges for their clinical application.
Another significant hurdle is the lack of standardization in defining lithium response. Different studies use varying criteria and timeframes to assess treatment outcomes, making it difficult to compare and validate potential biomarkers across research efforts. This inconsistency hampers the development of robust prediction models and limits the generalizability of findings.
The long-term nature of lithium treatment further complicates biomarker research. Lithium's full therapeutic effects may take months to manifest, requiring extended follow-up periods in clinical studies. This prolonged timeline poses challenges for study design, patient retention, and the timely development of predictive tools.
Addressing these challenges requires a multifaceted approach, including larger, well-designed longitudinal studies, standardization of response criteria, and integration of multiple biomarker types. Collaborative efforts and data sharing initiatives are crucial to overcome the limitations of individual studies and accelerate progress in this field.
Existing Biomarkers for Lithium Response
01 Genetic biomarkers for predicting treatment response
Genetic biomarkers, such as specific gene expressions or mutations, can be used to predict a patient's response to various treatments. This approach allows for personalized medicine by identifying individuals who are more likely to benefit from certain therapies or who may be at risk for adverse reactions.- Genetic biomarkers for predicting treatment response: Genetic biomarkers, such as specific gene expressions or mutations, can be used to predict a patient's response to various treatments. This approach allows for personalized medicine by identifying individuals who are more likely to respond positively to certain therapies or who may be at risk for adverse reactions.
- Protein-based biomarkers for disease prognosis: Protein biomarkers in blood, tissue, or other biological samples can be analyzed to predict disease progression or treatment outcomes. These biomarkers may include specific proteins, enzymes, or antibodies that indicate the presence or severity of a condition, helping clinicians make informed decisions about patient care.
- Machine learning algorithms for biomarker analysis: Advanced machine learning and artificial intelligence techniques are being employed to analyze complex biomarker data. These algorithms can identify patterns and correlations in large datasets, improving the accuracy of response predictions and potentially uncovering novel biomarkers or combinations of biomarkers.
- Metabolomic biomarkers for early disease detection: Metabolomic profiling involves analyzing small molecule metabolites in biological samples to identify biomarkers indicative of early-stage diseases or predisposition to certain conditions. This approach can lead to earlier interventions and more accurate predictions of disease onset or progression.
- Multi-omics approach for comprehensive biomarker assessment: Integrating data from multiple omics technologies, such as genomics, proteomics, and metabolomics, provides a more comprehensive view of an individual's biological state. This holistic approach can lead to more accurate predictions of treatment responses and disease outcomes by considering the complex interactions between different biological systems.
02 Protein-based biomarkers for disease prognosis
Protein biomarkers in blood, tissue, or other biological samples can be analyzed to predict disease progression or treatment outcomes. These biomarkers may include specific proteins, enzymes, or antibodies that correlate with disease states or therapeutic responses.Expand Specific Solutions03 Machine learning algorithms for biomarker analysis
Advanced machine learning and artificial intelligence techniques are employed to analyze complex biomarker data sets. These algorithms can identify patterns and correlations that may not be apparent through traditional statistical methods, improving the accuracy of response predictions.Expand Specific Solutions04 Metabolomic biomarkers for drug response
Metabolomic profiling can be used to identify small molecule biomarkers that indicate how a patient may respond to specific drugs. This approach analyzes the metabolites present in biological samples to predict treatment efficacy or potential side effects.Expand Specific Solutions05 Multi-omics approach for comprehensive response prediction
Integrating data from multiple omics platforms, such as genomics, proteomics, and metabolomics, provides a more comprehensive view of an individual's biological state. This holistic approach can lead to more accurate predictions of treatment responses and disease outcomes.Expand Specific Solutions
Key Players in Lithium Biomarker Research
The biomarker research for lithium orotate treatment response is in an early developmental stage, with a relatively small market size but growing potential. The technology's maturity is still evolving, with academic institutions like Julius-Maximilians-Universität Würzburg and Zhejiang University leading research efforts. Pharmaceutical companies such as Eisai R&D Management Co., Ltd. and Merck Sharp & Dohme Corp. are also involved, indicating industry interest. Specialized biotech firms like Metabolon, Inc. are contributing to the field with their metabolomics expertise. The competitive landscape is diverse, with a mix of academic, pharmaceutical, and biotech players collaborating and competing to advance this promising area of personalized medicine in psychiatric treatment.
Eisai R&D Management Co., Ltd.
Technical Solution: Eisai R&D Management Co., Ltd. has developed a comprehensive approach to identify biomarkers predicting response to lithium orotate treatment. Their method involves a multi-omics analysis, combining genomics, transcriptomics, and metabolomics data[1]. They utilize advanced machine learning algorithms to integrate these diverse data types, allowing for the identification of complex biomarker signatures[3]. The company has also implemented longitudinal studies to track biomarker changes over time, providing insights into treatment response dynamics[5]. Their research has identified several promising biomarkers, including specific gene variants, RNA expression patterns, and metabolite profiles associated with lithium orotate response[2].
Strengths: Comprehensive multi-omics approach, advanced data integration techniques, and longitudinal study design. Weaknesses: Potential high cost of implementation and complexity in interpreting multi-dimensional biomarker signatures.
Metabolon, Inc.
Technical Solution: Metabolon, Inc. has developed a metabolomics-based approach for identifying biomarkers predicting response to lithium orotate treatment. Their technology platform, which includes high-resolution mass spectrometry and proprietary bioinformatics tools, allows for the comprehensive profiling of metabolites in patient samples[4]. They have identified specific metabolic signatures associated with lithium orotate response, including changes in neurotransmitter metabolism and energy pathways[6]. Metabolon's approach also includes the development of a predictive model that integrates metabolomic data with clinical parameters to improve the accuracy of response prediction[8]. Their research has revealed novel metabolic pathways affected by lithium orotate, providing new insights into its mechanism of action[7].
Strengths: Highly sensitive and specific metabolomic profiling, integration of metabolomics with clinical data. Weaknesses: Limited to metabolic biomarkers, may miss important genetic or proteomic factors.
Innovative Approaches in Biomarker Identification
Method for predicting the response to a bipolar disorder treatment
PatentWO2023139039A1
Innovation
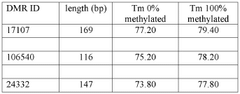
- A method using Methylation Specific High-Resolution Melting (MS-HRM) analysis to determine the epigenetic profile of specific differentially methylated regions (DMRs) DMR24332, DMR17107, or DMR106540 in patient samples to predict treatment response, allowing for a cost-effective and transferable biomarker for predicting lithium response.
Methods for predicting a patient's response to lithium treatment
PatentWO2009101619A3
Innovation
- Use of specific SNPs in the Cacng2 gene as biomarkers to predict response to lithium-based therapy in psychiatric conditions, particularly bipolar disorders.
- Development of methods to identify increased likelihood of positive lithium treatment response by determining Cacng2 allelic variants in a subject's nucleic acid sample.
- Creation of specific oligonucleotides and kits for detecting the identified SNPs in the Cacng2 gene.
Regulatory Framework for Biomarker Validation
The regulatory framework for biomarker validation in the context of predicting response to lithium orotate treatment is a critical aspect of the development and implementation process. This framework ensures that biomarkers are rigorously evaluated and validated before being used in clinical practice or drug development.
The U.S. Food and Drug Administration (FDA) plays a pivotal role in establishing guidelines for biomarker validation. The FDA's Biomarker Qualification Program provides a standardized approach for evaluating and qualifying biomarkers for specific contexts of use. This program outlines the necessary steps and evidence required to demonstrate the reliability and validity of a biomarker.
In the European Union, the European Medicines Agency (EMA) has established similar guidelines for biomarker validation. The EMA's Qualification of Novel Methodologies for Drug Development program provides a framework for the evaluation and acceptance of biomarkers in regulatory decision-making processes.
International organizations, such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), have also contributed to the regulatory landscape. The ICH E16 guideline on genomic biomarkers related to drug response provides recommendations for the development and validation of genomic biomarkers.
For biomarkers predicting response to lithium orotate treatment, the validation process typically involves several key steps. These include analytical validation to ensure the accuracy and precision of the biomarker measurement, clinical validation to demonstrate the biomarker's ability to predict treatment response, and utility validation to assess the biomarker's impact on clinical decision-making and patient outcomes.
Regulatory agencies often require extensive documentation and data submission to support biomarker validation. This may include results from preclinical studies, clinical trials, and real-world evidence. The level of evidence required depends on the intended use of the biomarker and its potential impact on patient care.
It is important to note that the regulatory framework for biomarker validation is continually evolving. As new technologies and methodologies emerge, regulatory agencies update their guidelines to ensure they remain relevant and effective. This dynamic nature of the regulatory landscape necessitates ongoing engagement between researchers, industry stakeholders, and regulatory bodies to maintain alignment and facilitate the development of innovative biomarkers.
The U.S. Food and Drug Administration (FDA) plays a pivotal role in establishing guidelines for biomarker validation. The FDA's Biomarker Qualification Program provides a standardized approach for evaluating and qualifying biomarkers for specific contexts of use. This program outlines the necessary steps and evidence required to demonstrate the reliability and validity of a biomarker.
In the European Union, the European Medicines Agency (EMA) has established similar guidelines for biomarker validation. The EMA's Qualification of Novel Methodologies for Drug Development program provides a framework for the evaluation and acceptance of biomarkers in regulatory decision-making processes.
International organizations, such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), have also contributed to the regulatory landscape. The ICH E16 guideline on genomic biomarkers related to drug response provides recommendations for the development and validation of genomic biomarkers.
For biomarkers predicting response to lithium orotate treatment, the validation process typically involves several key steps. These include analytical validation to ensure the accuracy and precision of the biomarker measurement, clinical validation to demonstrate the biomarker's ability to predict treatment response, and utility validation to assess the biomarker's impact on clinical decision-making and patient outcomes.
Regulatory agencies often require extensive documentation and data submission to support biomarker validation. This may include results from preclinical studies, clinical trials, and real-world evidence. The level of evidence required depends on the intended use of the biomarker and its potential impact on patient care.
It is important to note that the regulatory framework for biomarker validation is continually evolving. As new technologies and methodologies emerge, regulatory agencies update their guidelines to ensure they remain relevant and effective. This dynamic nature of the regulatory landscape necessitates ongoing engagement between researchers, industry stakeholders, and regulatory bodies to maintain alignment and facilitate the development of innovative biomarkers.
Ethical Implications of Predictive Biomarkers
The use of predictive biomarkers for lithium orotate treatment response raises significant ethical considerations that must be carefully addressed. One primary concern is the potential for discrimination based on genetic or biological factors. If certain biomarkers are found to indicate a higher likelihood of positive response to lithium orotate, individuals lacking these markers may face reduced access to treatment or insurance coverage, leading to healthcare disparities.
Privacy and data protection present another critical ethical challenge. The collection and storage of genetic information or other biological data for biomarker analysis require robust safeguards to prevent unauthorized access or misuse. There is a risk that such sensitive information could be exploited for purposes beyond medical treatment, such as employment decisions or insurance risk assessments.
The concept of informed consent becomes more complex in the context of predictive biomarkers. Patients must fully understand the implications of biomarker testing, including potential psychological impacts of learning about their predicted treatment response. Healthcare providers have an ethical obligation to ensure comprehensive counseling and support throughout the testing and treatment process.
There are also concerns about the potential for overreliance on biomarkers in treatment decisions. While biomarkers can provide valuable insights, they should not be the sole determinant of treatment plans. Ethical medical practice requires a holistic approach that considers individual patient circumstances, preferences, and alternative treatment options.
The development and validation of predictive biomarkers raise questions of equity in research. Ensuring diverse representation in biomarker studies is crucial to avoid biases that could exacerbate existing health disparities. Researchers and policymakers must prioritize inclusive study designs and equitable access to emerging biomarker technologies.
Lastly, the ethical use of predictive biomarkers necessitates ongoing evaluation and transparency. As our understanding of biomarkers evolves, there must be mechanisms in place to reassess their validity and utility. Open communication about the limitations and uncertainties of biomarker-based predictions is essential to maintain public trust and ensure responsible implementation in clinical practice.
Privacy and data protection present another critical ethical challenge. The collection and storage of genetic information or other biological data for biomarker analysis require robust safeguards to prevent unauthorized access or misuse. There is a risk that such sensitive information could be exploited for purposes beyond medical treatment, such as employment decisions or insurance risk assessments.
The concept of informed consent becomes more complex in the context of predictive biomarkers. Patients must fully understand the implications of biomarker testing, including potential psychological impacts of learning about their predicted treatment response. Healthcare providers have an ethical obligation to ensure comprehensive counseling and support throughout the testing and treatment process.
There are also concerns about the potential for overreliance on biomarkers in treatment decisions. While biomarkers can provide valuable insights, they should not be the sole determinant of treatment plans. Ethical medical practice requires a holistic approach that considers individual patient circumstances, preferences, and alternative treatment options.
The development and validation of predictive biomarkers raise questions of equity in research. Ensuring diverse representation in biomarker studies is crucial to avoid biases that could exacerbate existing health disparities. Researchers and policymakers must prioritize inclusive study designs and equitable access to emerging biomarker technologies.
Lastly, the ethical use of predictive biomarkers necessitates ongoing evaluation and transparency. As our understanding of biomarkers evolves, there must be mechanisms in place to reassess their validity and utility. Open communication about the limitations and uncertainties of biomarker-based predictions is essential to maintain public trust and ensure responsible implementation in clinical practice.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!