Lithium orotate's potential in addiction therapy frameworks
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate Background
Lithium orotate, a compound consisting of lithium and orotic acid, has gained attention in recent years for its potential therapeutic applications, particularly in the field of addiction treatment. This organic salt of lithium has a unique chemical structure that distinguishes it from more commonly used lithium salts, such as lithium carbonate or lithium citrate.
The history of lithium as a therapeutic agent dates back to the mid-19th century when it was first used to treat gout. However, it wasn't until the 1940s that its mood-stabilizing properties were discovered, leading to its widespread use in treating bipolar disorder. Lithium orotate, specifically, was introduced in the 1970s by Dr. Hans Nieper, a German physician who proposed that this form of lithium could be more easily absorbed by the body and cross the blood-brain barrier more efficiently than other lithium compounds.
The potential of lithium orotate in addiction therapy frameworks stems from lithium's known effects on neurotransmitter systems and neural plasticity. Lithium has been shown to modulate dopamine and serotonin pathways, which are crucial in addiction processes. Additionally, lithium's neuroprotective properties and its ability to enhance neurogenesis may contribute to its potential efficacy in treating addictive behaviors.
Recent research has focused on lithium orotate's lower dosage requirements compared to traditional lithium salts, potentially reducing side effects while maintaining therapeutic efficacy. This characteristic makes it an attractive option for long-term use in addiction treatment, where medication adherence is crucial.
The evolving understanding of addiction as a chronic brain disease has led to increased interest in medications that can address the underlying neurobiological changes associated with substance use disorders. Lithium orotate's potential to stabilize mood, reduce impulsivity, and promote neuroplasticity aligns well with current addiction treatment paradigms that emphasize both behavioral interventions and pharmacological support.
As the field of addiction medicine continues to advance, there is growing recognition of the need for novel therapeutic approaches. Lithium orotate represents a promising avenue for research, potentially offering a more tolerable and effective option for individuals struggling with addiction. Its unique properties and the preliminary evidence supporting its use in mental health contexts provide a foundation for further exploration of its role in comprehensive addiction therapy frameworks.
The history of lithium as a therapeutic agent dates back to the mid-19th century when it was first used to treat gout. However, it wasn't until the 1940s that its mood-stabilizing properties were discovered, leading to its widespread use in treating bipolar disorder. Lithium orotate, specifically, was introduced in the 1970s by Dr. Hans Nieper, a German physician who proposed that this form of lithium could be more easily absorbed by the body and cross the blood-brain barrier more efficiently than other lithium compounds.
The potential of lithium orotate in addiction therapy frameworks stems from lithium's known effects on neurotransmitter systems and neural plasticity. Lithium has been shown to modulate dopamine and serotonin pathways, which are crucial in addiction processes. Additionally, lithium's neuroprotective properties and its ability to enhance neurogenesis may contribute to its potential efficacy in treating addictive behaviors.
Recent research has focused on lithium orotate's lower dosage requirements compared to traditional lithium salts, potentially reducing side effects while maintaining therapeutic efficacy. This characteristic makes it an attractive option for long-term use in addiction treatment, where medication adherence is crucial.
The evolving understanding of addiction as a chronic brain disease has led to increased interest in medications that can address the underlying neurobiological changes associated with substance use disorders. Lithium orotate's potential to stabilize mood, reduce impulsivity, and promote neuroplasticity aligns well with current addiction treatment paradigms that emphasize both behavioral interventions and pharmacological support.
As the field of addiction medicine continues to advance, there is growing recognition of the need for novel therapeutic approaches. Lithium orotate represents a promising avenue for research, potentially offering a more tolerable and effective option for individuals struggling with addiction. Its unique properties and the preliminary evidence supporting its use in mental health contexts provide a foundation for further exploration of its role in comprehensive addiction therapy frameworks.
Addiction Therapy Market
The addiction therapy market has witnessed significant growth in recent years, driven by the increasing prevalence of substance abuse disorders and the growing awareness of mental health issues. This market encompasses a wide range of treatment options, including pharmacological interventions, behavioral therapies, and support programs. The global addiction treatment market size was valued at approximately $6.8 billion in 2020 and is projected to reach $10.2 billion by 2027, growing at a CAGR of 6.7% during the forecast period.
The market is segmented based on treatment type, drug type, and end-user. Pharmacological treatments, including medications for opioid addiction, alcohol dependence, and nicotine addiction, constitute a significant portion of the market. Behavioral therapies, such as cognitive-behavioral therapy (CBT) and motivational interviewing, are also gaining traction due to their effectiveness in addressing the psychological aspects of addiction.
North America dominates the addiction therapy market, accounting for the largest market share due to the high prevalence of substance abuse disorders and the presence of well-established healthcare infrastructure. Europe follows closely, with increasing government initiatives to combat addiction. The Asia-Pacific region is expected to witness the fastest growth, driven by rising awareness, improving healthcare systems, and increasing disposable income.
Key players in the addiction therapy market include Alkermes plc, Indivior PLC, Novartis AG, and Pfizer Inc. These companies are focusing on developing innovative treatment options and expanding their product portfolios through strategic collaborations and acquisitions. The market is also seeing the entry of new players with novel approaches to addiction treatment, including digital therapeutics and personalized medicine.
The COVID-19 pandemic has had a significant impact on the addiction therapy market. While it has disrupted traditional treatment delivery methods, it has also accelerated the adoption of telemedicine and digital health solutions for addiction treatment. This shift is expected to have long-lasting effects on the market landscape, potentially opening up new opportunities for growth and innovation.
Looking ahead, the addiction therapy market is poised for continued growth, driven by factors such as increasing government initiatives, rising healthcare expenditure, and growing public awareness about addiction and mental health. The integration of technology in addiction treatment, such as AI-powered monitoring systems and virtual reality-based therapies, is expected to further propel market growth and improve treatment outcomes.
The market is segmented based on treatment type, drug type, and end-user. Pharmacological treatments, including medications for opioid addiction, alcohol dependence, and nicotine addiction, constitute a significant portion of the market. Behavioral therapies, such as cognitive-behavioral therapy (CBT) and motivational interviewing, are also gaining traction due to their effectiveness in addressing the psychological aspects of addiction.
North America dominates the addiction therapy market, accounting for the largest market share due to the high prevalence of substance abuse disorders and the presence of well-established healthcare infrastructure. Europe follows closely, with increasing government initiatives to combat addiction. The Asia-Pacific region is expected to witness the fastest growth, driven by rising awareness, improving healthcare systems, and increasing disposable income.
Key players in the addiction therapy market include Alkermes plc, Indivior PLC, Novartis AG, and Pfizer Inc. These companies are focusing on developing innovative treatment options and expanding their product portfolios through strategic collaborations and acquisitions. The market is also seeing the entry of new players with novel approaches to addiction treatment, including digital therapeutics and personalized medicine.
The COVID-19 pandemic has had a significant impact on the addiction therapy market. While it has disrupted traditional treatment delivery methods, it has also accelerated the adoption of telemedicine and digital health solutions for addiction treatment. This shift is expected to have long-lasting effects on the market landscape, potentially opening up new opportunities for growth and innovation.
Looking ahead, the addiction therapy market is poised for continued growth, driven by factors such as increasing government initiatives, rising healthcare expenditure, and growing public awareness about addiction and mental health. The integration of technology in addiction treatment, such as AI-powered monitoring systems and virtual reality-based therapies, is expected to further propel market growth and improve treatment outcomes.
Current Challenges
The exploration of lithium orotate as a potential treatment for addiction faces several significant challenges in both research and clinical application. One of the primary obstacles is the limited body of scientific evidence supporting its efficacy in addiction therapy. While some studies have shown promising results, the overall research landscape remains sparse, particularly in comparison to more established treatments.
The lack of large-scale, randomized controlled trials presents a substantial hurdle in validating lithium orotate's effectiveness. This gap in clinical data makes it difficult for healthcare providers and regulatory bodies to confidently recommend or approve its use in addiction treatment frameworks. Additionally, the absence of standardized dosing protocols and long-term safety data further complicates its integration into mainstream therapy options.
Another challenge lies in the regulatory status of lithium orotate. Unlike lithium carbonate, which is FDA-approved for bipolar disorder, lithium orotate is classified as a dietary supplement in many countries. This classification limits its availability as a prescribed medication and raises concerns about quality control and consistency across different manufacturers.
The potential for side effects and interactions with other medications also poses a significant challenge. While lithium orotate is generally considered to have a milder side effect profile compared to lithium carbonate, the full spectrum of its interactions with other substances commonly used in addiction treatment remains unclear. This uncertainty creates hesitation among clinicians in incorporating it into existing treatment regimens.
Furthermore, the mechanism of action of lithium orotate in addressing addiction is not fully understood. While theories exist about its impact on neurotransmitter systems and brain chemistry, more research is needed to elucidate its precise role in mitigating addictive behaviors. This knowledge gap hinders the development of targeted therapies and optimal treatment strategies.
The stigma associated with lithium-based treatments, primarily due to the side effects and monitoring requirements of lithium carbonate, may also impact the acceptance of lithium orotate in addiction therapy. Overcoming this perception barrier among both patients and healthcare providers requires extensive education and awareness campaigns.
Lastly, the economic aspects of integrating lithium orotate into addiction treatment frameworks present challenges. The cost-effectiveness of this approach compared to existing therapies needs to be thoroughly evaluated. Additionally, the potential need for specialized monitoring and follow-up care could strain healthcare resources, particularly in settings with limited access to advanced medical facilities.
The lack of large-scale, randomized controlled trials presents a substantial hurdle in validating lithium orotate's effectiveness. This gap in clinical data makes it difficult for healthcare providers and regulatory bodies to confidently recommend or approve its use in addiction treatment frameworks. Additionally, the absence of standardized dosing protocols and long-term safety data further complicates its integration into mainstream therapy options.
Another challenge lies in the regulatory status of lithium orotate. Unlike lithium carbonate, which is FDA-approved for bipolar disorder, lithium orotate is classified as a dietary supplement in many countries. This classification limits its availability as a prescribed medication and raises concerns about quality control and consistency across different manufacturers.
The potential for side effects and interactions with other medications also poses a significant challenge. While lithium orotate is generally considered to have a milder side effect profile compared to lithium carbonate, the full spectrum of its interactions with other substances commonly used in addiction treatment remains unclear. This uncertainty creates hesitation among clinicians in incorporating it into existing treatment regimens.
Furthermore, the mechanism of action of lithium orotate in addressing addiction is not fully understood. While theories exist about its impact on neurotransmitter systems and brain chemistry, more research is needed to elucidate its precise role in mitigating addictive behaviors. This knowledge gap hinders the development of targeted therapies and optimal treatment strategies.
The stigma associated with lithium-based treatments, primarily due to the side effects and monitoring requirements of lithium carbonate, may also impact the acceptance of lithium orotate in addiction therapy. Overcoming this perception barrier among both patients and healthcare providers requires extensive education and awareness campaigns.
Lastly, the economic aspects of integrating lithium orotate into addiction treatment frameworks present challenges. The cost-effectiveness of this approach compared to existing therapies needs to be thoroughly evaluated. Additionally, the potential need for specialized monitoring and follow-up care could strain healthcare resources, particularly in settings with limited access to advanced medical facilities.
Existing Treatment Methods
01 Lithium orotate in battery technology
Lithium orotate is being explored in battery technology, particularly for its potential to improve the performance and efficiency of lithium-ion batteries. Research suggests that it may enhance electrode materials, electrolyte compositions, or serve as a precursor for advanced battery components.- Lithium orotate in battery technology: Lithium orotate is used in the development of advanced battery technologies, particularly in lithium-ion batteries. It may serve as a precursor or additive to improve battery performance, including enhanced energy density, longer cycle life, and improved safety characteristics.
- Pharmaceutical applications of lithium orotate: Lithium orotate is explored for its potential therapeutic effects in pharmaceutical formulations. It may be used in the treatment of mood disorders, neurological conditions, or as a supplement for mental health support. Research focuses on its bioavailability and efficacy compared to other lithium compounds.
- Lithium orotate in materials science: The compound is utilized in materials science applications, potentially for the development of advanced materials with unique properties. This may include its use in the synthesis of novel compounds, coatings, or as a component in composite materials with specific electrical or chemical characteristics.
- Lithium orotate in electrochemical processes: Lithium orotate is employed in various electrochemical processes and applications. This may involve its use in electrolytes, electrode materials, or as a catalyst in electrochemical reactions, potentially improving efficiency or enabling new electrochemical technologies.
- Analytical methods for lithium orotate: Development of analytical techniques for the detection, quantification, and characterization of lithium orotate in various matrices. This includes spectroscopic methods, chromatography, and other analytical approaches to assess purity, concentration, and structural properties of the compound in different applications.
02 Pharmaceutical applications of lithium orotate
Lithium orotate is being investigated for various pharmaceutical applications. It may have potential uses in treating mood disorders, neurological conditions, or as a supplement for mental health. Research is ongoing to determine its efficacy and safety profile compared to other lithium compounds.Expand Specific Solutions03 Lithium orotate in materials science
In materials science, lithium orotate is being studied for its potential applications in creating novel materials or improving existing ones. This could include its use in developing advanced ceramics, composites, or other functional materials with unique properties.Expand Specific Solutions04 Lithium orotate in energy storage systems
Beyond traditional batteries, lithium orotate is being explored for its potential role in next-generation energy storage systems. This could involve its use in supercapacitors, fuel cells, or other emerging energy storage technologies.Expand Specific Solutions05 Synthesis and production methods for lithium orotate
Research is being conducted on improved methods for synthesizing and producing lithium orotate. This includes developing more efficient, cost-effective, or environmentally friendly production processes, as well as exploring new precursors or reaction pathways.Expand Specific Solutions
Key Industry Players
The field of lithium orotate in addiction therapy frameworks is in an early developmental stage, with a growing market potential as researchers explore its efficacy. The technology's maturity is still evolving, with companies like Bristol Myers Squibb, Abbott Laboratories, and Janssen Pharmaceutica leading research efforts. Academic institutions such as Northwestern University and The University of North Carolina at Chapel Hill are also contributing to the knowledge base. The market size is expected to expand as more clinical trials are conducted and regulatory approvals are sought. However, competition is intensifying as pharmaceutical companies and research organizations invest in developing novel addiction therapies, potentially leveraging lithium orotate's unique properties.
Abbott Laboratories
Technical Solution: Abbott Laboratories has been exploring the potential of lithium orotate in addiction therapy frameworks. Their approach involves developing a novel formulation that combines lithium orotate with other compounds to enhance its efficacy in treating addiction. The company's research focuses on the neuroprotective properties of lithium orotate and its ability to modulate neurotransmitter systems involved in addiction[1]. Abbott's formulation aims to address the limitations of traditional lithium treatments, such as side effects and narrow therapeutic window, by leveraging the unique properties of the orotate salt[2]. The company is conducting preclinical studies to evaluate the safety and efficacy of their lithium orotate-based therapy for various types of addiction, including alcohol and opioid dependence[3].
Strengths: Innovative formulation, potential for reduced side effects, targeting multiple addiction types. Weaknesses: Early stage of development, limited clinical data, potential regulatory challenges.
Eli Lilly & Co.
Technical Solution: Eli Lilly & Co. is investigating lithium orotate as part of a multi-modal approach to addiction therapy. Their research combines lithium orotate with existing addiction treatments to create a synergistic effect. The company's strategy involves developing a controlled-release formulation of lithium orotate to maintain stable blood levels over time, potentially improving efficacy and reducing side effects[4]. Eli Lilly is also exploring the use of lithium orotate in combination with cognitive behavioral therapy (CBT) to enhance treatment outcomes. Preclinical studies have shown promising results in reducing drug-seeking behavior and improving cognitive function in animal models of addiction[5]. The company is preparing for early-phase clinical trials to assess the safety and efficacy of their lithium orotate-based therapy in human subjects with substance use disorders.
Strengths: Combination therapy approach, potential for improved patient compliance, integration with behavioral therapies. Weaknesses: Complex development process, potential drug interactions, need for extensive clinical trials.
Lithium Orotate Mechanisms
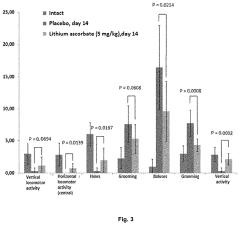
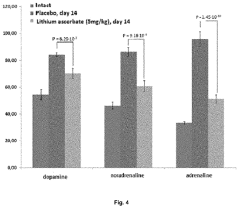
Use of lithium ascorbate to prevent and treat alcoholism and alcohol intoxication
PatentActiveUS20200147128A1
Innovation
- Lithium ascorbate is used as a prophylactic and therapeutic agent in doses of at least 5 mg/kg to regulate neuromediator balance and promote neuroadaptation, inhibiting the negative effects of alcohol on the central nervous system.
Diagnosis and treatment of addiction
PatentWO2021211540A1
Innovation
- A composition comprising a therapeutically effective amount of a carnitine derivative and short-chain fatty acids (SCFAs) is administered orally, which can be self-administered daily for up to 12 weeks, increasing specific microbial populations in the gut and reducing withdrawal symptoms, while allowing concurrent opioid use.
Regulatory Considerations
The regulatory landscape surrounding lithium orotate's use in addiction therapy frameworks is complex and evolving. Currently, lithium orotate is not approved by the FDA for any medical use, including addiction treatment. This lack of regulatory approval poses significant challenges for its integration into formal addiction therapy protocols.
In the United States, lithium orotate is classified as a dietary supplement, falling under the Dietary Supplement Health and Education Act (DSHEA) of 1994. This classification means it can be sold without proving safety or efficacy, provided no specific health claims are made. However, this also limits its use in clinical settings and prevents healthcare providers from prescribing it as a medication for addiction treatment.
Internationally, regulatory approaches to lithium orotate vary. Some countries have stricter controls, classifying it as a prescription medication or even banning its sale entirely. This regulatory inconsistency creates challenges for multinational research efforts and potential global market expansion.
For lithium orotate to gain acceptance in addiction therapy frameworks, extensive clinical trials would be necessary to demonstrate its safety and efficacy. These trials would need to adhere to stringent regulatory guidelines set by agencies such as the FDA, EMA, and other national health authorities. The process would likely involve multiple phases of clinical trials, potentially taking several years and requiring substantial financial investment.
Regulatory considerations also extend to manufacturing processes. Good Manufacturing Practices (GMP) would need to be established and maintained to ensure consistent quality and purity of lithium orotate products. This would involve developing standardized production methods, implementing quality control measures, and establishing traceability systems.
Safety monitoring and pharmacovigilance would be crucial regulatory requirements if lithium orotate were to be approved for addiction therapy. This would involve establishing systems for reporting and analyzing adverse events, conducting post-market surveillance studies, and regularly updating safety information.
Ethical considerations in clinical trials and patient consent processes would also fall under regulatory scrutiny. Given the vulnerable nature of individuals struggling with addiction, extra care would be needed to ensure that trials are conducted ethically and that participants fully understand the potential risks and benefits.
In conclusion, while lithium orotate shows potential in addiction therapy frameworks, significant regulatory hurdles must be overcome before it can be widely adopted in clinical practice. Addressing these regulatory challenges will require coordinated efforts from researchers, pharmaceutical companies, and regulatory bodies to establish a clear pathway for its evaluation and potential approval as a therapeutic agent in addiction treatment.
In the United States, lithium orotate is classified as a dietary supplement, falling under the Dietary Supplement Health and Education Act (DSHEA) of 1994. This classification means it can be sold without proving safety or efficacy, provided no specific health claims are made. However, this also limits its use in clinical settings and prevents healthcare providers from prescribing it as a medication for addiction treatment.
Internationally, regulatory approaches to lithium orotate vary. Some countries have stricter controls, classifying it as a prescription medication or even banning its sale entirely. This regulatory inconsistency creates challenges for multinational research efforts and potential global market expansion.
For lithium orotate to gain acceptance in addiction therapy frameworks, extensive clinical trials would be necessary to demonstrate its safety and efficacy. These trials would need to adhere to stringent regulatory guidelines set by agencies such as the FDA, EMA, and other national health authorities. The process would likely involve multiple phases of clinical trials, potentially taking several years and requiring substantial financial investment.
Regulatory considerations also extend to manufacturing processes. Good Manufacturing Practices (GMP) would need to be established and maintained to ensure consistent quality and purity of lithium orotate products. This would involve developing standardized production methods, implementing quality control measures, and establishing traceability systems.
Safety monitoring and pharmacovigilance would be crucial regulatory requirements if lithium orotate were to be approved for addiction therapy. This would involve establishing systems for reporting and analyzing adverse events, conducting post-market surveillance studies, and regularly updating safety information.
Ethical considerations in clinical trials and patient consent processes would also fall under regulatory scrutiny. Given the vulnerable nature of individuals struggling with addiction, extra care would be needed to ensure that trials are conducted ethically and that participants fully understand the potential risks and benefits.
In conclusion, while lithium orotate shows potential in addiction therapy frameworks, significant regulatory hurdles must be overcome before it can be widely adopted in clinical practice. Addressing these regulatory challenges will require coordinated efforts from researchers, pharmaceutical companies, and regulatory bodies to establish a clear pathway for its evaluation and potential approval as a therapeutic agent in addiction treatment.
Safety and Side Effects
The safety profile and potential side effects of lithium orotate in addiction therapy frameworks are crucial considerations for its clinical application. While lithium orotate is generally considered to have a lower toxicity profile compared to lithium carbonate, it is not without risks and potential adverse effects.
One of the primary safety concerns with lithium orotate is the lack of extensive clinical trials and long-term studies specifically focused on its use in addiction therapy. This gap in research makes it challenging to fully understand the potential risks and side effects associated with its prolonged use in this context.
Lithium orotate, like other lithium compounds, may cause gastrointestinal disturbances such as nausea, vomiting, and diarrhea. These side effects are typically mild and often subside as the body adjusts to the medication. However, in some cases, they may persist and require dose adjustments or discontinuation of treatment.
Neurological side effects have been reported with lithium use, including tremors, muscle weakness, and cognitive impairment. While these effects are generally less severe with lithium orotate compared to other lithium formulations, they still warrant careful monitoring, especially in patients with pre-existing neurological conditions.
Renal function is another area of concern when using lithium compounds. Although lithium orotate is believed to have a lower impact on kidney function than lithium carbonate, regular monitoring of renal function is still recommended to detect any potential adverse effects early.
Thyroid function may also be affected by lithium use, with some patients experiencing hypothyroidism. Regular thyroid function tests should be conducted to ensure early detection and management of any thyroid-related issues.
Weight gain has been associated with lithium use, which could be a significant concern for patients in addiction therapy, as weight changes can impact overall well-being and recovery. Monitoring of body weight and implementation of appropriate dietary and lifestyle interventions may be necessary.
It is important to note that lithium orotate can interact with other medications, particularly those affecting kidney function or electrolyte balance. This necessitates careful consideration of potential drug interactions when incorporating lithium orotate into addiction therapy frameworks.
The risk of lithium toxicity, although lower with lithium orotate, still exists. Symptoms of toxicity may include severe tremors, confusion, seizures, and in extreme cases, coma. Regular monitoring of lithium levels in the blood is essential to maintain therapeutic efficacy while minimizing the risk of toxicity.
One of the primary safety concerns with lithium orotate is the lack of extensive clinical trials and long-term studies specifically focused on its use in addiction therapy. This gap in research makes it challenging to fully understand the potential risks and side effects associated with its prolonged use in this context.
Lithium orotate, like other lithium compounds, may cause gastrointestinal disturbances such as nausea, vomiting, and diarrhea. These side effects are typically mild and often subside as the body adjusts to the medication. However, in some cases, they may persist and require dose adjustments or discontinuation of treatment.
Neurological side effects have been reported with lithium use, including tremors, muscle weakness, and cognitive impairment. While these effects are generally less severe with lithium orotate compared to other lithium formulations, they still warrant careful monitoring, especially in patients with pre-existing neurological conditions.
Renal function is another area of concern when using lithium compounds. Although lithium orotate is believed to have a lower impact on kidney function than lithium carbonate, regular monitoring of renal function is still recommended to detect any potential adverse effects early.
Thyroid function may also be affected by lithium use, with some patients experiencing hypothyroidism. Regular thyroid function tests should be conducted to ensure early detection and management of any thyroid-related issues.
Weight gain has been associated with lithium use, which could be a significant concern for patients in addiction therapy, as weight changes can impact overall well-being and recovery. Monitoring of body weight and implementation of appropriate dietary and lifestyle interventions may be necessary.
It is important to note that lithium orotate can interact with other medications, particularly those affecting kidney function or electrolyte balance. This necessitates careful consideration of potential drug interactions when incorporating lithium orotate into addiction therapy frameworks.
The risk of lithium toxicity, although lower with lithium orotate, still exists. Symptoms of toxicity may include severe tremors, confusion, seizures, and in extreme cases, coma. Regular monitoring of lithium levels in the blood is essential to maintain therapeutic efficacy while minimizing the risk of toxicity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!