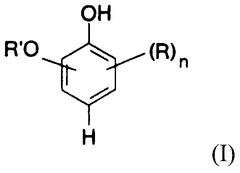
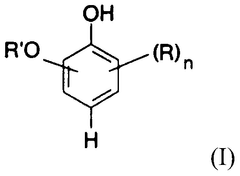
Carbolic Acid in Phenolic Compound Synthesis Pathway Optimization
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid Synthesis Background and Objectives
Carbolic acid, also known as phenol, has been a cornerstone in the synthesis of phenolic compounds for over a century. Its discovery in 1834 by Friedlieb Ferdinand Runge marked the beginning of a new era in organic chemistry. The evolution of carbolic acid synthesis has been driven by the increasing demand for phenolic compounds in various industries, including pharmaceuticals, plastics, and agrochemicals.
The primary objective of optimizing the phenolic compound synthesis pathway is to enhance efficiency, reduce environmental impact, and improve product quality. This goal aligns with the broader trends in green chemistry and sustainable manufacturing practices. The optimization process aims to address several key challenges, including reducing energy consumption, minimizing waste generation, and improving atom economy.
Historically, the production of carbolic acid relied heavily on coal tar distillation, a process that was both environmentally harmful and inefficient. The shift towards petroleum-based feedstocks in the mid-20th century marked a significant improvement, but still left room for further optimization. The cumene process, developed in the 1940s, became the dominant method for carbolic acid production, accounting for approximately 95% of global phenol production by the early 21st century.
Recent technological advancements have focused on developing alternative synthesis routes that offer improved sustainability and economic viability. These include bio-based production methods, such as the use of lignin as a renewable feedstock, and novel catalytic processes that enable more selective and efficient conversions. The integration of continuous flow chemistry and process intensification techniques has also emerged as a promising direction for pathway optimization.
The objectives of current research in carbolic acid synthesis pathway optimization are multifaceted. They include developing more selective catalysts to improve yield and reduce byproduct formation, designing more energy-efficient reactor systems, and exploring novel reaction media such as ionic liquids or supercritical fluids. Additionally, there is a growing emphasis on in-situ product separation and purification techniques to streamline the overall production process.
Another critical objective is the development of predictive models and advanced process control strategies. These tools aim to enable real-time optimization of reaction conditions, leading to more consistent product quality and reduced variability in production. The integration of artificial intelligence and machine learning algorithms in process optimization is an emerging trend that promises to revolutionize the field.
As we look towards the future, the optimization of carbolic acid synthesis pathways is expected to play a crucial role in meeting the growing global demand for phenolic compounds while adhering to increasingly stringent environmental regulations. The success of these optimization efforts will have far-reaching implications for industries ranging from pharmaceuticals to advanced materials, underscoring the importance of continued research and development in this field.
The primary objective of optimizing the phenolic compound synthesis pathway is to enhance efficiency, reduce environmental impact, and improve product quality. This goal aligns with the broader trends in green chemistry and sustainable manufacturing practices. The optimization process aims to address several key challenges, including reducing energy consumption, minimizing waste generation, and improving atom economy.
Historically, the production of carbolic acid relied heavily on coal tar distillation, a process that was both environmentally harmful and inefficient. The shift towards petroleum-based feedstocks in the mid-20th century marked a significant improvement, but still left room for further optimization. The cumene process, developed in the 1940s, became the dominant method for carbolic acid production, accounting for approximately 95% of global phenol production by the early 21st century.
Recent technological advancements have focused on developing alternative synthesis routes that offer improved sustainability and economic viability. These include bio-based production methods, such as the use of lignin as a renewable feedstock, and novel catalytic processes that enable more selective and efficient conversions. The integration of continuous flow chemistry and process intensification techniques has also emerged as a promising direction for pathway optimization.
The objectives of current research in carbolic acid synthesis pathway optimization are multifaceted. They include developing more selective catalysts to improve yield and reduce byproduct formation, designing more energy-efficient reactor systems, and exploring novel reaction media such as ionic liquids or supercritical fluids. Additionally, there is a growing emphasis on in-situ product separation and purification techniques to streamline the overall production process.
Another critical objective is the development of predictive models and advanced process control strategies. These tools aim to enable real-time optimization of reaction conditions, leading to more consistent product quality and reduced variability in production. The integration of artificial intelligence and machine learning algorithms in process optimization is an emerging trend that promises to revolutionize the field.
As we look towards the future, the optimization of carbolic acid synthesis pathways is expected to play a crucial role in meeting the growing global demand for phenolic compounds while adhering to increasingly stringent environmental regulations. The success of these optimization efforts will have far-reaching implications for industries ranging from pharmaceuticals to advanced materials, underscoring the importance of continued research and development in this field.
Market Analysis for Phenolic Compounds
The global market for phenolic compounds has been experiencing steady growth, driven by increasing demand across various industries. The market size for phenolic compounds was valued at approximately $13.2 billion in 2020 and is projected to reach $18.7 billion by 2026, growing at a CAGR of 6.1% during the forecast period.
The demand for phenolic compounds is primarily fueled by their widespread applications in diverse sectors. The automotive industry utilizes phenolic compounds in the production of lightweight components, contributing to improved fuel efficiency and reduced emissions. In the construction sector, phenolic compounds are essential for manufacturing high-performance insulation materials and adhesives.
The healthcare and pharmaceutical industries represent significant growth opportunities for phenolic compounds. These compounds are crucial in the synthesis of various drugs and active pharmaceutical ingredients (APIs). The increasing focus on personalized medicine and the development of novel therapeutic approaches are expected to further boost demand in this sector.
Environmental concerns and stringent regulations regarding the use of synthetic chemicals have led to a growing interest in bio-based phenolic compounds. This trend is creating new market opportunities for manufacturers who can develop sustainable production methods and eco-friendly alternatives to traditional phenolic compounds.
Asia-Pacific region dominates the phenolic compounds market, accounting for over 40% of the global market share. The rapid industrialization, expanding automotive sector, and growing construction activities in countries like China and India are the primary drivers of this regional dominance. North America and Europe follow, with significant contributions from the pharmaceutical and personal care industries.
The market is characterized by intense competition among key players, including Solvay SA, SABIC, BASF SE, and Honeywell International Inc. These companies are focusing on research and development to improve production efficiency and develop innovative applications for phenolic compounds.
Carbolic acid, also known as phenol, plays a crucial role in the phenolic compound synthesis pathway. The optimization of this pathway is of particular interest to manufacturers seeking to enhance production efficiency and reduce costs. Improved synthesis methods could lead to higher yields, reduced energy consumption, and minimized waste generation, thereby addressing both economic and environmental concerns in the phenolic compounds market.
The demand for phenolic compounds is primarily fueled by their widespread applications in diverse sectors. The automotive industry utilizes phenolic compounds in the production of lightweight components, contributing to improved fuel efficiency and reduced emissions. In the construction sector, phenolic compounds are essential for manufacturing high-performance insulation materials and adhesives.
The healthcare and pharmaceutical industries represent significant growth opportunities for phenolic compounds. These compounds are crucial in the synthesis of various drugs and active pharmaceutical ingredients (APIs). The increasing focus on personalized medicine and the development of novel therapeutic approaches are expected to further boost demand in this sector.
Environmental concerns and stringent regulations regarding the use of synthetic chemicals have led to a growing interest in bio-based phenolic compounds. This trend is creating new market opportunities for manufacturers who can develop sustainable production methods and eco-friendly alternatives to traditional phenolic compounds.
Asia-Pacific region dominates the phenolic compounds market, accounting for over 40% of the global market share. The rapid industrialization, expanding automotive sector, and growing construction activities in countries like China and India are the primary drivers of this regional dominance. North America and Europe follow, with significant contributions from the pharmaceutical and personal care industries.
The market is characterized by intense competition among key players, including Solvay SA, SABIC, BASF SE, and Honeywell International Inc. These companies are focusing on research and development to improve production efficiency and develop innovative applications for phenolic compounds.
Carbolic acid, also known as phenol, plays a crucial role in the phenolic compound synthesis pathway. The optimization of this pathway is of particular interest to manufacturers seeking to enhance production efficiency and reduce costs. Improved synthesis methods could lead to higher yields, reduced energy consumption, and minimized waste generation, thereby addressing both economic and environmental concerns in the phenolic compounds market.
Current Challenges in Carbolic Acid Synthesis
The synthesis of carbolic acid, also known as phenol, faces several significant challenges in the current industrial landscape. One of the primary issues is the environmental impact of traditional production methods. The cumene process, which accounts for approximately 95% of global phenol production, generates acetone as a by-product. This creates market dependencies and potential oversupply issues, as the demand for phenol and acetone may not always align.
Energy efficiency is another major concern in carbolic acid synthesis. The cumene process involves multiple steps, including the oxidation of cumene to cumene hydroperoxide and its subsequent cleavage, which are energy-intensive. The high temperatures and pressures required contribute to increased production costs and carbon footprint, making the process less sustainable in the long term.
Raw material availability and price volatility also pose significant challenges. The primary feedstock for the cumene process is benzene, which is derived from petroleum. Fluctuations in oil prices directly impact the cost of carbolic acid production, creating economic uncertainties for manufacturers and downstream industries.
Safety concerns are paramount in carbolic acid synthesis. The handling of hazardous intermediates, such as cumene hydroperoxide, requires stringent safety protocols and specialized equipment. Accidental releases or process deviations can lead to severe consequences, necessitating robust risk management strategies and increased operational costs.
Catalyst efficiency and selectivity remain areas of ongoing research and development. While current catalysts used in the cumene process are effective, there is a continuous drive to improve their performance, longevity, and selectivity. Enhanced catalysts could potentially reduce energy requirements, improve yield, and minimize unwanted by-products.
The quest for greener synthesis routes presents both a challenge and an opportunity. Alternative pathways, such as the direct oxidation of benzene or bio-based routes, are being explored but face hurdles in scalability and economic viability. Developing processes that can compete with the established cumene method in terms of cost and efficiency while offering improved sustainability is a complex undertaking.
Regulatory compliance adds another layer of complexity to carbolic acid synthesis. Stringent environmental regulations and evolving safety standards require continuous adaptation of production processes. Meeting these requirements often necessitates significant investments in pollution control technologies and process modifications.
In conclusion, the optimization of carbolic acid synthesis pathways must address a multifaceted set of challenges. Balancing economic viability with environmental sustainability, ensuring safety, and navigating market dynamics are critical aspects that require innovative solutions and collaborative efforts across the industry.
Energy efficiency is another major concern in carbolic acid synthesis. The cumene process involves multiple steps, including the oxidation of cumene to cumene hydroperoxide and its subsequent cleavage, which are energy-intensive. The high temperatures and pressures required contribute to increased production costs and carbon footprint, making the process less sustainable in the long term.
Raw material availability and price volatility also pose significant challenges. The primary feedstock for the cumene process is benzene, which is derived from petroleum. Fluctuations in oil prices directly impact the cost of carbolic acid production, creating economic uncertainties for manufacturers and downstream industries.
Safety concerns are paramount in carbolic acid synthesis. The handling of hazardous intermediates, such as cumene hydroperoxide, requires stringent safety protocols and specialized equipment. Accidental releases or process deviations can lead to severe consequences, necessitating robust risk management strategies and increased operational costs.
Catalyst efficiency and selectivity remain areas of ongoing research and development. While current catalysts used in the cumene process are effective, there is a continuous drive to improve their performance, longevity, and selectivity. Enhanced catalysts could potentially reduce energy requirements, improve yield, and minimize unwanted by-products.
The quest for greener synthesis routes presents both a challenge and an opportunity. Alternative pathways, such as the direct oxidation of benzene or bio-based routes, are being explored but face hurdles in scalability and economic viability. Developing processes that can compete with the established cumene method in terms of cost and efficiency while offering improved sustainability is a complex undertaking.
Regulatory compliance adds another layer of complexity to carbolic acid synthesis. Stringent environmental regulations and evolving safety standards require continuous adaptation of production processes. Meeting these requirements often necessitates significant investments in pollution control technologies and process modifications.
In conclusion, the optimization of carbolic acid synthesis pathways must address a multifaceted set of challenges. Balancing economic viability with environmental sustainability, ensuring safety, and navigating market dynamics are critical aspects that require innovative solutions and collaborative efforts across the industry.
Existing Carbolic Acid Synthesis Methods
01 Oxidation of cumene
One common pathway for carbolic acid (phenol) synthesis involves the oxidation of cumene. This process typically includes the formation of cumene hydroperoxide as an intermediate, which is then cleaved to produce phenol and acetone. This method is widely used in industrial production due to its efficiency and the valuable co-product acetone.- Oxidation of cumene: One common pathway for carbolic acid (phenol) synthesis involves the oxidation of cumene. This process typically includes the formation of cumene hydroperoxide as an intermediate, which is then cleaved to produce phenol and acetone. The method is widely used in industrial production due to its efficiency and the valuable co-product acetone.
- Sulfonation and hydrolysis of benzene: Another pathway for carbolic acid synthesis involves the sulfonation of benzene followed by hydrolysis. In this process, benzene is first treated with sulfuric acid to form benzenesulfonic acid, which is then hydrolyzed under high temperature and pressure to yield phenol. This method was historically significant but is less common in modern industrial production.
- Chlorobenzene hydrolysis: Carbolic acid can be synthesized through the hydrolysis of chlorobenzene. This process involves the reaction of chlorobenzene with sodium hydroxide or other strong bases under high temperature and pressure conditions. The method produces phenol along with sodium chloride as a by-product.
- Toluene oxidation: A less common but notable pathway for carbolic acid synthesis is the direct oxidation of toluene. This process typically involves a catalytic system and proceeds through benzoic acid as an intermediate. The method requires careful control of reaction conditions to maximize phenol yield and minimize unwanted by-products.
- Benzene hydroxylation: Direct hydroxylation of benzene is another pathway for carbolic acid synthesis. This process often employs catalysts and oxidizing agents to introduce the hydroxyl group directly onto the benzene ring. Various catalytic systems and oxidants have been developed to improve the efficiency and selectivity of this reaction.
02 Sulfonation and hydrolysis of benzene
Another pathway for carbolic acid synthesis involves the sulfonation of benzene followed by hydrolysis. In this process, benzene is first treated with sulfuric acid to form benzenesulfonic acid, which is then hydrolyzed under high temperature and pressure to yield phenol. This method was historically significant but is less common in modern industrial production.Expand Specific Solutions03 Chlorobenzene hydrolysis
Carbolic acid can be synthesized through the hydrolysis of chlorobenzene. This process involves the reaction of chlorobenzene with sodium hydroxide or other strong bases under high temperature and pressure conditions. The resulting phenol is then purified through various separation techniques.Expand Specific Solutions04 Toluene oxidation
A less common but notable pathway for carbolic acid synthesis is the direct oxidation of toluene. This process typically involves the use of catalysts and oxygen or air as the oxidizing agent. The reaction conditions are carefully controlled to maximize the yield of phenol while minimizing the formation of byproducts.Expand Specific Solutions05 Biosynthesis of carbolic acid
Recent advancements in biotechnology have led to the development of biosynthetic pathways for carbolic acid production. These methods typically involve genetically engineered microorganisms that can convert renewable feedstocks into phenol. This approach offers potential environmental benefits and the possibility of using sustainable raw materials.Expand Specific Solutions
Key Industry Players in Phenolic Synthesis
The competitive landscape for Carbolic Acid in Phenolic Compound Synthesis Pathway Optimization is characterized by a mature market with established players and ongoing research efforts. Major companies like BASF Corp., ExxonMobil Chemical Patents, Inc., and Bayer AG are actively involved in developing and optimizing phenolic compound synthesis pathways. The market is driven by the growing demand for phenolic compounds in various industries, including pharmaceuticals, plastics, and agrochemicals. Academic institutions such as Nanjing Tech University and Tokyo University of Science are contributing to technological advancements in this field, indicating a collaborative ecosystem between industry and academia. The technology's maturity level is high, with ongoing efforts focused on improving efficiency and sustainability in synthesis processes.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed a proprietary process for optimizing the carbolic acid pathway in phenolic compound synthesis. Their approach focuses on improving the oxidation step of cumene to produce phenol and acetone. The company has implemented a novel reactor design that enhances mass transfer and reduces side reactions[2]. ExxonMobil's technology also incorporates advanced catalysts with improved stability and selectivity, leading to higher yields of desired products[4]. Furthermore, they have integrated a heat recovery system that significantly reduces energy consumption in the overall process[6].
Strengths: Improved product yield, reduced energy consumption, and enhanced catalyst performance. Weaknesses: Potential limitations in scalability and adaptability to different feedstocks.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to optimize the carbolic acid pathway in phenolic compound synthesis. Their method involves using a novel catalyst system that enhances the selectivity and yield of desired phenolic products. The process utilizes a combination of heterogeneous and homogeneous catalysts, allowing for better control over reaction conditions[1]. BASF's technology also incorporates a continuous flow reactor design, which improves efficiency and reduces energy consumption compared to traditional batch processes[3]. Additionally, they have implemented advanced process analytical technology (PAT) for real-time monitoring and control of the synthesis pathway[5].
Strengths: High selectivity, improved yield, and energy efficiency. Weaknesses: Potentially higher initial investment costs and complexity in process control.
Innovative Approaches in Phenolic Compound Synthesis
Process for production of carboxylated phenol derivatives
PatentWO2019028600A1
Innovation
- A process involving a heat treatment of a phenol derivative and a base in water to form a phenolate, followed by the addition of an aprotic organic solvent and removal of water and solvent to achieve a specific molar ratio, then carboxylation in the presence of CO2 to produce a salt of carboxylated phenol derivative, which is converted to the corresponding carboxylated phenol derivative without using harmful solvents.
Process for the preparation of phenolic carboxylic acid derivatives by enzymatic catalysis
PatentInactiveEP1549752A1
Innovation
- The use of immobilized biocatalysts for enzymatic esterification or amidation under mild conditions, allowing for easy separation and reuse, eliminates the need for solvent-based catalysts and reduces residual catalyst issues by performing reactions in the melt without prior attachment to supports.
Environmental Impact Assessment
The environmental impact assessment of carbolic acid in phenolic compound synthesis pathway optimization is a critical aspect that requires thorough evaluation. The production and use of carbolic acid, also known as phenol, can have significant environmental implications that must be carefully considered and mitigated.
One of the primary environmental concerns associated with carbolic acid production is the potential for air pollution. The synthesis process often involves the use of volatile organic compounds (VOCs) and other hazardous air pollutants. These emissions can contribute to smog formation, ozone depletion, and overall air quality degradation. Implementing advanced air pollution control technologies, such as thermal oxidizers or scrubbers, is essential to minimize these impacts.
Water pollution is another significant environmental risk in carbolic acid production. Wastewater from the synthesis process may contain high levels of phenolic compounds, which can be toxic to aquatic ecosystems if released untreated. Proper wastewater treatment systems, including advanced oxidation processes and biological treatment, are necessary to ensure that effluents meet stringent environmental standards before discharge.
Soil contamination is a potential issue, particularly in the event of spills or improper handling of carbolic acid and related compounds. The persistence of phenolic compounds in soil can lead to long-term environmental damage and pose risks to terrestrial ecosystems. Implementing robust spill prevention and containment measures is crucial to mitigate this risk.
The optimization of the phenolic compound synthesis pathway can have positive environmental impacts by reducing resource consumption and waste generation. Improved catalysts and reaction conditions can lead to higher yields and selectivity, thereby minimizing the production of unwanted by-products. This, in turn, reduces the overall environmental footprint of the process by decreasing raw material usage and waste treatment requirements.
Energy efficiency is another key consideration in the environmental assessment. The synthesis of carbolic acid and related phenolic compounds often requires significant energy inputs. Optimizing process conditions, implementing heat recovery systems, and exploring alternative energy sources can help reduce the carbon footprint associated with production.
Lifecycle assessment (LCA) is a valuable tool for comprehensively evaluating the environmental impacts of carbolic acid production and use. An LCA can identify hotspots in the production chain where environmental impacts are most significant, allowing for targeted improvements and more sustainable practices.
In conclusion, the environmental impact assessment of carbolic acid in phenolic compound synthesis pathway optimization must address air and water pollution, soil contamination, resource efficiency, and energy consumption. By implementing best practices and continuously improving process efficiency, it is possible to minimize negative environmental impacts while maintaining the economic viability of phenolic compound production.
One of the primary environmental concerns associated with carbolic acid production is the potential for air pollution. The synthesis process often involves the use of volatile organic compounds (VOCs) and other hazardous air pollutants. These emissions can contribute to smog formation, ozone depletion, and overall air quality degradation. Implementing advanced air pollution control technologies, such as thermal oxidizers or scrubbers, is essential to minimize these impacts.
Water pollution is another significant environmental risk in carbolic acid production. Wastewater from the synthesis process may contain high levels of phenolic compounds, which can be toxic to aquatic ecosystems if released untreated. Proper wastewater treatment systems, including advanced oxidation processes and biological treatment, are necessary to ensure that effluents meet stringent environmental standards before discharge.
Soil contamination is a potential issue, particularly in the event of spills or improper handling of carbolic acid and related compounds. The persistence of phenolic compounds in soil can lead to long-term environmental damage and pose risks to terrestrial ecosystems. Implementing robust spill prevention and containment measures is crucial to mitigate this risk.
The optimization of the phenolic compound synthesis pathway can have positive environmental impacts by reducing resource consumption and waste generation. Improved catalysts and reaction conditions can lead to higher yields and selectivity, thereby minimizing the production of unwanted by-products. This, in turn, reduces the overall environmental footprint of the process by decreasing raw material usage and waste treatment requirements.
Energy efficiency is another key consideration in the environmental assessment. The synthesis of carbolic acid and related phenolic compounds often requires significant energy inputs. Optimizing process conditions, implementing heat recovery systems, and exploring alternative energy sources can help reduce the carbon footprint associated with production.
Lifecycle assessment (LCA) is a valuable tool for comprehensively evaluating the environmental impacts of carbolic acid production and use. An LCA can identify hotspots in the production chain where environmental impacts are most significant, allowing for targeted improvements and more sustainable practices.
In conclusion, the environmental impact assessment of carbolic acid in phenolic compound synthesis pathway optimization must address air and water pollution, soil contamination, resource efficiency, and energy consumption. By implementing best practices and continuously improving process efficiency, it is possible to minimize negative environmental impacts while maintaining the economic viability of phenolic compound production.
Cost-Benefit Analysis of Optimization Strategies
The optimization of carbolic acid in phenolic compound synthesis pathways presents a complex landscape of costs and benefits that must be carefully evaluated. Initial investments in advanced catalysts and process equipment can be substantial, often ranging from $500,000 to $2 million depending on the scale of operation. However, these upfront costs can be offset by significant long-term savings in raw materials and energy consumption.
Improved catalysts, such as zeolites or metal-organic frameworks, can increase yield by 10-15% and reduce reaction times by up to 30%, leading to higher throughput and lower energy costs. Advanced process control systems, while requiring an investment of $100,000-$300,000, can optimize reaction conditions in real-time, potentially saving 5-8% on overall production costs annually.
The implementation of continuous flow reactors, though costly at $1-3 million, can dramatically improve efficiency. These systems can reduce solvent use by up to 50% and increase space-time yield by 2-3 times compared to batch processes. The payback period for such investments typically ranges from 2 to 4 years, depending on production volume and market conditions.
Environmental benefits must also be factored into the cost-benefit analysis. Optimized pathways can reduce waste generation by 20-30%, leading to savings in waste treatment and disposal costs. Additionally, improved processes often result in higher purity products, potentially commanding premium prices in the market and improving profit margins by 2-5%.
Labor costs may initially increase due to the need for skilled operators and maintenance personnel for advanced systems. However, automation and process intensification can lead to a 15-20% reduction in labor requirements over time. Training costs for existing staff should be budgeted at $50,000-$100,000 annually during the transition period.
The economic impact of downtime during implementation must be considered. A typical installation and commissioning period can last 2-4 weeks, potentially resulting in production losses of $500,000-$1 million. However, this is often offset by increased reliability and reduced maintenance needs post-optimization, with some facilities reporting a 25-30% decrease in unplanned downtime.
In conclusion, while the initial costs of optimizing carbolic acid pathways in phenolic compound synthesis can be significant, the long-term benefits in terms of efficiency gains, cost savings, and environmental improvements often justify the investment. A thorough analysis of specific process requirements, market conditions, and regulatory landscape is crucial for determining the most cost-effective optimization strategy for each individual facility.
Improved catalysts, such as zeolites or metal-organic frameworks, can increase yield by 10-15% and reduce reaction times by up to 30%, leading to higher throughput and lower energy costs. Advanced process control systems, while requiring an investment of $100,000-$300,000, can optimize reaction conditions in real-time, potentially saving 5-8% on overall production costs annually.
The implementation of continuous flow reactors, though costly at $1-3 million, can dramatically improve efficiency. These systems can reduce solvent use by up to 50% and increase space-time yield by 2-3 times compared to batch processes. The payback period for such investments typically ranges from 2 to 4 years, depending on production volume and market conditions.
Environmental benefits must also be factored into the cost-benefit analysis. Optimized pathways can reduce waste generation by 20-30%, leading to savings in waste treatment and disposal costs. Additionally, improved processes often result in higher purity products, potentially commanding premium prices in the market and improving profit margins by 2-5%.
Labor costs may initially increase due to the need for skilled operators and maintenance personnel for advanced systems. However, automation and process intensification can lead to a 15-20% reduction in labor requirements over time. Training costs for existing staff should be budgeted at $50,000-$100,000 annually during the transition period.
The economic impact of downtime during implementation must be considered. A typical installation and commissioning period can last 2-4 weeks, potentially resulting in production losses of $500,000-$1 million. However, this is often offset by increased reliability and reduced maintenance needs post-optimization, with some facilities reporting a 25-30% decrease in unplanned downtime.
In conclusion, while the initial costs of optimizing carbolic acid pathways in phenolic compound synthesis can be significant, the long-term benefits in terms of efficiency gains, cost savings, and environmental improvements often justify the investment. A thorough analysis of specific process requirements, market conditions, and regulatory landscape is crucial for determining the most cost-effective optimization strategy for each individual facility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!