Coatings For Cathode–Electrolyte Interfaces In Chloride-Based ASSLBs
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloride-Based ASSLB Coating Technology Background and Objectives
All-solid-state lithium batteries (ASSLBs) represent a significant advancement in energy storage technology, offering potential solutions to the safety and performance limitations of conventional lithium-ion batteries. Within this domain, chloride-based solid electrolytes have emerged as promising candidates due to their high ionic conductivity, favorable electrochemical stability, and cost-effectiveness compared to other solid electrolyte systems.
The evolution of ASSLB technology can be traced back to the early 2000s, with significant acceleration in research and development occurring over the past decade. Chloride-based systems, particularly those utilizing lithium chloride compounds, have gained attention since approximately 2015, when their potential for high ionic conductivity at room temperature was first demonstrated in laboratory settings.
Current technological trends indicate a growing focus on interface engineering, as the cathode-electrolyte interface represents one of the most critical challenges in realizing commercially viable chloride-based ASSLBs. The high reactivity of chloride electrolytes with conventional cathode materials creates interfacial resistance issues that significantly impair battery performance and longevity.
Protective coating technologies have emerged as a promising approach to mitigate these interfacial challenges. The development trajectory has progressed from simple oxide coatings to more sophisticated multi-functional coating architectures designed to simultaneously address multiple interface-related issues, including chemical incompatibility, mechanical stress, and ion transport barriers.
The primary technical objectives for cathode coating technologies in chloride-based ASSLBs include: reducing interfacial resistance to enhance rate capability; preventing undesired side reactions between cathode materials and chloride electrolytes; maintaining mechanical integrity during cycling; and ensuring stable long-term performance under various operating conditions.
Additionally, these coating technologies aim to be scalable and cost-effective for industrial implementation, as commercial viability remains a key consideration for widespread adoption. Current research is particularly focused on developing coating materials and processes that can be integrated into existing battery manufacturing workflows without introducing prohibitive costs or complexity.
Looking forward, the field is moving toward atomic-level interface design, with increasing emphasis on understanding and controlling the fundamental mechanisms of ion transport across coated interfaces. Advanced characterization techniques and computational modeling are playing crucial roles in accelerating this understanding and guiding the rational design of next-generation coating solutions for chloride-based ASSLB systems.
The evolution of ASSLB technology can be traced back to the early 2000s, with significant acceleration in research and development occurring over the past decade. Chloride-based systems, particularly those utilizing lithium chloride compounds, have gained attention since approximately 2015, when their potential for high ionic conductivity at room temperature was first demonstrated in laboratory settings.
Current technological trends indicate a growing focus on interface engineering, as the cathode-electrolyte interface represents one of the most critical challenges in realizing commercially viable chloride-based ASSLBs. The high reactivity of chloride electrolytes with conventional cathode materials creates interfacial resistance issues that significantly impair battery performance and longevity.
Protective coating technologies have emerged as a promising approach to mitigate these interfacial challenges. The development trajectory has progressed from simple oxide coatings to more sophisticated multi-functional coating architectures designed to simultaneously address multiple interface-related issues, including chemical incompatibility, mechanical stress, and ion transport barriers.
The primary technical objectives for cathode coating technologies in chloride-based ASSLBs include: reducing interfacial resistance to enhance rate capability; preventing undesired side reactions between cathode materials and chloride electrolytes; maintaining mechanical integrity during cycling; and ensuring stable long-term performance under various operating conditions.
Additionally, these coating technologies aim to be scalable and cost-effective for industrial implementation, as commercial viability remains a key consideration for widespread adoption. Current research is particularly focused on developing coating materials and processes that can be integrated into existing battery manufacturing workflows without introducing prohibitive costs or complexity.
Looking forward, the field is moving toward atomic-level interface design, with increasing emphasis on understanding and controlling the fundamental mechanisms of ion transport across coated interfaces. Advanced characterization techniques and computational modeling are playing crucial roles in accelerating this understanding and guiding the rational design of next-generation coating solutions for chloride-based ASSLB systems.
Market Analysis for Chloride-Based ASSLB Applications
The global market for all-solid-state lithium batteries (ASSLBs) is experiencing significant growth, with chloride-based systems emerging as a promising segment. Current market projections indicate that the overall ASSLB market will reach approximately $2 billion by 2025, with chloride-based systems potentially capturing 15-20% of this market due to their unique advantages in energy density and safety profiles.
Chloride-based ASSLBs are particularly attractive for electric vehicle applications, where their enhanced safety characteristics and potential for higher energy density provide competitive advantages over conventional lithium-ion batteries. The automotive sector represents the largest potential market for these technologies, with premium electric vehicle manufacturers showing increased interest in integrating solid-state battery technology into their upcoming models.
Consumer electronics constitutes another significant market segment, where the compact size and safety benefits of chloride-based ASSLBs could drive adoption in high-end portable devices. Market research suggests that this segment could grow at a compound annual growth rate of 25% over the next five years, outpacing the broader battery market.
Geographically, Asia-Pacific dominates the development and production landscape, with Japan, South Korea, and China leading in patents and commercial development efforts. North America and Europe are rapidly expanding their research initiatives, particularly through university-industry partnerships focused on overcoming the cathode-electrolyte interface challenges.
Market barriers for chloride-based ASSLBs include higher production costs compared to conventional lithium-ion batteries and technical challenges related to interface stability. The cost premium currently stands at approximately 30-40% higher than conventional systems, though economies of scale are expected to reduce this gap as production volumes increase.
Investment in chloride-based ASSLB technology has seen substantial growth, with venture capital funding in this specific segment increasing by nearly 45% year-over-year. Major battery manufacturers and automotive OEMs have announced strategic investments totaling over $500 million specifically for chloride-based solid electrolyte research and development in the past two years.
Customer demand analysis reveals that while energy density remains the primary performance metric of interest, cycle life stability and fast-charging capabilities are increasingly important factors driving market adoption. The development of effective cathode coatings that address the interface challenges in chloride-based systems could potentially accelerate market penetration by addressing these key performance indicators.
Chloride-based ASSLBs are particularly attractive for electric vehicle applications, where their enhanced safety characteristics and potential for higher energy density provide competitive advantages over conventional lithium-ion batteries. The automotive sector represents the largest potential market for these technologies, with premium electric vehicle manufacturers showing increased interest in integrating solid-state battery technology into their upcoming models.
Consumer electronics constitutes another significant market segment, where the compact size and safety benefits of chloride-based ASSLBs could drive adoption in high-end portable devices. Market research suggests that this segment could grow at a compound annual growth rate of 25% over the next five years, outpacing the broader battery market.
Geographically, Asia-Pacific dominates the development and production landscape, with Japan, South Korea, and China leading in patents and commercial development efforts. North America and Europe are rapidly expanding their research initiatives, particularly through university-industry partnerships focused on overcoming the cathode-electrolyte interface challenges.
Market barriers for chloride-based ASSLBs include higher production costs compared to conventional lithium-ion batteries and technical challenges related to interface stability. The cost premium currently stands at approximately 30-40% higher than conventional systems, though economies of scale are expected to reduce this gap as production volumes increase.
Investment in chloride-based ASSLB technology has seen substantial growth, with venture capital funding in this specific segment increasing by nearly 45% year-over-year. Major battery manufacturers and automotive OEMs have announced strategic investments totaling over $500 million specifically for chloride-based solid electrolyte research and development in the past two years.
Customer demand analysis reveals that while energy density remains the primary performance metric of interest, cycle life stability and fast-charging capabilities are increasingly important factors driving market adoption. The development of effective cathode coatings that address the interface challenges in chloride-based systems could potentially accelerate market penetration by addressing these key performance indicators.
Current Challenges in Cathode-Electrolyte Interface Coatings
Despite significant advancements in all-solid-state lithium batteries (ASSLBs) with chloride-based solid electrolytes, the cathode-electrolyte interface remains a critical bottleneck for commercial viability. The high reactivity between cathode active materials and chloride solid electrolytes creates interfacial instability, leading to increased impedance and capacity degradation during cycling.
One major challenge is the chemical incompatibility between high-voltage cathode materials (such as NMC and LCO) and chloride-based electrolytes. During battery operation, transition metals from cathodes can dissolve and migrate into the electrolyte, while chloride ions can attack the cathode structure, forming resistive interphases that impede lithium-ion transport.
Mechanical issues present another significant hurdle. The volume changes during lithiation/delithiation cycles create contact loss between cathode particles and the solid electrolyte, increasing interfacial resistance. This problem is particularly pronounced in chloride systems due to their relatively rigid nature compared to sulfide electrolytes, yet they lack the mechanical robustness of oxide systems.
Current coating technologies face limitations in achieving uniform and conformal coverage on cathode particles. Traditional wet-chemistry methods often result in non-uniform coatings with varying thickness, while atomic layer deposition (ALD), though precise, faces scalability challenges for mass production. The ideal coating must be thin enough to maintain high ionic conductivity while being robust enough to prevent direct contact between cathode and electrolyte.
Stability at high voltages (>4V) remains problematic for most coating materials. Many coating candidates that show excellent protection at moderate voltages degrade when batteries operate at higher voltages needed for high energy density applications. This voltage instability often manifests as gas evolution, electrolyte decomposition, or coating breakdown.
The long-term durability of interface coatings presents another challenge. While many coating solutions demonstrate promising results in short-term cycling tests, their performance often deteriorates during extended cycling or under extreme temperature conditions. This raises questions about the viability of current coating technologies for applications requiring 10+ years of operational life.
Cost-effectiveness and scalability of coating processes represent significant barriers to commercialization. Many laboratory-scale coating techniques utilize expensive precursors or complex equipment that may not be economically viable for mass production. Finding coating materials and processes that balance performance with manufacturing practicality remains a critical challenge.
One major challenge is the chemical incompatibility between high-voltage cathode materials (such as NMC and LCO) and chloride-based electrolytes. During battery operation, transition metals from cathodes can dissolve and migrate into the electrolyte, while chloride ions can attack the cathode structure, forming resistive interphases that impede lithium-ion transport.
Mechanical issues present another significant hurdle. The volume changes during lithiation/delithiation cycles create contact loss between cathode particles and the solid electrolyte, increasing interfacial resistance. This problem is particularly pronounced in chloride systems due to their relatively rigid nature compared to sulfide electrolytes, yet they lack the mechanical robustness of oxide systems.
Current coating technologies face limitations in achieving uniform and conformal coverage on cathode particles. Traditional wet-chemistry methods often result in non-uniform coatings with varying thickness, while atomic layer deposition (ALD), though precise, faces scalability challenges for mass production. The ideal coating must be thin enough to maintain high ionic conductivity while being robust enough to prevent direct contact between cathode and electrolyte.
Stability at high voltages (>4V) remains problematic for most coating materials. Many coating candidates that show excellent protection at moderate voltages degrade when batteries operate at higher voltages needed for high energy density applications. This voltage instability often manifests as gas evolution, electrolyte decomposition, or coating breakdown.
The long-term durability of interface coatings presents another challenge. While many coating solutions demonstrate promising results in short-term cycling tests, their performance often deteriorates during extended cycling or under extreme temperature conditions. This raises questions about the viability of current coating technologies for applications requiring 10+ years of operational life.
Cost-effectiveness and scalability of coating processes represent significant barriers to commercialization. Many laboratory-scale coating techniques utilize expensive precursors or complex equipment that may not be economically viable for mass production. Finding coating materials and processes that balance performance with manufacturing practicality remains a critical challenge.
Current Coating Solutions for Chloride-Based Electrolytes
01 Protective coating materials for cathode-electrolyte interfaces
Various materials can be applied as protective coatings at the cathode-electrolyte interface to enhance stability. These materials include metal oxides, fluorides, and polymers that form a physical barrier between the cathode and electrolyte, preventing unwanted reactions. The coatings help minimize cathode dissolution, electrolyte decomposition, and interfacial resistance, thereby improving the overall battery performance and longevity.- Protective coating materials for cathode-electrolyte interfaces: Various materials can be applied as protective coatings at the cathode-electrolyte interface to enhance stability. These materials include metal oxides, fluorides, and polymeric compounds that form a physical barrier between the cathode and electrolyte. The coatings help prevent unwanted side reactions, reduce interfacial resistance, and mitigate degradation mechanisms that occur at the interface during battery operation.
- Artificial solid electrolyte interphase (SEI) formation: Engineered artificial SEI layers can be created on cathode surfaces to improve interface stability. These artificial SEI layers are designed to have specific properties such as high ionic conductivity while blocking electron transfer, which helps prevent continuous electrolyte decomposition. The artificial SEI can be formed through pre-treatment processes or in-situ during initial battery cycling, providing a stable interface that enhances battery performance and longevity.
- Atomic layer deposition (ALD) and other thin film coating techniques: Advanced deposition techniques such as atomic layer deposition (ALD), chemical vapor deposition (CVD), and pulsed laser deposition (PLD) are employed to create ultrathin, conformal coatings on cathode surfaces. These techniques allow for precise control of coating thickness at the nanometer scale, ensuring uniform coverage even on complex cathode structures. The resulting thin films effectively stabilize the cathode-electrolyte interface while maintaining efficient ion transport.
- Composite and gradient coatings for enhanced interface stability: Composite and gradient coatings consisting of multiple materials or layers with gradually changing compositions can provide superior interface stability. These sophisticated coating structures combine the benefits of different materials to address multiple degradation mechanisms simultaneously. The gradient nature of these coatings enables smooth transitions between materials with different properties, reducing interfacial stress and improving adhesion while maintaining efficient ion transport.
- Surface modification with functional groups and dopants: Chemical modification of cathode surfaces through the introduction of functional groups or dopant elements can significantly improve interface stability. These modifications alter the surface chemistry of the cathode material, making it more compatible with the electrolyte and less prone to unwanted side reactions. Techniques include fluorination, phosphorylation, and doping with elements that can scavenge harmful species or create favorable binding sites for stable interface formation.
02 Artificial solid electrolyte interphase (SEI) formation
Artificial solid electrolyte interphase layers can be deliberately created on cathode surfaces to improve interface stability. These engineered SEI layers serve as protective barriers that allow ion transport while preventing parasitic reactions. Various methods for creating artificial SEI include pre-lithiation treatments, electrolyte additives, and surface modifications that promote the formation of stable interfacial films with optimal ionic conductivity and mechanical properties.Expand Specific Solutions03 Composite and gradient interface structures
Composite and gradient structures at the cathode-electrolyte interface can significantly enhance stability. These structures typically involve multiple layers with gradually changing compositions or properties, which help mitigate mechanical stress and chemical incompatibilities. The gradient design allows for better accommodation of volume changes during cycling and provides a smoother transition between the cathode and electrolyte materials, reducing interfacial resistance.Expand Specific Solutions04 Atomic layer deposition (ALD) and other thin-film coating techniques
Advanced deposition techniques such as atomic layer deposition (ALD), pulsed laser deposition, and chemical vapor deposition are employed to create ultrathin, conformal coatings on cathode surfaces. These techniques allow for precise control over coating thickness and composition, enabling the formation of uniform protective layers that maintain intimate contact with the cathode material while providing effective protection against electrolyte attack and unwanted side reactions.Expand Specific Solutions05 Functional additives and surface modifiers
Various functional additives and surface modifiers can be incorporated at the cathode-electrolyte interface to enhance stability. These include ionic conductors, scavengers for harmful species, and compounds that promote favorable interfacial reactions. By strategically selecting additives that can neutralize acidic species, trap metal ions, or facilitate Li-ion transport across the interface, the chemical and electrochemical stability of the cathode-electrolyte interface can be significantly improved.Expand Specific Solutions
Leading Companies and Research Institutions in ASSLB Coatings
The all-solid-state lithium battery (ASSLB) market with chloride-based electrolytes is in an early growth phase, characterized by intensive R&D activities rather than mass commercialization. The global ASSLB market is projected to reach $2-3 billion by 2030, with chloride-based systems representing an emerging segment due to their promising ionic conductivity advantages. Technology maturity varies significantly among key players: established materials companies like Corning and Murata Manufacturing are leveraging their expertise in ceramic technologies; academic institutions including Northeastern University and Rice University are pioneering fundamental research; while specialized battery developers such as A123 Systems and Factorial are focusing on practical implementation challenges. The competitive landscape shows a collaborative ecosystem where materials science expertise from research institutions complements the manufacturing capabilities of industrial players.
Factorial, Inc.
Technical Solution: Factorial has developed proprietary solid-state electrolyte coatings specifically designed for chloride-based all-solid-state lithium batteries (ASSLBs). Their approach involves applying nanometer-thick protective layers on cathode materials that effectively mitigate interfacial degradation issues common in chloride electrolyte systems. The company utilizes atomic layer deposition (ALD) techniques to create uniform, conformal coatings that prevent direct contact between active cathode materials and aggressive chloride ions while maintaining excellent ionic conductivity. Factorial's coating technology incorporates lithium-conductive materials such as lithium phosphates and lithium niobium oxides that form a stable interface with both the cathode and the chloride solid electrolyte. This interface engineering approach has demonstrated significant improvements in cycling stability, with coated cathodes showing capacity retention above 80% after 500 cycles compared to rapid degradation in uncoated samples.
Strengths: Provides exceptional protection against chloride corrosion while maintaining high ionic conductivity; scalable ALD process suitable for industrial production; significantly extends battery cycle life. Weaknesses: May add additional manufacturing complexity and cost; coating thickness must be precisely controlled to avoid increasing internal resistance; potential challenges in ensuring uniform coating coverage on complex cathode particle morphologies.
Tianmu Lake Institute of Advanced Energy Storage Technologies Co., Ltd.
Technical Solution: Tianmu Lake Institute has developed an innovative surface modification technology for cathode materials in chloride-based ASSLBs using lithium-rich anti-perovskite compounds as protective coatings. Their approach involves synthesizing Li3OCl-based coating materials doped with aluminum and magnesium to enhance stability against chloride electrolytes while maintaining high lithium-ion conductivity (>10^-4 S/cm at room temperature). The coating is applied through a controlled sol-gel process followed by low-temperature crystallization, creating a 5-20nm thick protective layer that effectively isolates the cathode active material from direct contact with aggressive chloride species. This coating technology has been demonstrated to significantly reduce interfacial resistance growth during cycling, with impedance measurements showing minimal increase even after 1000 hours of storage at elevated temperatures. The chemical compatibility between the anti-perovskite coating and chloride solid electrolytes creates a seamless interface that facilitates rapid lithium-ion transport while preventing detrimental side reactions that typically lead to capacity fade and increased cell resistance.
Strengths: Excellent chemical compatibility with chloride electrolytes due to similar chemical composition; maintains high ionic conductivity across the interface; demonstrated long-term stability under storage and cycling conditions. Weaknesses: Requires precise control of coating stoichiometry and thickness; potential challenges in scaling up the sol-gel process for mass production; limited data on performance under high-rate charging conditions.
Key Innovations in Cathode-Electrolyte Interface Engineering
Coated Cathode For Solid State Batteries
PatentPendingUS20210408539A1
Innovation
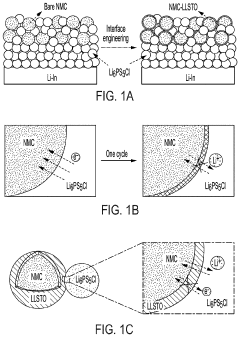
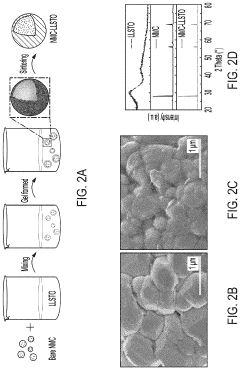
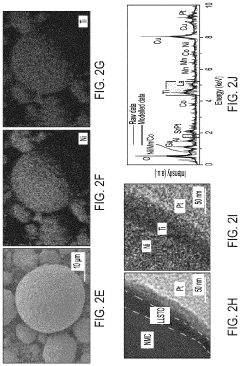
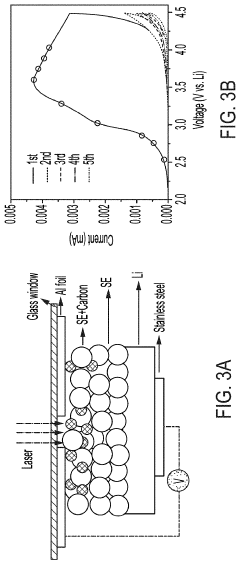
- A thin amorphous Li0.35La0.5Sr0.05TiO3 (LLSTO) coating layer is applied via a wet chemical method to stabilize the interface between the Li6PS5Cl electrolyte and the LiNi1/3Mn1/3Co1/3O2 (NMC) cathode, enhancing ionic conductivity and preventing decomposition, thereby extending cycling performance.
High-energy all-solid-state lithium batteries
PatentWO2024009303A3
Innovation
- Development of cost-effective sulfide-based cathode materials as alternatives to expensive cobalt-based materials for high-energy ASSLBs.
- Implementation of high-pressure synthesis technique for creating protective layers on cathode materials to enhance stability at the cathode-electrolyte interface.
- Utilization of atomic layer deposition (ALD) technique to create high-quality interphase coatings that improve interface stability and fast interfacial transport dynamics.
Material Compatibility and Degradation Mechanisms
The compatibility between cathode materials and chloride-based solid electrolytes represents a critical challenge in all-solid-state lithium batteries (ASSLBs). When these materials come into contact, interfacial reactions often occur that lead to the formation of high-impedance layers, significantly hampering ion transport and overall battery performance. These reactions are particularly pronounced in chloride-based systems due to the high chemical reactivity of chloride ions with transition metal oxides commonly used as cathode materials.
Chemical degradation at the cathode-electrolyte interface typically manifests through several mechanisms. The most prevalent is the exchange of cations between the cathode and the chloride electrolyte, resulting in the formation of transition metal chlorides and other undesirable compounds. This process not only depletes active cathode material but also creates resistive interfacial layers that impede lithium-ion migration.
Electrochemical degradation presents another significant challenge, particularly during cycling. The high oxidation potential at the cathode surface can lead to oxidative decomposition of chloride electrolytes, releasing chlorine gas in severe cases. This decomposition is accelerated at elevated temperatures and higher voltage operations, limiting the practical voltage window of chloride-based ASSLBs.
Mechanical degradation also occurs at these interfaces due to volume changes during lithiation/delithiation cycles. The resulting microcracks create new reactive surfaces, accelerating chemical degradation in a self-reinforcing cycle. The brittle nature of most solid electrolytes exacerbates this issue, as they cannot accommodate the strain generated during cycling.
Thermodynamic instability between high-voltage cathode materials and chloride electrolytes presents a fundamental challenge. Density functional theory (DFT) calculations have demonstrated that many common cathode materials, such as LiCoO2 and NMC, have unfavorable interfacial energies when paired with chloride electrolytes, predicting spontaneous decomposition even before electrochemical cycling begins.
Environmental factors further influence degradation mechanisms. Trace moisture can accelerate interfacial reactions through hydrolysis of chloride electrolytes, while oxygen exposure can lead to oxidation of both cathode materials and electrolyte components. These factors necessitate stringent manufacturing conditions and packaging requirements for chloride-based ASSLBs.
Understanding these complex degradation mechanisms is essential for developing effective coating strategies. Ideal coating materials must simultaneously address chemical, electrochemical, and mechanical degradation while maintaining excellent ionic conductivity. This multifunctional requirement explains why single-component coatings often fail to provide comprehensive protection in practical applications.
Chemical degradation at the cathode-electrolyte interface typically manifests through several mechanisms. The most prevalent is the exchange of cations between the cathode and the chloride electrolyte, resulting in the formation of transition metal chlorides and other undesirable compounds. This process not only depletes active cathode material but also creates resistive interfacial layers that impede lithium-ion migration.
Electrochemical degradation presents another significant challenge, particularly during cycling. The high oxidation potential at the cathode surface can lead to oxidative decomposition of chloride electrolytes, releasing chlorine gas in severe cases. This decomposition is accelerated at elevated temperatures and higher voltage operations, limiting the practical voltage window of chloride-based ASSLBs.
Mechanical degradation also occurs at these interfaces due to volume changes during lithiation/delithiation cycles. The resulting microcracks create new reactive surfaces, accelerating chemical degradation in a self-reinforcing cycle. The brittle nature of most solid electrolytes exacerbates this issue, as they cannot accommodate the strain generated during cycling.
Thermodynamic instability between high-voltage cathode materials and chloride electrolytes presents a fundamental challenge. Density functional theory (DFT) calculations have demonstrated that many common cathode materials, such as LiCoO2 and NMC, have unfavorable interfacial energies when paired with chloride electrolytes, predicting spontaneous decomposition even before electrochemical cycling begins.
Environmental factors further influence degradation mechanisms. Trace moisture can accelerate interfacial reactions through hydrolysis of chloride electrolytes, while oxygen exposure can lead to oxidation of both cathode materials and electrolyte components. These factors necessitate stringent manufacturing conditions and packaging requirements for chloride-based ASSLBs.
Understanding these complex degradation mechanisms is essential for developing effective coating strategies. Ideal coating materials must simultaneously address chemical, electrochemical, and mechanical degradation while maintaining excellent ionic conductivity. This multifunctional requirement explains why single-component coatings often fail to provide comprehensive protection in practical applications.
Scalability and Manufacturing Considerations for Coating Technologies
The scalability and manufacturing considerations for coating technologies in chloride-based ASSLBs represent a critical factor in their commercial viability. Current laboratory-scale coating methods, while effective for research purposes, face significant challenges when transitioning to industrial production. Physical vapor deposition (PVD) techniques, commonly used for high-quality interface coatings, require expensive vacuum equipment and demonstrate limited throughput, making them prohibitive for large-scale battery manufacturing.
Solution-based coating methods offer more promising scalability pathways. Techniques such as spray coating, dip coating, and spin coating can be adapted to continuous roll-to-roll processing, potentially enabling high-volume production. However, these methods currently struggle with achieving uniform nanometer-thick coatings across large-area cathode materials, particularly for the complex geometries of composite cathodes.
The economic viability of coating technologies depends heavily on material costs and process efficiency. Atomic layer deposition (ALD), while providing excellent conformality and thickness control, remains cost-prohibitive for mass production due to slow deposition rates and expensive precursors. Recent developments in spatial ALD show promise for increasing throughput, but further optimization is needed to meet commercial production requirements.
Integration of coating processes into existing battery manufacturing lines presents another significant challenge. Ideally, cathode coating should be seamlessly incorporated between cathode synthesis and cell assembly steps. This requires careful consideration of process compatibility, including temperature constraints, solvent selection, and handling protocols to prevent contamination or degradation of chloride-based solid electrolytes.
Quality control and process monitoring represent additional manufacturing hurdles. Unlike liquid electrolyte systems, defects in interface coatings for solid-state batteries cannot self-heal, making robust in-line inspection methods essential. Advanced characterization techniques such as optical monitoring systems and impedance spectroscopy need adaptation for high-speed production environments.
Environmental and safety considerations also impact manufacturing scalability. Some coating precursors used in ALD or CVD processes may present toxicity or reactivity concerns, necessitating additional containment measures. Water-based or environmentally benign coating solutions are being explored as alternatives, though their performance often lags behind conventional approaches for chloride-based systems.
Recent industry trends show increasing investment in pilot-scale coating facilities specifically designed for solid-state battery components. These intermediate-scale operations aim to bridge the gap between laboratory research and full commercial production, allowing for process optimization while reducing financial risk associated with immediate large-scale deployment.
Solution-based coating methods offer more promising scalability pathways. Techniques such as spray coating, dip coating, and spin coating can be adapted to continuous roll-to-roll processing, potentially enabling high-volume production. However, these methods currently struggle with achieving uniform nanometer-thick coatings across large-area cathode materials, particularly for the complex geometries of composite cathodes.
The economic viability of coating technologies depends heavily on material costs and process efficiency. Atomic layer deposition (ALD), while providing excellent conformality and thickness control, remains cost-prohibitive for mass production due to slow deposition rates and expensive precursors. Recent developments in spatial ALD show promise for increasing throughput, but further optimization is needed to meet commercial production requirements.
Integration of coating processes into existing battery manufacturing lines presents another significant challenge. Ideally, cathode coating should be seamlessly incorporated between cathode synthesis and cell assembly steps. This requires careful consideration of process compatibility, including temperature constraints, solvent selection, and handling protocols to prevent contamination or degradation of chloride-based solid electrolytes.
Quality control and process monitoring represent additional manufacturing hurdles. Unlike liquid electrolyte systems, defects in interface coatings for solid-state batteries cannot self-heal, making robust in-line inspection methods essential. Advanced characterization techniques such as optical monitoring systems and impedance spectroscopy need adaptation for high-speed production environments.
Environmental and safety considerations also impact manufacturing scalability. Some coating precursors used in ALD or CVD processes may present toxicity or reactivity concerns, necessitating additional containment measures. Water-based or environmentally benign coating solutions are being explored as alternatives, though their performance often lags behind conventional approaches for chloride-based systems.
Recent industry trends show increasing investment in pilot-scale coating facilities specifically designed for solid-state battery components. These intermediate-scale operations aim to bridge the gap between laboratory research and full commercial production, allowing for process optimization while reducing financial risk associated with immediate large-scale deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!