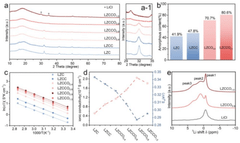
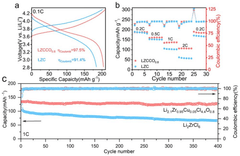
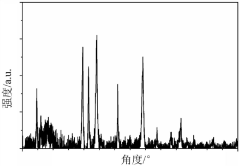
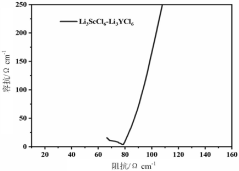
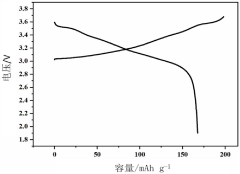
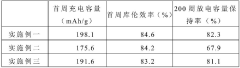
Synthesis Of Li3MCl6 And Li2ZrCl6: Structure, Ion Transport, And Scalability
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid Electrolyte Development Background and Objectives
The development of solid-state electrolytes represents a pivotal advancement in battery technology, offering potential solutions to the safety and performance limitations of conventional liquid electrolytes. The exploration of halide solid electrolytes, particularly Li3MCl6 and Li2ZrCl6 compounds, has emerged as a promising research direction due to their unique structural properties and ion transport capabilities.
Historically, battery technology has evolved from primary cells to rechargeable lithium-ion batteries with liquid electrolytes. However, these conventional systems face inherent challenges including flammability risks, limited electrochemical stability windows, and dendrite formation. The pursuit of solid electrolytes began in earnest during the 1970s with the discovery of fast ion conductors, but significant progress in halide-based systems has only materialized in the past decade.
Li3MCl6 (where M represents various transition metals) and Li2ZrCl6 compounds have attracted particular attention due to their crystallographic structures that facilitate lithium-ion mobility. These materials feature three-dimensional frameworks with interconnected pathways for lithium-ion migration, potentially enabling high ionic conductivity at room temperature—a critical requirement for practical solid-state batteries.
The technical objectives for these halide solid electrolytes encompass several dimensions. Primary goals include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, maintaining electrochemical stability windows wider than 4V, and demonstrating compatibility with both lithium metal anodes and high-voltage cathodes. Additionally, these materials must exhibit mechanical stability to prevent dendrite penetration while maintaining chemical stability against electrode materials.
Current research trends indicate growing interest in understanding structure-property relationships in these compounds, with particular focus on how atomic arrangements influence ion transport mechanisms. Computational studies have begun mapping potential energy landscapes for ion migration, while experimental work has focused on synthesis optimization and performance characterization.
The scalability of synthesis processes represents another critical objective, as laboratory-scale production methods must evolve toward industrially viable techniques. This includes developing cost-effective precursors, reducing energy-intensive processing steps, and ensuring consistent quality across larger batch sizes—all essential for eventual commercialization.
Looking forward, the field is moving toward multi-component systems and compositional engineering to fine-tune properties. The integration of these materials into full-cell configurations represents the next major milestone, requiring innovations in interfacial engineering and composite electrolyte designs to address remaining challenges in mechanical and chemical compatibility.
Historically, battery technology has evolved from primary cells to rechargeable lithium-ion batteries with liquid electrolytes. However, these conventional systems face inherent challenges including flammability risks, limited electrochemical stability windows, and dendrite formation. The pursuit of solid electrolytes began in earnest during the 1970s with the discovery of fast ion conductors, but significant progress in halide-based systems has only materialized in the past decade.
Li3MCl6 (where M represents various transition metals) and Li2ZrCl6 compounds have attracted particular attention due to their crystallographic structures that facilitate lithium-ion mobility. These materials feature three-dimensional frameworks with interconnected pathways for lithium-ion migration, potentially enabling high ionic conductivity at room temperature—a critical requirement for practical solid-state batteries.
The technical objectives for these halide solid electrolytes encompass several dimensions. Primary goals include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, maintaining electrochemical stability windows wider than 4V, and demonstrating compatibility with both lithium metal anodes and high-voltage cathodes. Additionally, these materials must exhibit mechanical stability to prevent dendrite penetration while maintaining chemical stability against electrode materials.
Current research trends indicate growing interest in understanding structure-property relationships in these compounds, with particular focus on how atomic arrangements influence ion transport mechanisms. Computational studies have begun mapping potential energy landscapes for ion migration, while experimental work has focused on synthesis optimization and performance characterization.
The scalability of synthesis processes represents another critical objective, as laboratory-scale production methods must evolve toward industrially viable techniques. This includes developing cost-effective precursors, reducing energy-intensive processing steps, and ensuring consistent quality across larger batch sizes—all essential for eventual commercialization.
Looking forward, the field is moving toward multi-component systems and compositional engineering to fine-tune properties. The integration of these materials into full-cell configurations represents the next major milestone, requiring innovations in interfacial engineering and composite electrolyte designs to address remaining challenges in mechanical and chemical compatibility.
Market Analysis for Solid-State Battery Electrolytes
The global solid-state battery market is experiencing significant growth, driven by increasing demand for high-performance energy storage solutions across multiple sectors. Current market valuations place the solid-state battery market at approximately $500 million in 2023, with projections indicating potential growth to reach $3.4 billion by 2030, representing a compound annual growth rate (CAGR) of 31.2% during this forecast period.
Solid-state electrolytes, particularly those based on lithium halides such as Li3MCl6 and Li2ZrCl6, are positioned as critical components in this expanding market. These materials address key limitations of conventional liquid electrolytes, offering enhanced safety profiles by eliminating flammable components and providing superior thermal stability under extreme operating conditions.
The automotive sector represents the largest market segment for solid-state battery electrolytes, accounting for roughly 45% of current demand. Major automotive manufacturers including Toyota, Volkswagen, and BMW have announced significant investments in solid-state battery technology, with commercial deployment timelines targeted between 2025-2028. This automotive push is primarily driven by the need for batteries with higher energy density, faster charging capabilities, and improved safety profiles.
Consumer electronics constitutes the second-largest market segment at approximately 30% of current demand, with manufacturers seeking batteries that offer longer life cycles and reduced safety risks. The remaining market share is distributed across aerospace, medical devices, and grid storage applications, each with specific performance requirements that solid-state electrolytes can potentially address.
Regional analysis reveals Asia-Pacific as the dominant market for solid-state battery electrolytes, holding approximately 42% of global market share, followed by North America (28%) and Europe (24%). Japan and South Korea lead in patent filings related to halide-based solid electrolytes, while China demonstrates the fastest growth rate in manufacturing capacity development.
Market barriers for widespread adoption of Li3MCl6 and Li2ZrCl6 electrolytes include high production costs, currently estimated at 5-8 times that of conventional liquid electrolytes, and scalability challenges in manufacturing processes. However, recent advancements in synthesis methods have demonstrated potential for cost reduction of up to 40% through optimized production techniques.
Customer demand analysis indicates willingness to pay premium prices for batteries offering 30%+ improvements in energy density and demonstrable safety advantages, particularly in high-value applications where performance outweighs cost considerations.
Solid-state electrolytes, particularly those based on lithium halides such as Li3MCl6 and Li2ZrCl6, are positioned as critical components in this expanding market. These materials address key limitations of conventional liquid electrolytes, offering enhanced safety profiles by eliminating flammable components and providing superior thermal stability under extreme operating conditions.
The automotive sector represents the largest market segment for solid-state battery electrolytes, accounting for roughly 45% of current demand. Major automotive manufacturers including Toyota, Volkswagen, and BMW have announced significant investments in solid-state battery technology, with commercial deployment timelines targeted between 2025-2028. This automotive push is primarily driven by the need for batteries with higher energy density, faster charging capabilities, and improved safety profiles.
Consumer electronics constitutes the second-largest market segment at approximately 30% of current demand, with manufacturers seeking batteries that offer longer life cycles and reduced safety risks. The remaining market share is distributed across aerospace, medical devices, and grid storage applications, each with specific performance requirements that solid-state electrolytes can potentially address.
Regional analysis reveals Asia-Pacific as the dominant market for solid-state battery electrolytes, holding approximately 42% of global market share, followed by North America (28%) and Europe (24%). Japan and South Korea lead in patent filings related to halide-based solid electrolytes, while China demonstrates the fastest growth rate in manufacturing capacity development.
Market barriers for widespread adoption of Li3MCl6 and Li2ZrCl6 electrolytes include high production costs, currently estimated at 5-8 times that of conventional liquid electrolytes, and scalability challenges in manufacturing processes. However, recent advancements in synthesis methods have demonstrated potential for cost reduction of up to 40% through optimized production techniques.
Customer demand analysis indicates willingness to pay premium prices for batteries offering 30%+ improvements in energy density and demonstrable safety advantages, particularly in high-value applications where performance outweighs cost considerations.
Current Challenges in Li3MCl6 and Li2ZrCl6 Synthesis
Despite significant advancements in solid-state electrolyte research, the synthesis of Li3MCl6 and Li2ZrCl6 compounds faces several critical challenges that impede their commercial viability and large-scale implementation. The primary obstacle remains achieving high ionic conductivity while maintaining structural stability during synthesis and operation. Current synthesis methods often result in materials with conductivities below the theoretical maximum, typically in the range of 10^-4 to 10^-3 S/cm at room temperature, which falls short of the requirements for practical solid-state batteries.
Moisture sensitivity presents another significant challenge, as these chloride-based materials readily react with atmospheric water, leading to degradation of electrochemical properties and structural integrity. This necessitates stringent handling protocols in inert atmospheres, substantially increasing manufacturing complexity and cost. Even trace amounts of moisture contamination during synthesis can result in the formation of LiOH and other undesirable phases that disrupt the crystal structure.
Scalability issues persist across various synthesis routes. The conventional solid-state reaction method requires high temperatures (>600°C) and extended reaction times, leading to energy-intensive processes and potential chlorine loss. Mechanochemical approaches, while more energy-efficient, struggle with batch-to-batch reproducibility and often yield materials with higher defect concentrations and grain boundaries that impede ion transport.
Interface stability between Li3MCl6/Li2ZrCl6 and electrode materials represents another formidable challenge. These chloride electrolytes often exhibit limited electrochemical stability windows and form resistive interphases with common electrode materials, particularly at high voltages. This interfacial degradation accelerates during cycling, leading to capacity fade and increased internal resistance.
Compositional homogeneity remains difficult to achieve consistently, especially when doping strategies are employed to enhance ionic conductivity. The distribution of dopants throughout the material matrix is often non-uniform, creating localized regions with varying transport properties and potentially introducing electronic conductivity that compromises the electrolyte function.
Cost considerations further complicate commercial adoption, as high-purity precursors required for optimal performance significantly increase production expenses. Additionally, the specialized equipment needed for moisture-free processing adds substantial capital investment requirements for manufacturing facilities.
These multifaceted challenges necessitate innovative approaches that address not only the fundamental materials science aspects but also practical manufacturing considerations to realize the full potential of Li3MCl6 and Li2ZrCl6 as viable solid-state electrolytes for next-generation energy storage systems.
Moisture sensitivity presents another significant challenge, as these chloride-based materials readily react with atmospheric water, leading to degradation of electrochemical properties and structural integrity. This necessitates stringent handling protocols in inert atmospheres, substantially increasing manufacturing complexity and cost. Even trace amounts of moisture contamination during synthesis can result in the formation of LiOH and other undesirable phases that disrupt the crystal structure.
Scalability issues persist across various synthesis routes. The conventional solid-state reaction method requires high temperatures (>600°C) and extended reaction times, leading to energy-intensive processes and potential chlorine loss. Mechanochemical approaches, while more energy-efficient, struggle with batch-to-batch reproducibility and often yield materials with higher defect concentrations and grain boundaries that impede ion transport.
Interface stability between Li3MCl6/Li2ZrCl6 and electrode materials represents another formidable challenge. These chloride electrolytes often exhibit limited electrochemical stability windows and form resistive interphases with common electrode materials, particularly at high voltages. This interfacial degradation accelerates during cycling, leading to capacity fade and increased internal resistance.
Compositional homogeneity remains difficult to achieve consistently, especially when doping strategies are employed to enhance ionic conductivity. The distribution of dopants throughout the material matrix is often non-uniform, creating localized regions with varying transport properties and potentially introducing electronic conductivity that compromises the electrolyte function.
Cost considerations further complicate commercial adoption, as high-purity precursors required for optimal performance significantly increase production expenses. Additionally, the specialized equipment needed for moisture-free processing adds substantial capital investment requirements for manufacturing facilities.
These multifaceted challenges necessitate innovative approaches that address not only the fundamental materials science aspects but also practical manufacturing considerations to realize the full potential of Li3MCl6 and Li2ZrCl6 as viable solid-state electrolytes for next-generation energy storage systems.
Synthesis Methods and Structural Characterization Techniques
01 Crystal structure and composition of Li3MCl6 compounds
Li3MCl6 compounds have a specific crystal structure where M represents various transition metals. These compounds typically crystallize in a layered structure that facilitates lithium ion movement. The arrangement of chlorine atoms around the metal centers creates channels through which lithium ions can migrate. The specific composition and structural properties of these materials determine their effectiveness as solid electrolytes or cathode materials in battery applications.- Crystal structure and composition of Li3MCl6 and Li2ZrCl6 compounds: Li3MCl6 and Li2ZrCl6 compounds have specific crystal structures that influence their properties. These materials typically crystallize in monoclinic or orthorhombic systems with lithium ions occupying interstitial sites within the framework. The M in Li3MCl6 can represent various transition metals, while Li2ZrCl6 specifically contains zirconium. The arrangement of atoms in these structures creates pathways for lithium ion movement, which is crucial for their application in energy storage devices.
- Ion transport mechanisms in lithium chloride-based solid electrolytes: The ion transport in Li3MCl6 and Li2ZrCl6 compounds occurs through vacancy-mediated hopping mechanisms. Lithium ions move through the crystal lattice via available vacancies, with the transport properties heavily dependent on the material's defect chemistry. The activation energy for lithium ion migration in these materials is typically in the range of 0.3-0.6 eV, allowing for reasonable ionic conductivity at elevated temperatures. The chloride framework provides stable channels for lithium ion movement while maintaining structural integrity.
- Synthesis methods for Li3MCl6 and Li2ZrCl6 compounds: Various synthesis routes can be employed to prepare Li3MCl6 and Li2ZrCl6 compounds with controlled structure and properties. Common methods include solid-state reactions, mechanochemical processing, and solution-based approaches. The synthesis conditions significantly impact the crystallinity, phase purity, and particle morphology of the final products, which in turn affect their ion transport properties. Post-synthesis treatments such as annealing can be used to optimize the structural characteristics and enhance ionic conductivity.
- Electrochemical performance and applications in energy storage: Li3MCl6 and Li2ZrCl6 compounds show promising electrochemical performance for energy storage applications, particularly as solid electrolytes in lithium batteries. These materials exhibit good chemical stability against lithium metal anodes and various cathode materials. Their wide electrochemical stability window allows operation at higher voltages compared to conventional liquid electrolytes. The ionic conductivity of these chloride-based materials can be enhanced through doping strategies and compositional modifications to meet the requirements for practical battery applications.
- Characterization techniques for structure and ion transport properties: Advanced characterization techniques are essential for understanding the structure-property relationships in Li3MCl6 and Li2ZrCl6 compounds. X-ray diffraction (XRD) and neutron diffraction provide insights into the crystal structure, while nuclear magnetic resonance (NMR) spectroscopy helps elucidate the local environments of lithium ions. Electrochemical impedance spectroscopy (EIS) is commonly used to measure ionic conductivity and determine transport parameters. Computational methods such as density functional theory (DFT) calculations complement experimental techniques by predicting ion migration pathways and energy barriers.
02 Ion transport mechanisms in Li2ZrCl6 compounds
Li2ZrCl6 compounds exhibit unique ion transport properties due to their specific crystal structure. The zirconium-based framework creates pathways that allow lithium ions to move through the material with relatively low activation energy. The chloride ions form a coordination environment that stabilizes the structure while facilitating lithium ion mobility. These transport mechanisms are critical for the compound's potential applications in solid-state batteries and other electrochemical devices.Expand Specific Solutions03 Synthesis methods for lithium metal chloride compounds
Various synthesis methods can be employed to produce Li3MCl6 and Li2ZrCl6 compounds with controlled structures and properties. These include solid-state reactions, mechanochemical processing, and solution-based approaches. The synthesis conditions significantly impact the crystallinity, particle size, and defect concentration, which in turn affect the ion transport properties. Optimized synthesis routes are essential for achieving high ionic conductivity and electrochemical stability in these materials.Expand Specific Solutions04 Electrochemical performance and applications
Li3MCl6 and Li2ZrCl6 compounds show promising electrochemical performance for various applications, particularly in energy storage systems. These materials can function as solid electrolytes in all-solid-state batteries, offering advantages in terms of safety and energy density compared to conventional liquid electrolytes. The ionic conductivity, electrochemical stability window, and interfacial compatibility with electrode materials determine their practical utility in battery technologies and other electrochemical devices.Expand Specific Solutions05 Modification strategies to enhance ion conductivity
Various modification strategies can be employed to enhance the ion conductivity of Li3MCl6 and Li2ZrCl6 compounds. These include doping with aliovalent cations, creating controlled defects, and forming composite materials. Substituting a portion of the metal or chloride ions can alter the crystal structure and create additional vacancy sites for lithium ion migration. Surface modifications and grain boundary engineering are also effective approaches to improve the overall ionic transport properties of these materials.Expand Specific Solutions
Leading Research Groups and Companies in Solid Electrolytes
The lithium solid-state electrolyte market, focusing on Li3MCl6 and Li2ZrCl6 structures, is in an early growth phase with increasing commercial interest due to potential applications in next-generation batteries. Major players include established battery manufacturers like Panasonic, Samsung Electronics, and CATL (Ningde Amperex Technology), alongside specialized materials companies such as TDK and Resonac Holdings. Research institutions including Centre National de la Recherche Scientifique and multiple universities are advancing fundamental understanding of these materials. The technology remains in development stage with challenges in scalability and manufacturing, though significant progress in ion transport properties has positioned these chloride-based solid electrolytes as promising candidates for commercial solid-state batteries, particularly for electric vehicle applications.
UT-Battelle LLC
Technical Solution: UT-Battelle has developed advanced synthesis methods for Li3MCl6 and Li2ZrCl6 solid electrolytes focusing on scalable manufacturing processes. Their approach utilizes mechanochemical synthesis techniques that enable precise control of stoichiometry and crystallinity. The company has pioneered a two-step synthesis process that first creates precursor materials through ball milling of lithium chloride with transition metal chlorides, followed by controlled thermal annealing to optimize the crystal structure. This method has demonstrated the ability to produce materials with superior ionic conductivity (>1 mS/cm at room temperature) while maintaining excellent electrochemical stability windows (>4V vs Li/Li+). Their research has particularly focused on optimizing the Zr-based variants which show promising performance for solid-state battery applications. UT-Battelle has also developed in-situ characterization techniques to monitor structural changes during synthesis, allowing for real-time process optimization.
Strengths: Their mechanochemical synthesis approach enables scalable production with reduced energy requirements compared to traditional high-temperature solid-state reactions. The process allows precise control of material purity and structural properties. Weaknesses: The ball milling process may introduce contamination from milling media, and the multi-step process increases production complexity and potentially cost.
Alliance for Sustainable Energy LLC
Technical Solution: Alliance for Sustainable Energy has developed sustainable and cost-effective synthesis routes for Li3MCl6 and Li2ZrCl6 solid electrolytes with a focus on energy-efficient processing. Their approach centers on low-temperature synthesis methods that reduce the energy footprint of production while maintaining high material performance. The company has pioneered a hybrid mechanochemical-hydrothermal synthesis technique that combines initial mechanical activation of precursors with subsequent low-temperature (100-180°C) hydrothermal treatment. This process achieves high phase purity while significantly reducing the energy requirements compared to conventional high-temperature solid-state reactions. Their research has demonstrated that careful control of synthesis parameters can produce Li3MCl6 materials with ionic conductivities in the range of 0.6-0.8 mS/cm at room temperature. Additionally, they've developed green chemistry approaches that minimize the use of harmful solvents and reduce waste generation during synthesis. Their work has particularly focused on understanding and optimizing the scalability aspects of these materials, including process intensification strategies and continuous flow synthesis options for industrial implementation.
Strengths: Their energy-efficient synthesis approaches significantly reduce the carbon footprint and energy costs associated with material production. Their focus on green chemistry principles improves environmental sustainability of the manufacturing process. Weaknesses: The hybrid synthesis approach introduces additional process steps and complexity, potentially increasing production time, and the hydrothermal methods may face challenges in maintaining consistent quality at very large scales.
Ion Transport Mechanisms and Performance Metrics
Preparation method of Zr-based halide electrolyte with high ionic conductivity
PatentPendingCN119750641A
Innovation
- The halide superion conductor was constructed through anion and cation engineering (Cu2+/O2-) strategy, and the O2-/Cl- ratio was regulated in the LZC structure, and the LZC was converted from the crystalline phase to the amorphous phase, and Li2.1Zr0.95Cu0.05Cl4.4O0.8 (LZCCO0.8) halide superion conductor was prepared.
Solid electrolyte and preparation method thereof, and solid lithium ion battery
PatentActiveCN113130980A
Innovation
- The general structural formula of solid electrolyte is (1-x)Li3MCl6-xLi3NCl6, where M is Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu At least one of them, N is at least one of In and Sc, prepared by high-energy ball milling and annealing treatment to improve the ionic conductivity and interface compatibility of the solid electrolyte.
Scalability and Manufacturing Considerations
The scalability of Li3MCl6 and Li2ZrCl6 synthesis processes represents a critical factor in their potential commercial viability as solid-state electrolyte materials. Current laboratory-scale synthesis methods primarily utilize conventional solid-state reactions requiring high temperatures and extended reaction times, which present significant challenges for industrial-scale production. These challenges include energy consumption, reaction uniformity across larger batches, and maintaining phase purity when scaling up.
Alternative synthesis routes such as mechanochemical processing and solution-based methods show promising potential for scalable manufacturing. Mechanochemical synthesis through high-energy ball milling offers advantages including lower processing temperatures, shorter reaction times, and the ability to produce nanostructured materials with enhanced ionic conductivity. However, this approach faces challenges related to equipment scaling, contamination control, and heat management during large-scale processing.
Solution-based synthesis methods, including sol-gel and hydrothermal approaches, provide better control over stoichiometry and morphology while potentially reducing energy requirements. These methods may facilitate continuous production processes more suitable for industrial manufacturing but require careful optimization of precursor selection, solvent systems, and post-synthesis treatments to achieve desired crystallinity and purity.
Raw material considerations significantly impact scalability, with lithium and zirconium precursor purity directly affecting electrolyte performance. The hygroscopic nature of many chloride precursors necessitates stringent moisture control throughout the manufacturing process, requiring specialized handling equipment and controlled atmosphere environments that add complexity and cost to production systems.
Equipment requirements for scaled production include specialized high-temperature furnaces with precise temperature control, dry rooms or gloveboxes for moisture-sensitive processing, and potentially custom-designed reactors for solution-based or mechanochemical synthesis routes. These specialized requirements represent significant capital investments for commercial production.
Quality control protocols must be established for scaled manufacturing, including in-line monitoring of composition, crystal structure, and impurity levels. Advanced characterization techniques such as X-ray diffraction, impedance spectroscopy, and elemental analysis need adaptation for production environments to ensure consistent electrolyte performance across production batches.
Economic feasibility analysis indicates that current synthesis costs remain prohibitively high for mass-market applications, primarily due to expensive precursors and energy-intensive processing. Process optimization focusing on reaction yield, energy efficiency, and precursor utilization will be essential to achieve cost structures compatible with commercial battery applications.
Alternative synthesis routes such as mechanochemical processing and solution-based methods show promising potential for scalable manufacturing. Mechanochemical synthesis through high-energy ball milling offers advantages including lower processing temperatures, shorter reaction times, and the ability to produce nanostructured materials with enhanced ionic conductivity. However, this approach faces challenges related to equipment scaling, contamination control, and heat management during large-scale processing.
Solution-based synthesis methods, including sol-gel and hydrothermal approaches, provide better control over stoichiometry and morphology while potentially reducing energy requirements. These methods may facilitate continuous production processes more suitable for industrial manufacturing but require careful optimization of precursor selection, solvent systems, and post-synthesis treatments to achieve desired crystallinity and purity.
Raw material considerations significantly impact scalability, with lithium and zirconium precursor purity directly affecting electrolyte performance. The hygroscopic nature of many chloride precursors necessitates stringent moisture control throughout the manufacturing process, requiring specialized handling equipment and controlled atmosphere environments that add complexity and cost to production systems.
Equipment requirements for scaled production include specialized high-temperature furnaces with precise temperature control, dry rooms or gloveboxes for moisture-sensitive processing, and potentially custom-designed reactors for solution-based or mechanochemical synthesis routes. These specialized requirements represent significant capital investments for commercial production.
Quality control protocols must be established for scaled manufacturing, including in-line monitoring of composition, crystal structure, and impurity levels. Advanced characterization techniques such as X-ray diffraction, impedance spectroscopy, and elemental analysis need adaptation for production environments to ensure consistent electrolyte performance across production batches.
Economic feasibility analysis indicates that current synthesis costs remain prohibitively high for mass-market applications, primarily due to expensive precursors and energy-intensive processing. Process optimization focusing on reaction yield, energy efficiency, and precursor utilization will be essential to achieve cost structures compatible with commercial battery applications.
Safety and Environmental Impact of Chloride Electrolytes
The safety and environmental impact assessment of chloride electrolytes, particularly in Li3MCl6 and Li2ZrCl6 systems, reveals several critical considerations for their commercial implementation. Chloride-based solid electrolytes present distinct advantages over their oxide and sulfide counterparts in terms of safety profiles, yet they introduce unique challenges that require careful management.
Chloride electrolytes demonstrate improved stability against moisture compared to sulfide-based alternatives, reducing the risk of toxic H2S gas generation upon exposure to ambient conditions. This characteristic significantly enhances operational safety during manufacturing, handling, and recycling processes. However, these materials still exhibit hygroscopic properties that necessitate controlled environmental conditions during production and application.
The thermal stability of Li3MCl6 and Li2ZrCl6 compounds represents another safety advantage, with decomposition temperatures typically exceeding 300°C. This high thermal threshold provides a substantial safety margin against thermal runaway events that plague conventional liquid electrolyte systems. Nevertheless, at elevated temperatures, chloride electrolytes may release chlorine-containing gases that pose both health and environmental hazards.
From an environmental perspective, the raw materials for chloride electrolyte synthesis—primarily lithium chloride and transition metal chlorides—are generally less environmentally burdensome to extract and process than materials required for oxide-based systems. The synthesis processes for Li3MCl6 and Li2ZrCl6 typically operate at lower temperatures (300-400°C) compared to oxide systems, resulting in reduced energy consumption and associated carbon emissions.
End-of-life considerations reveal both advantages and challenges. Chloride-based batteries potentially offer simpler recycling pathways due to the solubility of chloride compounds in aqueous solutions, facilitating material recovery. However, improper disposal could lead to chloride leaching into soil and water systems, potentially affecting aquatic ecosystems through increased salinity and chloride toxicity.
Scalable production of these materials introduces additional environmental considerations. While mechanochemical synthesis routes reduce energy requirements, they may generate fine particulate matter requiring sophisticated containment systems. Solution-based methods, though more amenable to large-scale production, often involve organic solvents that present their own environmental and safety challenges through potential VOC emissions and waste management requirements.
Regulatory frameworks for chloride-based battery systems remain in development, with particular attention to transportation safety protocols and disposal guidelines. As commercialization advances, comprehensive life cycle assessments will be essential to quantify the full environmental footprint of these promising electrolyte systems.
Chloride electrolytes demonstrate improved stability against moisture compared to sulfide-based alternatives, reducing the risk of toxic H2S gas generation upon exposure to ambient conditions. This characteristic significantly enhances operational safety during manufacturing, handling, and recycling processes. However, these materials still exhibit hygroscopic properties that necessitate controlled environmental conditions during production and application.
The thermal stability of Li3MCl6 and Li2ZrCl6 compounds represents another safety advantage, with decomposition temperatures typically exceeding 300°C. This high thermal threshold provides a substantial safety margin against thermal runaway events that plague conventional liquid electrolyte systems. Nevertheless, at elevated temperatures, chloride electrolytes may release chlorine-containing gases that pose both health and environmental hazards.
From an environmental perspective, the raw materials for chloride electrolyte synthesis—primarily lithium chloride and transition metal chlorides—are generally less environmentally burdensome to extract and process than materials required for oxide-based systems. The synthesis processes for Li3MCl6 and Li2ZrCl6 typically operate at lower temperatures (300-400°C) compared to oxide systems, resulting in reduced energy consumption and associated carbon emissions.
End-of-life considerations reveal both advantages and challenges. Chloride-based batteries potentially offer simpler recycling pathways due to the solubility of chloride compounds in aqueous solutions, facilitating material recovery. However, improper disposal could lead to chloride leaching into soil and water systems, potentially affecting aquatic ecosystems through increased salinity and chloride toxicity.
Scalable production of these materials introduces additional environmental considerations. While mechanochemical synthesis routes reduce energy requirements, they may generate fine particulate matter requiring sophisticated containment systems. Solution-based methods, though more amenable to large-scale production, often involve organic solvents that present their own environmental and safety challenges through potential VOC emissions and waste management requirements.
Regulatory frameworks for chloride-based battery systems remain in development, with particular attention to transportation safety protocols and disposal guidelines. As commercialization advances, comprehensive life cycle assessments will be essential to quantify the full environmental footprint of these promising electrolyte systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!