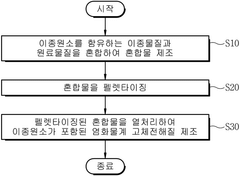
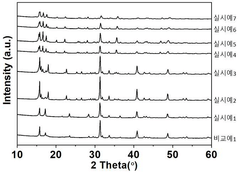
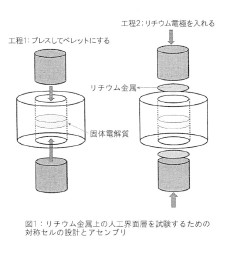
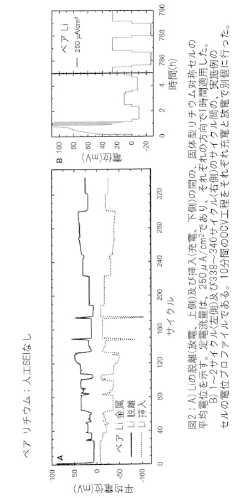
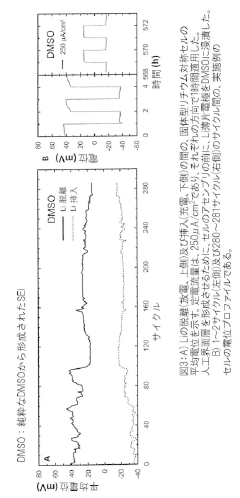
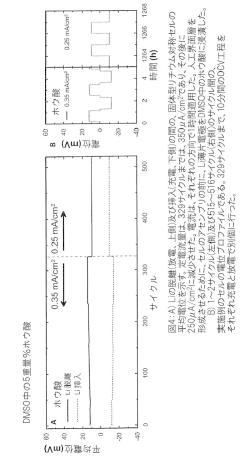
Interfacial Engineering Between Lithium Metal And Chloride Solid Electrolytes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium-Chloride Interface Engineering Background and Objectives
The development of lithium-ion batteries has revolutionized portable electronics and electric vehicles, yet current technologies face limitations in energy density, safety, and cycle life. Solid-state batteries using lithium metal anodes and solid electrolytes represent a promising next-generation energy storage solution, offering potentially higher energy densities and improved safety profiles. Among various solid electrolytes, chloride-based systems have emerged as particularly promising candidates due to their high ionic conductivity and favorable electrochemical stability.
The interface between lithium metal and chloride solid electrolytes represents a critical frontier in solid-state battery development. Historically, this interface has been plagued by high impedance, chemical instability, and mechanical degradation during cycling. These challenges stem from the reactive nature of lithium metal, the formation of interphases with varying ionic conductivity, and volume changes during lithium plating and stripping processes.
Recent technological evolution has focused on understanding the fundamental mechanisms of interfacial degradation and developing strategies to mitigate these issues. Early approaches relied primarily on physical modifications, while more recent advancements have incorporated chemical treatments, interlayers, and novel electrolyte formulations to stabilize the interface.
The primary objective of interfacial engineering between lithium metal and chloride solid electrolytes is to create a stable, low-impedance interface that maintains consistent performance over extended cycling. This involves minimizing chemical reactions that form resistive layers, ensuring uniform lithium deposition to prevent dendrite formation, and accommodating volume changes during cycling.
Secondary objectives include enhancing the mechanical properties of the interface to prevent delamination, reducing manufacturing complexity to enable commercial viability, and ensuring compatibility with existing battery production infrastructure. These goals must be achieved while maintaining or improving the inherent advantages of solid-state systems, such as safety and energy density.
The technological trajectory suggests a convergence of multiple approaches, including atomic layer deposition techniques, engineered interlayers, and surface chemistry modifications. Emerging research indicates that tailored chloride solid electrolytes with specific surface functionalities may offer superior interfacial properties compared to conventional formulations.
Understanding and controlling the lithium-chloride interface represents not only a scientific challenge but also a critical enabler for next-generation energy storage technologies that could power everything from consumer electronics to electric vehicles and grid-scale storage systems. Success in this domain could accelerate the transition away from fossil fuels and toward renewable energy systems by providing higher-capacity, faster-charging, and safer energy storage solutions.
The interface between lithium metal and chloride solid electrolytes represents a critical frontier in solid-state battery development. Historically, this interface has been plagued by high impedance, chemical instability, and mechanical degradation during cycling. These challenges stem from the reactive nature of lithium metal, the formation of interphases with varying ionic conductivity, and volume changes during lithium plating and stripping processes.
Recent technological evolution has focused on understanding the fundamental mechanisms of interfacial degradation and developing strategies to mitigate these issues. Early approaches relied primarily on physical modifications, while more recent advancements have incorporated chemical treatments, interlayers, and novel electrolyte formulations to stabilize the interface.
The primary objective of interfacial engineering between lithium metal and chloride solid electrolytes is to create a stable, low-impedance interface that maintains consistent performance over extended cycling. This involves minimizing chemical reactions that form resistive layers, ensuring uniform lithium deposition to prevent dendrite formation, and accommodating volume changes during cycling.
Secondary objectives include enhancing the mechanical properties of the interface to prevent delamination, reducing manufacturing complexity to enable commercial viability, and ensuring compatibility with existing battery production infrastructure. These goals must be achieved while maintaining or improving the inherent advantages of solid-state systems, such as safety and energy density.
The technological trajectory suggests a convergence of multiple approaches, including atomic layer deposition techniques, engineered interlayers, and surface chemistry modifications. Emerging research indicates that tailored chloride solid electrolytes with specific surface functionalities may offer superior interfacial properties compared to conventional formulations.
Understanding and controlling the lithium-chloride interface represents not only a scientific challenge but also a critical enabler for next-generation energy storage technologies that could power everything from consumer electronics to electric vehicles and grid-scale storage systems. Success in this domain could accelerate the transition away from fossil fuels and toward renewable energy systems by providing higher-capacity, faster-charging, and safer energy storage solutions.
Market Analysis for Solid-State Battery Technologies
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple industries. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating potential growth to reach $8-10 billion by 2030, representing a compound annual growth rate (CAGR) of over 35%.
Electric vehicles constitute the primary market driver, with automotive manufacturers investing heavily in solid-state technology to overcome the limitations of conventional lithium-ion batteries. Major automakers including Toyota, Volkswagen, and BMW have announced significant investments totaling over $13 billion in solid-state battery development, specifically targeting lithium metal anodes paired with solid electrolytes.
Consumer electronics represents the second largest application segment, where demand for longer-lasting, safer batteries continues to grow at 25% annually. The aerospace and defense sectors are emerging as high-value niche markets, with specialized requirements driving premium pricing for advanced solid-state solutions.
Regionally, Asia Pacific dominates the market landscape, accounting for approximately 45% of global development activities, with Japan and South Korea leading in patent filings related to lithium metal and chloride solid electrolyte interfaces. North America follows at 30% market share, with significant research clusters in California and Massachusetts focusing on interfacial engineering solutions.
The market for chloride-based solid electrolytes specifically has seen 40% year-over-year growth since 2020, outpacing other solid electrolyte chemistries. This acceleration is attributed to their superior ionic conductivity and compatibility with lithium metal anodes when properly engineered at the interface.
Venture capital funding in startups focused on interfacial engineering between lithium metal and solid electrolytes has reached $2.1 billion in the past three years, with five companies securing funding rounds exceeding $100 million each in 2022-2023.
Market barriers include high manufacturing costs, with current solid-state cells costing 3-5 times more than conventional lithium-ion batteries, though economies of scale are expected to reduce this premium to 1.5 times by 2028. Technical challenges at the lithium-chloride electrolyte interface remain the critical bottleneck, with companies that successfully address interfacial stability issues positioned to capture disproportionate market share.
Customer surveys indicate 85% of EV manufacturers consider solid-state technology "extremely important" to their future product roadmaps, with 70% specifically citing lithium metal anodes as critical to achieving next-generation energy density targets above 400 Wh/kg.
Electric vehicles constitute the primary market driver, with automotive manufacturers investing heavily in solid-state technology to overcome the limitations of conventional lithium-ion batteries. Major automakers including Toyota, Volkswagen, and BMW have announced significant investments totaling over $13 billion in solid-state battery development, specifically targeting lithium metal anodes paired with solid electrolytes.
Consumer electronics represents the second largest application segment, where demand for longer-lasting, safer batteries continues to grow at 25% annually. The aerospace and defense sectors are emerging as high-value niche markets, with specialized requirements driving premium pricing for advanced solid-state solutions.
Regionally, Asia Pacific dominates the market landscape, accounting for approximately 45% of global development activities, with Japan and South Korea leading in patent filings related to lithium metal and chloride solid electrolyte interfaces. North America follows at 30% market share, with significant research clusters in California and Massachusetts focusing on interfacial engineering solutions.
The market for chloride-based solid electrolytes specifically has seen 40% year-over-year growth since 2020, outpacing other solid electrolyte chemistries. This acceleration is attributed to their superior ionic conductivity and compatibility with lithium metal anodes when properly engineered at the interface.
Venture capital funding in startups focused on interfacial engineering between lithium metal and solid electrolytes has reached $2.1 billion in the past three years, with five companies securing funding rounds exceeding $100 million each in 2022-2023.
Market barriers include high manufacturing costs, with current solid-state cells costing 3-5 times more than conventional lithium-ion batteries, though economies of scale are expected to reduce this premium to 1.5 times by 2028. Technical challenges at the lithium-chloride electrolyte interface remain the critical bottleneck, with companies that successfully address interfacial stability issues positioned to capture disproportionate market share.
Customer surveys indicate 85% of EV manufacturers consider solid-state technology "extremely important" to their future product roadmaps, with 70% specifically citing lithium metal anodes as critical to achieving next-generation energy density targets above 400 Wh/kg.
Current Challenges in Li-Metal/Chloride Solid Electrolyte Interfaces
The interface between lithium metal anodes and chloride solid electrolytes represents one of the most critical challenges in the development of all-solid-state batteries. Despite the promising ionic conductivity of chloride solid electrolytes, their chemical and electrochemical stability against lithium metal remains problematic. The high reactivity of lithium metal with chloride-based electrolytes leads to the formation of interphases that significantly increase interfacial resistance, hampering efficient lithium ion transport.
A primary challenge is the continuous decomposition of chloride solid electrolytes when in contact with lithium metal. This reaction forms lithium chloride (LiCl) and other decomposition products at the interface, creating a resistive layer that grows over time. Unlike the beneficial solid electrolyte interphase (SEI) formed in liquid electrolyte systems, this interfacial layer in solid-state batteries often lacks sufficient ionic conductivity, leading to increased cell impedance and capacity fade.
Mechanical issues further complicate the interface stability. During cycling, lithium metal undergoes significant volume changes, creating mechanical stress at the interface. The brittle nature of most chloride solid electrolytes means they cannot accommodate these volume fluctuations, resulting in contact loss and increased interfacial resistance. This mechanical mismatch accelerates degradation and can lead to dendrite formation through cracks in the solid electrolyte.
The heterogeneous nature of the interface presents another significant challenge. Local current density variations cause uneven lithium deposition and dissolution, leading to dendrite growth even in solid-state systems. These dendrites can propagate through grain boundaries or defects in the chloride electrolyte, eventually causing short circuits and catastrophic battery failure.
Chemical compatibility issues extend beyond simple decomposition reactions. Impurities in either the lithium metal or the chloride electrolyte can accelerate interfacial degradation. Trace amounts of moisture or oxygen can trigger side reactions that consume active lithium and degrade the electrolyte structure, particularly at elevated temperatures commonly used to improve ionic conductivity in solid-state systems.
The high interfacial resistance also necessitates elevated operating temperatures, typically above 60°C, to achieve practical performance levels. This temperature requirement limits the practical application of these systems and introduces additional thermal management complexities in battery design.
Recent research has identified that many chloride solid electrolytes exhibit narrow electrochemical stability windows, making them thermodynamically unstable against lithium metal. This fundamental limitation suggests that interface engineering approaches must focus not only on improving contact but also on creating artificial interphases with enhanced stability and conductivity properties.
A primary challenge is the continuous decomposition of chloride solid electrolytes when in contact with lithium metal. This reaction forms lithium chloride (LiCl) and other decomposition products at the interface, creating a resistive layer that grows over time. Unlike the beneficial solid electrolyte interphase (SEI) formed in liquid electrolyte systems, this interfacial layer in solid-state batteries often lacks sufficient ionic conductivity, leading to increased cell impedance and capacity fade.
Mechanical issues further complicate the interface stability. During cycling, lithium metal undergoes significant volume changes, creating mechanical stress at the interface. The brittle nature of most chloride solid electrolytes means they cannot accommodate these volume fluctuations, resulting in contact loss and increased interfacial resistance. This mechanical mismatch accelerates degradation and can lead to dendrite formation through cracks in the solid electrolyte.
The heterogeneous nature of the interface presents another significant challenge. Local current density variations cause uneven lithium deposition and dissolution, leading to dendrite growth even in solid-state systems. These dendrites can propagate through grain boundaries or defects in the chloride electrolyte, eventually causing short circuits and catastrophic battery failure.
Chemical compatibility issues extend beyond simple decomposition reactions. Impurities in either the lithium metal or the chloride electrolyte can accelerate interfacial degradation. Trace amounts of moisture or oxygen can trigger side reactions that consume active lithium and degrade the electrolyte structure, particularly at elevated temperatures commonly used to improve ionic conductivity in solid-state systems.
The high interfacial resistance also necessitates elevated operating temperatures, typically above 60°C, to achieve practical performance levels. This temperature requirement limits the practical application of these systems and introduces additional thermal management complexities in battery design.
Recent research has identified that many chloride solid electrolytes exhibit narrow electrochemical stability windows, making them thermodynamically unstable against lithium metal. This fundamental limitation suggests that interface engineering approaches must focus not only on improving contact but also on creating artificial interphases with enhanced stability and conductivity properties.
State-of-the-Art Interface Modification Strategies
01 Protective coating layers for lithium-chloride interfaces
Protective coating layers can be applied at the interface between lithium metal and chloride solid electrolytes to enhance stability and prevent unwanted reactions. These coatings act as buffer layers that mitigate the chemical and electrochemical reactions occurring at the interface. Materials such as lithium phosphorus oxynitride (LiPON), lithium nitride, or thin metal films can be used as protective layers to improve the interfacial stability and reduce impedance growth during battery cycling.- Protective coating layers for lithium-chloride interfaces: Applying protective coating layers between lithium metal anodes and chloride solid electrolytes can significantly improve interface stability and reduce interfacial resistance. These coatings act as buffer layers that prevent direct chemical reactions between lithium metal and chloride electrolytes, which often lead to degradation. Materials such as lithium nitride, lithium phosphorus oxynitride, and certain polymers can be used as effective protective layers that allow lithium ion transport while preventing side reactions at the interface.
- Interfacial modification with lithium-containing compounds: Introducing lithium-containing compounds at the interface between lithium metal and chloride solid electrolytes can enhance interfacial stability and improve electrochemical performance. These compounds, such as lithium halides, lithium phosphates, and lithium oxides, can form a stable solid electrolyte interphase (SEI) that facilitates lithium ion transport while suppressing dendrite formation. The modified interface reduces impedance and enables more uniform lithium deposition and stripping during battery cycling.
- Composite interlayers for enhanced ion conductivity: Developing composite interlayers that combine multiple materials with complementary properties can significantly improve the interface between lithium metal and chloride solid electrolytes. These composite interlayers typically contain a mixture of ionic conductors, electronic insulators, and mechanically robust components that work together to facilitate lithium ion transport while preventing electronic short circuits. The composite structure can accommodate volume changes during cycling and maintain intimate contact between the lithium metal and solid electrolyte.
- Surface treatment techniques for chloride electrolytes: Various surface treatment techniques can be applied to chloride solid electrolytes to improve their compatibility with lithium metal anodes. These treatments include thermal annealing, chemical etching, plasma treatment, and mechanical polishing, which can modify the surface chemistry and morphology of the electrolyte. Treated surfaces typically exhibit reduced interfacial resistance, improved wettability with lithium, and enhanced electrochemical stability, leading to better battery performance and longer cycle life.
- In-situ formed interfaces through electrolyte additives: Incorporating specific additives into the battery system can promote the in-situ formation of stable interfaces between lithium metal and chloride solid electrolytes during battery operation. These additives can include fluorinated compounds, boron-containing molecules, and certain salts that decompose preferentially to form a protective layer at the interface. The in-situ formed interface can self-heal during cycling and adapt to the changing electrode surface, providing long-term stability and improved cycling performance.
02 Interfacial modification with additives and dopants
Chemical additives and dopants can be incorporated at the lithium-chloride solid electrolyte interface to improve compatibility and reduce interfacial resistance. These additives can include lithium salts, polymers, or ceramic particles that enhance ion transport across the interface while suppressing dendrite formation. The strategic introduction of dopants can alter the electrochemical properties of the interface, leading to improved cycling stability and enhanced battery performance.Expand Specific Solutions03 Composite interlayers for enhanced ion transport
Composite interlayers consisting of multiple materials can be engineered between lithium metal and chloride solid electrolytes to facilitate smooth lithium ion transport. These interlayers typically combine polymers, ceramics, or gel materials that provide both mechanical flexibility and ionic conductivity. The composite structure helps to maintain intimate contact between the lithium metal and solid electrolyte while accommodating volume changes during cycling, thereby reducing interfacial resistance and improving battery performance.Expand Specific Solutions04 Surface treatment of lithium metal electrodes
Surface treatments can be applied to lithium metal electrodes before assembly with chloride solid electrolytes to improve interfacial compatibility. These treatments may include mechanical polishing, chemical etching, or plasma treatment to remove surface impurities and create a more reactive surface for bonding with the electrolyte. Such treatments can significantly reduce the initial interfacial resistance and improve the wetting behavior between lithium metal and the solid electrolyte, leading to more uniform lithium deposition during battery operation.Expand Specific Solutions05 Pressure-assisted interfacial engineering
Applying controlled pressure during battery assembly or operation can improve the contact between lithium metal and chloride solid electrolytes. This approach helps to maintain intimate physical contact at the interface, reducing void formation and minimizing interfacial resistance. Pressure-assisted techniques can include stack pressure during cell assembly, external pressure application during cycling, or the use of specially designed cell housings that maintain consistent pressure on the interface throughout battery operation.Expand Specific Solutions
Leading Research Groups and Companies in Solid-State Battery Field
The interfacial engineering between lithium metal and chloride solid electrolytes market is in an early growth phase, characterized by intensive R&D activities across academic institutions and industry players. The global solid-state battery market, which this technology supports, is projected to reach $8-10 billion by 2030, with a CAGR exceeding 30%. Technical maturity varies significantly among key players: established corporations like Toyota, GM, and IBM are advancing commercial applications, while specialized companies such as PolyPlus Battery and Saft Groupe are developing proprietary interface engineering solutions. Chinese institutions (Shanghai Institute of Ceramics, Xidian University) are rapidly closing the technology gap through government-backed research initiatives, while academic-industrial collaborations between universities and companies like Corning and Applied Materials are accelerating innovation in electrode-electrolyte interface design.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has developed a proprietary "Protected Lithium Electrode" (PLE) technology specifically designed for compatibility with chloride solid electrolytes. Their approach uses a polymer-ceramic composite interlayer that serves as both an artificial solid electrolyte interphase (SEI) and a physical barrier between lithium metal and chloride-based solid electrolytes. The engineered interface consists of a lithium-ion conducting polymer matrix embedded with ceramic nanoparticles that enhance mechanical stability and ionic conductivity. This composite layer is chemically modified to be compatible with chloride-containing environments, preventing the formation of LiCl and other detrimental reaction products at the interface. PolyPlus's technology also incorporates specialized surface treatments of the lithium metal that create a lithium-rich phosphate or nitride layer prior to interface formation, further enhancing stability. Their approach has demonstrated significant improvements in cycling efficiency and interfacial stability, with test cells achieving over 500 cycles with minimal capacity degradation.
Strengths: Specialized expertise in lithium protection technologies; scalable manufacturing processes adaptable to existing production lines; demonstrated stability in various chloride electrolyte systems. Weaknesses: Polymer components may limit high-temperature operation; potential long-term degradation of polymer matrix in contact with lithium metal.
Shanghai Institute of Ceramics, Chinese Academy of Sciences
Technical Solution: The Shanghai Institute of Ceramics has developed an innovative interfacial engineering approach focusing on in-situ formed protective layers between lithium metal and chloride solid electrolytes. Their technology utilizes controlled reactivity principles to create beneficial interfacial compounds rather than trying to completely prevent reactions. The institute's researchers have pioneered the use of lithium-containing additives (such as Li3N, Li2S-P2S5, and LiF) that are strategically incorporated at the interface to promote the formation of a stable, ion-conducting interphase. These additives react preferentially with the chloride electrolyte to form a self-limiting interfacial layer that prevents further degradation while maintaining high lithium-ion conductivity. The institute has also developed specialized surface modification techniques for chloride electrolytes, including the creation of lithium-rich surface layers through ion exchange processes that improve compatibility with lithium metal. Their approach combines computational modeling with advanced characterization techniques to optimize the interfacial chemistry for specific chloride electrolyte compositions.
Strengths: Innovative approach leveraging controlled reactivity rather than complete prevention; deep expertise in ceramic materials science; cost-effective compared to complex coating technologies. Weaknesses: Interface properties may vary with different chloride electrolyte compositions; potential challenges in precisely controlling in-situ formed interfaces in mass production.
Key Patents and Publications on Li-Chloride Interface Engineering
Chloride-based solid electrolyte, all-solid batteries and manufacturing method thereof
PatentActiveKR1020220072088A
Innovation
- A chloride-based solid electrolyte is developed by substituting part of yttrium with hafnium, prepared in a moisture-free environment, using Li6-xY1-xHf xCl6 (0<x≤0.7), enhancing lithium ion conductivity and stability.
Interface layer between lithium metal and solid electrolyte
PatentActiveJP2019125578A
Innovation
- An artificial interfacial layer formed by reacting lithium metal with an acid in a non-aqueous solvent creates a lithium-ion conducting but electronically non-conducting layer on the lithium metal surface, preventing electrolyte decomposition and stabilizing the electrode.
Safety and Performance Metrics for Solid-State Battery Interfaces
Safety and performance metrics for solid-state battery interfaces represent critical evaluation parameters that determine the viability of lithium metal batteries with chloride solid electrolytes. These metrics must be systematically assessed to ensure both operational safety and optimal performance across various conditions.
Interface stability metrics include chemical compatibility between lithium metal and chloride electrolytes, which can be quantified through impedance growth rates during cycling. Successful interfacial engineering should demonstrate impedance increases of less than 10% over 100 cycles. Mechanical stability at the interface requires measurement of critical pressure thresholds before dendrite propagation occurs, with superior interfaces withstanding pressures above 10 MPa.
Electrochemical performance metrics focus on interfacial resistance, typically measured via electrochemical impedance spectroscopy. State-of-the-art engineered interfaces between lithium and chloride electrolytes achieve area-specific resistances below 10 Ω·cm². Coulombic efficiency serves as another crucial metric, with well-engineered interfaces demonstrating values exceeding 99.9% over extended cycling.
Safety assessment protocols must include thermal runaway tests, where interfaces should maintain stability at temperatures up to 100°C above normal operating conditions. Short-circuit prevention capabilities are evaluated through dendrite penetration tests, with effective interfaces preventing lithium dendrite formation even at current densities exceeding 3 mA/cm².
Cycle life durability represents a comprehensive performance indicator, with advanced interfacial engineering solutions enabling over 1000 cycles with less than 20% capacity degradation. Rate capability metrics assess how interfaces perform under high current densities, with superior designs maintaining at least 80% capacity at 3C rates compared to 0.1C performance.
Environmental resilience testing evaluates interface stability across temperature ranges (-20°C to 60°C) and humidity conditions. Properly engineered interfaces should demonstrate less than 15% performance variation across this temperature spectrum. Additionally, manufacturing scalability metrics assess whether interfacial engineering approaches can be implemented in industrial settings with reproducible performance characteristics.
Standardized testing protocols are emerging to enable direct comparison between different interfacial engineering strategies. These include accelerated aging tests, pulse power capability measurements, and in-situ characterization techniques that provide real-time monitoring of interface evolution during battery operation.
Interface stability metrics include chemical compatibility between lithium metal and chloride electrolytes, which can be quantified through impedance growth rates during cycling. Successful interfacial engineering should demonstrate impedance increases of less than 10% over 100 cycles. Mechanical stability at the interface requires measurement of critical pressure thresholds before dendrite propagation occurs, with superior interfaces withstanding pressures above 10 MPa.
Electrochemical performance metrics focus on interfacial resistance, typically measured via electrochemical impedance spectroscopy. State-of-the-art engineered interfaces between lithium and chloride electrolytes achieve area-specific resistances below 10 Ω·cm². Coulombic efficiency serves as another crucial metric, with well-engineered interfaces demonstrating values exceeding 99.9% over extended cycling.
Safety assessment protocols must include thermal runaway tests, where interfaces should maintain stability at temperatures up to 100°C above normal operating conditions. Short-circuit prevention capabilities are evaluated through dendrite penetration tests, with effective interfaces preventing lithium dendrite formation even at current densities exceeding 3 mA/cm².
Cycle life durability represents a comprehensive performance indicator, with advanced interfacial engineering solutions enabling over 1000 cycles with less than 20% capacity degradation. Rate capability metrics assess how interfaces perform under high current densities, with superior designs maintaining at least 80% capacity at 3C rates compared to 0.1C performance.
Environmental resilience testing evaluates interface stability across temperature ranges (-20°C to 60°C) and humidity conditions. Properly engineered interfaces should demonstrate less than 15% performance variation across this temperature spectrum. Additionally, manufacturing scalability metrics assess whether interfacial engineering approaches can be implemented in industrial settings with reproducible performance characteristics.
Standardized testing protocols are emerging to enable direct comparison between different interfacial engineering strategies. These include accelerated aging tests, pulse power capability measurements, and in-situ characterization techniques that provide real-time monitoring of interface evolution during battery operation.
Manufacturing Scalability of Interfacial Engineering Solutions
The scalability of interfacial engineering solutions represents a critical challenge in transitioning lithium metal-chloride solid electrolyte systems from laboratory demonstrations to commercial production. Current laboratory-scale interfacial treatments often involve complex processes such as atomic layer deposition, physical vapor deposition, or solution-based chemical treatments that are difficult to implement in high-volume manufacturing environments.
Vacuum-based deposition techniques, while offering precise control over interfacial layers, face significant throughput limitations when scaled to industrial production. The equipment required for these processes demands substantial capital investment and typically operates in batch mode rather than continuous processing, creating production bottlenecks. Additionally, maintaining uniform deposition across large-area substrates presents technical challenges that impact product consistency.
Solution-based approaches offer potentially higher throughput but struggle with precise thickness control and uniform coverage across the lithium metal-solid electrolyte interface. These methods often require careful control of process parameters such as concentration, temperature, and reaction time, which become increasingly difficult to maintain consistently at larger scales.
Cost considerations further complicate manufacturing scalability. Many interfacial engineering solutions utilize expensive materials such as noble metals or specialized organic compounds. The economic viability of these approaches diminishes significantly when projected to commercial production volumes, necessitating the development of alternative materials that maintain performance while reducing costs.
Energy consumption during manufacturing represents another scaling challenge. High-temperature processes or extended treatment times increase production costs and carbon footprint, contradicting sustainability goals for clean energy technologies. Developing low-energy processing routes becomes essential for commercial viability.
Quality control and characterization methods must also evolve to accommodate high-volume production. Current analytical techniques for evaluating interfacial properties are often time-consuming and destructive, making them unsuitable for in-line quality assurance in manufacturing environments. Non-destructive, rapid characterization methods are needed to ensure consistent interface quality during mass production.
Recent advances in roll-to-roll processing and solution-based coating technologies show promise for scaling certain interfacial engineering approaches. These continuous manufacturing methods could potentially reduce production costs while maintaining the critical interfacial properties necessary for optimal battery performance. However, significant research and development efforts are still required to adapt laboratory-proven interfacial engineering solutions to these manufacturing platforms.
Vacuum-based deposition techniques, while offering precise control over interfacial layers, face significant throughput limitations when scaled to industrial production. The equipment required for these processes demands substantial capital investment and typically operates in batch mode rather than continuous processing, creating production bottlenecks. Additionally, maintaining uniform deposition across large-area substrates presents technical challenges that impact product consistency.
Solution-based approaches offer potentially higher throughput but struggle with precise thickness control and uniform coverage across the lithium metal-solid electrolyte interface. These methods often require careful control of process parameters such as concentration, temperature, and reaction time, which become increasingly difficult to maintain consistently at larger scales.
Cost considerations further complicate manufacturing scalability. Many interfacial engineering solutions utilize expensive materials such as noble metals or specialized organic compounds. The economic viability of these approaches diminishes significantly when projected to commercial production volumes, necessitating the development of alternative materials that maintain performance while reducing costs.
Energy consumption during manufacturing represents another scaling challenge. High-temperature processes or extended treatment times increase production costs and carbon footprint, contradicting sustainability goals for clean energy technologies. Developing low-energy processing routes becomes essential for commercial viability.
Quality control and characterization methods must also evolve to accommodate high-volume production. Current analytical techniques for evaluating interfacial properties are often time-consuming and destructive, making them unsuitable for in-line quality assurance in manufacturing environments. Non-destructive, rapid characterization methods are needed to ensure consistent interface quality during mass production.
Recent advances in roll-to-roll processing and solution-based coating technologies show promise for scaling certain interfacial engineering approaches. These continuous manufacturing methods could potentially reduce production costs while maintaining the critical interfacial properties necessary for optimal battery performance. However, significant research and development efforts are still required to adapt laboratory-proven interfacial engineering solutions to these manufacturing platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!