Modeling Ion Transport In Disordered Chloride Lattices
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion Transport Background and Research Objectives
Ion transport in disordered chloride lattices represents a critical area of research with significant implications for materials science, energy storage, and environmental applications. The study of ion movement through these complex structures has evolved considerably over the past decades, transitioning from simplified models to sophisticated computational approaches that can account for structural disorder and dynamic interactions.
Historically, ion transport research began with idealized crystalline models that failed to capture the complexity of real-world materials. The recognition that disorder plays a fundamental role in determining transport properties marked a paradigm shift in the field during the 1980s and 1990s. Since then, advances in computational methods and experimental techniques have enabled increasingly accurate characterization of ion mobility in disordered systems.
Chloride lattices present particular interest due to their prevalence in numerous technological applications, including solid-state batteries, fuel cells, desalination membranes, and environmental remediation technologies. The transport behavior of various ions (including Na+, K+, Li+, Mg2+) through chloride-containing structures significantly impacts the performance and efficiency of these technologies.
Current research trends indicate growing interest in multi-scale modeling approaches that bridge atomic-level phenomena with macroscopic transport properties. Machine learning techniques are increasingly being integrated with traditional molecular dynamics simulations to predict ion transport in complex disordered systems with greater accuracy and computational efficiency.
The primary objectives of this technical research include developing comprehensive models that can accurately predict ion transport mechanisms in disordered chloride lattices across different temperature ranges and concentration gradients. These models must account for various types of disorder, including compositional, positional, and topological variations that occur in real materials.
Additionally, we aim to establish correlations between structural characteristics and transport properties to enable rational design of materials with enhanced ion conductivity. This includes identifying key structural motifs and defect configurations that facilitate or hinder ion movement through the lattice.
Another critical goal is to develop predictive capabilities for how external factors—such as temperature fluctuations, applied electric fields, and mechanical stress—influence ion transport dynamics in these disordered systems. This understanding will support the optimization of materials for specific operational conditions.
The research also seeks to bridge the gap between theoretical predictions and experimental observations by developing models that can be validated against measurable parameters, thereby establishing a feedback loop for continuous refinement of both theoretical frameworks and experimental methodologies.
Historically, ion transport research began with idealized crystalline models that failed to capture the complexity of real-world materials. The recognition that disorder plays a fundamental role in determining transport properties marked a paradigm shift in the field during the 1980s and 1990s. Since then, advances in computational methods and experimental techniques have enabled increasingly accurate characterization of ion mobility in disordered systems.
Chloride lattices present particular interest due to their prevalence in numerous technological applications, including solid-state batteries, fuel cells, desalination membranes, and environmental remediation technologies. The transport behavior of various ions (including Na+, K+, Li+, Mg2+) through chloride-containing structures significantly impacts the performance and efficiency of these technologies.
Current research trends indicate growing interest in multi-scale modeling approaches that bridge atomic-level phenomena with macroscopic transport properties. Machine learning techniques are increasingly being integrated with traditional molecular dynamics simulations to predict ion transport in complex disordered systems with greater accuracy and computational efficiency.
The primary objectives of this technical research include developing comprehensive models that can accurately predict ion transport mechanisms in disordered chloride lattices across different temperature ranges and concentration gradients. These models must account for various types of disorder, including compositional, positional, and topological variations that occur in real materials.
Additionally, we aim to establish correlations between structural characteristics and transport properties to enable rational design of materials with enhanced ion conductivity. This includes identifying key structural motifs and defect configurations that facilitate or hinder ion movement through the lattice.
Another critical goal is to develop predictive capabilities for how external factors—such as temperature fluctuations, applied electric fields, and mechanical stress—influence ion transport dynamics in these disordered systems. This understanding will support the optimization of materials for specific operational conditions.
The research also seeks to bridge the gap between theoretical predictions and experimental observations by developing models that can be validated against measurable parameters, thereby establishing a feedback loop for continuous refinement of both theoretical frameworks and experimental methodologies.
Market Applications for Disordered Chloride Lattice Technologies
The market for technologies based on disordered chloride lattices is experiencing significant growth across multiple sectors, driven by the unique ion transport properties these materials exhibit. Energy storage represents the primary application domain, with next-generation batteries utilizing disordered chloride lattices showing promise for higher energy densities and faster charging capabilities. These advanced materials could potentially overcome current lithium-ion battery limitations, particularly in electric vehicles and grid-scale storage systems where energy density and charging speed remain critical challenges.
Semiconductor manufacturing presents another substantial market opportunity. The controlled ion transport properties of disordered chloride lattices make them valuable for developing novel doping techniques and creating specialized semiconductor materials with customized electrical properties. This application is particularly relevant as the semiconductor industry continues its pursuit of smaller, more efficient devices beyond traditional silicon-based technologies.
Environmental remediation technologies represent a growing application area, with disordered chloride lattice materials showing effectiveness in selective ion capture systems for water purification and waste treatment. Their ability to selectively transport specific ions makes them ideal for removing heavy metals and other contaminants from industrial wastewater and contaminated groundwater sources.
The medical technology sector is exploring these materials for drug delivery systems and biosensors. The controlled ion transport mechanisms can be leveraged to create smart drug delivery platforms that release therapeutic agents in response to specific physiological conditions. Similarly, biosensors utilizing these materials can detect minute changes in ion concentrations, enabling more sensitive diagnostic tools.
Emerging applications in quantum computing are also noteworthy, where the unique quantum properties of certain disordered chloride lattices could potentially serve as platforms for quantum bits or quantum information processing. Though still in early research stages, this represents a high-value future market with significant growth potential.
The telecommunications industry is investigating these materials for next-generation signal processing components, where controlled ion movement could enable novel switching mechanisms and signal modulation techniques. This could lead to more efficient and compact communication devices with enhanced performance characteristics.
Market analysis indicates that while energy storage applications currently dominate research funding and commercial interest, the diversification across multiple sectors suggests a robust long-term market outlook. The interdisciplinary nature of these applications also creates opportunities for technology transfer and cross-sector innovation, potentially accelerating commercialization timelines and expanding market reach.
Semiconductor manufacturing presents another substantial market opportunity. The controlled ion transport properties of disordered chloride lattices make them valuable for developing novel doping techniques and creating specialized semiconductor materials with customized electrical properties. This application is particularly relevant as the semiconductor industry continues its pursuit of smaller, more efficient devices beyond traditional silicon-based technologies.
Environmental remediation technologies represent a growing application area, with disordered chloride lattice materials showing effectiveness in selective ion capture systems for water purification and waste treatment. Their ability to selectively transport specific ions makes them ideal for removing heavy metals and other contaminants from industrial wastewater and contaminated groundwater sources.
The medical technology sector is exploring these materials for drug delivery systems and biosensors. The controlled ion transport mechanisms can be leveraged to create smart drug delivery platforms that release therapeutic agents in response to specific physiological conditions. Similarly, biosensors utilizing these materials can detect minute changes in ion concentrations, enabling more sensitive diagnostic tools.
Emerging applications in quantum computing are also noteworthy, where the unique quantum properties of certain disordered chloride lattices could potentially serve as platforms for quantum bits or quantum information processing. Though still in early research stages, this represents a high-value future market with significant growth potential.
The telecommunications industry is investigating these materials for next-generation signal processing components, where controlled ion movement could enable novel switching mechanisms and signal modulation techniques. This could lead to more efficient and compact communication devices with enhanced performance characteristics.
Market analysis indicates that while energy storage applications currently dominate research funding and commercial interest, the diversification across multiple sectors suggests a robust long-term market outlook. The interdisciplinary nature of these applications also creates opportunities for technology transfer and cross-sector innovation, potentially accelerating commercialization timelines and expanding market reach.
Current Challenges in Modeling Disordered Ionic Systems
The modeling of ion transport in disordered chloride lattices presents several significant challenges that continue to impede progress in this field. Traditional computational approaches often struggle with accurately representing the complex dynamics of ionic movement through disordered structures, particularly when dealing with chloride ions which exhibit unique transport behaviors compared to other halides.
One fundamental challenge lies in capturing the multi-scale nature of ion transport phenomena. Molecular dynamics simulations can provide atomic-level insights but are computationally expensive for long time scales relevant to macroscopic transport properties. Conversely, continuum models may overlook critical microscopic interactions that govern transport mechanisms in disordered systems.
The representation of structural disorder itself poses a substantial hurdle. Unlike crystalline materials with well-defined periodic structures, disordered chloride lattices exhibit significant local variations in coordination environments, defect concentrations, and energy landscapes. Current models often rely on oversimplified representations that fail to capture the full complexity of these disordered environments, particularly the correlation between structural disorder and transport pathways.
Accurately modeling the energetics of ion-ion and ion-lattice interactions remains problematic. The long-range Coulombic interactions, short-range repulsions, and polarization effects all contribute to the complex potential energy surface that governs ion mobility. Existing force fields frequently struggle to balance computational efficiency with the accuracy needed to predict transport properties in disordered systems.
Temperature effects introduce additional complexity, as thermal fluctuations can significantly alter the energy landscape and activation barriers for ion migration. Most current models inadequately account for the temperature dependence of transport parameters, limiting their predictive capability across different operating conditions relevant to practical applications.
The coupling between different transport mechanisms presents another challenge. In real disordered systems, multiple transport pathways may operate simultaneously, including vacancy-mediated diffusion, interstitial mechanisms, and cooperative movements. Current modeling approaches often focus on a single dominant mechanism, neglecting the interplay between different transport modes.
Furthermore, the integration of experimental validation with computational predictions remains difficult. The time and length scales accessible to experimental techniques often differ from those of simulations, making direct comparisons challenging. This validation gap undermines confidence in model predictions and hinders the development of more accurate theoretical frameworks for ion transport in disordered chloride lattices.
One fundamental challenge lies in capturing the multi-scale nature of ion transport phenomena. Molecular dynamics simulations can provide atomic-level insights but are computationally expensive for long time scales relevant to macroscopic transport properties. Conversely, continuum models may overlook critical microscopic interactions that govern transport mechanisms in disordered systems.
The representation of structural disorder itself poses a substantial hurdle. Unlike crystalline materials with well-defined periodic structures, disordered chloride lattices exhibit significant local variations in coordination environments, defect concentrations, and energy landscapes. Current models often rely on oversimplified representations that fail to capture the full complexity of these disordered environments, particularly the correlation between structural disorder and transport pathways.
Accurately modeling the energetics of ion-ion and ion-lattice interactions remains problematic. The long-range Coulombic interactions, short-range repulsions, and polarization effects all contribute to the complex potential energy surface that governs ion mobility. Existing force fields frequently struggle to balance computational efficiency with the accuracy needed to predict transport properties in disordered systems.
Temperature effects introduce additional complexity, as thermal fluctuations can significantly alter the energy landscape and activation barriers for ion migration. Most current models inadequately account for the temperature dependence of transport parameters, limiting their predictive capability across different operating conditions relevant to practical applications.
The coupling between different transport mechanisms presents another challenge. In real disordered systems, multiple transport pathways may operate simultaneously, including vacancy-mediated diffusion, interstitial mechanisms, and cooperative movements. Current modeling approaches often focus on a single dominant mechanism, neglecting the interplay between different transport modes.
Furthermore, the integration of experimental validation with computational predictions remains difficult. The time and length scales accessible to experimental techniques often differ from those of simulations, making direct comparisons challenging. This validation gap undermines confidence in model predictions and hinders the development of more accurate theoretical frameworks for ion transport in disordered chloride lattices.
State-of-the-Art Computational Approaches
01 Ion transport mechanisms in disordered chloride lattices
Research on the fundamental mechanisms of ion transport in disordered chloride lattice structures, including the study of ion mobility, diffusion pathways, and conductivity properties. These studies examine how structural disorder affects ion movement through the lattice and the resulting electrical properties, which is crucial for developing advanced materials for energy storage and conversion applications.- Ion transport mechanisms in disordered chloride lattices: Research on the fundamental mechanisms of ion transport in disordered chloride lattice structures, including studies on ion mobility, conductivity, and diffusion pathways. These investigations examine how structural disorder affects ion movement through chloride-based materials and the relationship between lattice defects and transport properties.
- Measurement and characterization techniques for ion transport: Advanced analytical methods and instrumentation for measuring ion transport phenomena in chloride lattices, including spectroscopic techniques, impedance analysis, and computational modeling approaches. These techniques enable quantitative assessment of ion mobility, concentration, and distribution within disordered lattice structures.
- Materials engineering for enhanced ion conductivity: Development of novel materials with engineered chloride lattices designed to optimize ion transport properties. This includes doping strategies, composite materials, and nanostructured systems that enhance ionic conductivity through controlled disorder in the crystal structure.
- Applications in energy storage and conversion systems: Implementation of disordered chloride lattice materials in energy-related applications such as batteries, fuel cells, and electrochemical devices. These applications leverage the unique ion transport properties of disordered chloride systems to improve energy efficiency, storage capacity, and device performance.
- Environmental and sensing applications of ion transport: Utilization of ion transport phenomena in disordered chloride lattices for environmental monitoring, chemical sensing, and remediation technologies. These applications exploit the selective ion transport properties of chloride-based materials for detection and separation of specific ionic species in complex environments.
02 Measurement and characterization techniques for ion transport
Various analytical and measurement techniques used to characterize ion transport phenomena in chloride-containing materials. These include spectroscopic methods, electrochemical impedance spectroscopy, and computational modeling approaches that help quantify ion mobility, identify transport mechanisms, and evaluate material performance for specific applications.Expand Specific Solutions03 Applications in energy storage and conversion devices
Implementation of chloride-based materials with specific ion transport properties in energy storage and conversion devices such as batteries, fuel cells, and supercapacitors. The controlled movement of ions through these materials enables efficient energy storage and release, making them valuable components in renewable energy systems and portable electronics.Expand Specific Solutions04 Environmental and industrial applications of ion transport
Utilization of ion transport phenomena in chloride lattices for environmental remediation, waste treatment, and industrial processes. These applications leverage selective ion transport properties for separations, purifications, and transformations in various industrial settings, including water treatment, chemical manufacturing, and resource recovery.Expand Specific Solutions05 Novel materials and structures for enhanced ion conductivity
Development of new materials and engineered structures with disordered chloride lattices designed to enhance ion transport properties. These innovations include composite materials, doped compounds, and nanostructured architectures that facilitate faster ion movement, improved stability, and better performance in various applications requiring efficient ion transport.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
# Modeling Ion Transport In Disordered Chloride Lattices: Competitive Landscape Analysis
The field of ion transport modeling in disordered chloride lattices is in its growth phase, with an estimated market size of $2.5-3 billion and expanding at 7-9% annually. The competitive landscape features academic institutions (University of California, Texas A&M, Hohai University) conducting fundamental research alongside commercial players developing practical applications. Leading companies like Thermo Fisher Scientific and Agilent Technologies are investing in advanced analytical instruments, while specialized firms such as Ionwerks focus on mass spectrometry innovations. The technology maturity varies significantly across applications, with analytical instrumentation being relatively mature while computational modeling tools remain in early development stages. Research collaboration between academia and industry is driving innovation, with CNRS and pharmaceutical companies like AbbVie exploring therapeutic applications of ion transport mechanisms.
The field of ion transport modeling in disordered chloride lattices is in its growth phase, with an estimated market size of $2.5-3 billion and expanding at 7-9% annually. The competitive landscape features academic institutions (University of California, Texas A&M, Hohai University) conducting fundamental research alongside commercial players developing practical applications. Leading companies like Thermo Fisher Scientific and Agilent Technologies are investing in advanced analytical instruments, while specialized firms such as Ionwerks focus on mass spectrometry innovations. The technology maturity varies significantly across applications, with analytical instrumentation being relatively mature while computational modeling tools remain in early development stages. Research collaboration between academia and industry is driving innovation, with CNRS and pharmaceutical companies like AbbVie exploring therapeutic applications of ion transport mechanisms.
The Regents of the University of California
Technical Solution: The University of California has developed advanced computational models for ion transport in disordered chloride lattices using molecular dynamics simulations combined with density functional theory (DFT). Their approach incorporates both classical and quantum mechanical methods to accurately predict ion diffusion pathways through complex chloride-containing materials. The research team has created algorithms that can account for lattice distortions and defects that significantly influence ion mobility. Their models incorporate temperature-dependent effects and can simulate systems with varying degrees of disorder, from slightly perturbed crystalline structures to highly amorphous materials. The university's research has particularly focused on solid-state electrolytes for battery applications, where understanding chloride ion transport mechanisms is crucial for developing next-generation energy storage solutions. Their computational framework allows for high-throughput screening of potential chloride-based materials with optimized ion conductivity properties.
Strengths: Exceptional integration of multi-scale modeling techniques from atomic to macroscopic levels; strong validation against experimental data; ability to predict transport properties in previously unexplored materials. Weaknesses: Computationally intensive simulations limiting the size and time scales of systems that can be modeled; challenges in accurately representing complex interfaces and grain boundaries in polycrystalline materials.
Hohai University
Technical Solution: Hohai University has pioneered a hybrid modeling approach for ion transport in disordered chloride lattices that combines machine learning algorithms with traditional molecular dynamics. Their research focuses particularly on hydrated chloride systems relevant to environmental applications and water treatment technologies. The university has developed specialized force fields that accurately capture the interactions between chloride ions and various cations in disordered environments, accounting for the effects of hydration shells and hydrogen bonding networks. Their models incorporate Monte Carlo methods to efficiently sample configuration space in highly disordered systems where traditional molecular dynamics might be trapped in local energy minima. Hohai's approach is particularly notable for its ability to model chloride transport across interfaces between ordered and disordered regions, which is critical for understanding real-world materials with heterogeneous structures. The university has also created visualization tools that help identify preferential transport pathways in complex chloride-containing materials.
Strengths: Excellent handling of hydrated systems and environmental applications; innovative integration of machine learning to accelerate simulations; strong focus on practical applications in water treatment and environmental remediation. Weaknesses: Models sometimes sacrifice atomic-level detail for computational efficiency; limited application to high-temperature systems where water is not present.
Key Theoretical Frameworks for Chloride Lattice Dynamics
Use of glucosidase inhibitors for therapy of mucovisidosis
PatentInactiveUS8242136B2
Innovation
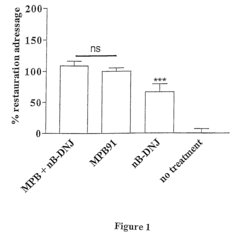
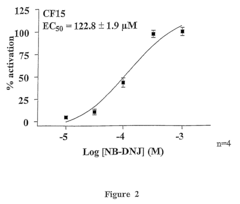
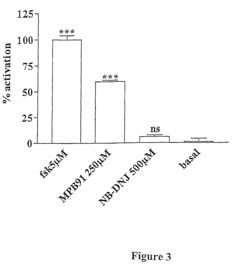
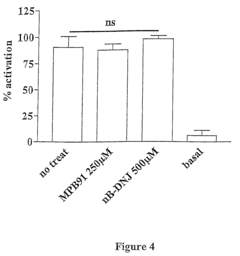
- The use of glucosidase inhibitors, such as N-butyl-deoxynojirimycin (NB-DNJ), which inhibit glucosidase I and II enzymes, allowing delF508 to correctly fold and reach the cell membrane without affecting other chloride channels or cell viability, enabling ion transport activity.
Materials Science Implications and Opportunities
The advancements in modeling ion transport in disordered chloride lattices present significant implications for materials science that extend beyond theoretical understanding. These models enable precise prediction of how chloride ions move through complex crystalline structures with defects, opening new pathways for materials engineering and optimization.
The ability to accurately model ion transport mechanisms provides materials scientists with powerful tools to design novel solid-state electrolytes with enhanced ionic conductivity. By understanding how structural disorder affects ion mobility at the atomic level, researchers can deliberately introduce specific defects or dopants to create fast ion conductors for next-generation batteries and fuel cells.
These modeling approaches bridge the gap between computational predictions and experimental observations, allowing for more efficient materials discovery processes. Rather than relying solely on trial-and-error experimentation, materials scientists can now use simulation-guided design to narrow down candidate materials before synthesis, significantly accelerating development timelines and reducing research costs.
For structural materials exposed to chloride-rich environments, these models offer unprecedented insights into corrosion mechanisms. Understanding how chloride ions penetrate and interact with protective oxide layers on metals enables the development of more corrosion-resistant alloys and protective coatings, extending the service life of critical infrastructure in marine and industrial settings.
The cross-disciplinary nature of this research creates opportunities for collaboration between computational scientists, materials engineers, and electrochemists. Such partnerships can lead to breakthroughs in solid-state ionics, with applications ranging from energy storage to sensing technologies and smart materials that respond to ionic stimuli.
From a manufacturing perspective, these models help optimize processing conditions for materials where ion transport properties are crucial to performance. By simulating how different synthesis routes affect lattice disorder and consequently ion mobility, manufacturers can fine-tune production parameters to achieve desired material properties with greater consistency and efficiency.
Looking forward, the integration of machine learning approaches with ion transport models presents opportunities to discover entirely new classes of materials with unprecedented ionic conductivity properties, potentially revolutionizing fields such as solid-state batteries, desalination membranes, and ion-selective sensors.
The ability to accurately model ion transport mechanisms provides materials scientists with powerful tools to design novel solid-state electrolytes with enhanced ionic conductivity. By understanding how structural disorder affects ion mobility at the atomic level, researchers can deliberately introduce specific defects or dopants to create fast ion conductors for next-generation batteries and fuel cells.
These modeling approaches bridge the gap between computational predictions and experimental observations, allowing for more efficient materials discovery processes. Rather than relying solely on trial-and-error experimentation, materials scientists can now use simulation-guided design to narrow down candidate materials before synthesis, significantly accelerating development timelines and reducing research costs.
For structural materials exposed to chloride-rich environments, these models offer unprecedented insights into corrosion mechanisms. Understanding how chloride ions penetrate and interact with protective oxide layers on metals enables the development of more corrosion-resistant alloys and protective coatings, extending the service life of critical infrastructure in marine and industrial settings.
The cross-disciplinary nature of this research creates opportunities for collaboration between computational scientists, materials engineers, and electrochemists. Such partnerships can lead to breakthroughs in solid-state ionics, with applications ranging from energy storage to sensing technologies and smart materials that respond to ionic stimuli.
From a manufacturing perspective, these models help optimize processing conditions for materials where ion transport properties are crucial to performance. By simulating how different synthesis routes affect lattice disorder and consequently ion mobility, manufacturers can fine-tune production parameters to achieve desired material properties with greater consistency and efficiency.
Looking forward, the integration of machine learning approaches with ion transport models presents opportunities to discover entirely new classes of materials with unprecedented ionic conductivity properties, potentially revolutionizing fields such as solid-state batteries, desalination membranes, and ion-selective sensors.
Energy Storage Integration Potential
The integration of ion transport modeling in disordered chloride lattices presents significant opportunities for advancing energy storage technologies. These models can directly inform the development of next-generation batteries, particularly solid-state electrolytes where chloride-based materials show promising ionic conductivity characteristics while maintaining structural stability under various operating conditions.
When incorporated into energy storage systems, the insights gained from chloride lattice transport models enable optimization of ion mobility pathways, potentially increasing charge/discharge rates and overall battery efficiency. The ability to predict ion behavior in disordered structures allows for targeted material design that maximizes conductivity while minimizing degradation mechanisms that typically limit battery lifespan.
Computational models of chloride lattice transport can be integrated into broader battery management systems, enabling real-time prediction of performance under varying conditions. This predictive capability supports adaptive control strategies that optimize energy storage operation based on environmental factors and usage patterns, ultimately extending device longevity and reliability in field applications.
The thermal stability advantages of many chloride-based materials make them particularly valuable for high-temperature applications where conventional lithium-ion technologies face significant safety challenges. Transport models that accurately capture behavior across temperature ranges can facilitate the development of energy storage solutions for extreme environments, including industrial processes and specialized transportation sectors.
Grid-scale energy storage represents another promising integration pathway, where chloride-based systems could offer cost advantages through abundant material resources. The scalability of these systems depends critically on understanding ion transport mechanisms at interfaces and across large-format cells, areas where advanced modeling capabilities provide essential design guidance.
Hybrid energy storage systems that combine chloride-based components with other technologies may leverage complementary performance characteristics. Transport models that account for multi-material interfaces and heterogeneous structures can inform the design of these hybrid systems, potentially achieving performance metrics unattainable with single-material approaches.
The integration potential extends beyond batteries to include supercapacitors, fuel cells, and electrochemical sensors, where ion transport characteristics similarly determine device performance. Cross-disciplinary application of chloride lattice transport models could accelerate innovation across the broader energy technology landscape, creating synergistic development opportunities.
When incorporated into energy storage systems, the insights gained from chloride lattice transport models enable optimization of ion mobility pathways, potentially increasing charge/discharge rates and overall battery efficiency. The ability to predict ion behavior in disordered structures allows for targeted material design that maximizes conductivity while minimizing degradation mechanisms that typically limit battery lifespan.
Computational models of chloride lattice transport can be integrated into broader battery management systems, enabling real-time prediction of performance under varying conditions. This predictive capability supports adaptive control strategies that optimize energy storage operation based on environmental factors and usage patterns, ultimately extending device longevity and reliability in field applications.
The thermal stability advantages of many chloride-based materials make them particularly valuable for high-temperature applications where conventional lithium-ion technologies face significant safety challenges. Transport models that accurately capture behavior across temperature ranges can facilitate the development of energy storage solutions for extreme environments, including industrial processes and specialized transportation sectors.
Grid-scale energy storage represents another promising integration pathway, where chloride-based systems could offer cost advantages through abundant material resources. The scalability of these systems depends critically on understanding ion transport mechanisms at interfaces and across large-format cells, areas where advanced modeling capabilities provide essential design guidance.
Hybrid energy storage systems that combine chloride-based components with other technologies may leverage complementary performance characteristics. Transport models that account for multi-material interfaces and heterogeneous structures can inform the design of these hybrid systems, potentially achieving performance metrics unattainable with single-material approaches.
The integration potential extends beyond batteries to include supercapacitors, fuel cells, and electrochemical sensors, where ion transport characteristics similarly determine device performance. Cross-disciplinary application of chloride lattice transport models could accelerate innovation across the broader energy technology landscape, creating synergistic development opportunities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!