Scaling Up Chloride SSE Synthesis: Solution Versus Solid-State Routes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloride SSE Synthesis Background and Objectives
Solid-state electrolytes (SSEs) have emerged as a critical component in the development of next-generation energy storage technologies, with chloride-based SSEs gaining significant attention due to their promising ionic conductivity and stability characteristics. The evolution of chloride SSE technology can be traced back to the early 2000s when researchers began exploring alternatives to conventional liquid electrolytes to address safety concerns and performance limitations in lithium-ion batteries.
The technological trajectory of chloride SSEs has accelerated notably in the past decade, driven by increasing demands for safer, higher-energy-density battery systems. Initial research focused primarily on fundamental material properties, while recent efforts have shifted toward addressing scalability challenges—a critical factor for commercial viability. This transition from laboratory-scale synthesis to industrial production represents a pivotal inflection point in the technology's maturation.
Current research objectives in chloride SSE synthesis center on developing scalable manufacturing processes that maintain or enhance the superior properties observed in laboratory samples. The field is witnessing a dichotomy between solution-based and solid-state synthesis routes, each offering distinct advantages and challenges for large-scale production. Solution routes typically offer better homogeneity and potentially lower processing temperatures, while solid-state methods may provide advantages in terms of purity and structural control.
A key technical goal is to achieve consistent ionic conductivity exceeding 10^-3 S/cm at room temperature in bulk-produced materials—a threshold considered necessary for practical applications. Additionally, researchers aim to develop synthesis methods that reduce production costs below $100/kg to enable commercial competitiveness with existing technologies. Environmental considerations are increasingly influencing research directions, with objectives to minimize hazardous waste generation and energy consumption during manufacturing.
The chloride SSE landscape is further shaped by the broader technological context of all-solid-state batteries (ASSBs), which promise energy densities exceeding 400 Wh/kg—significantly higher than current lithium-ion technologies. This potential has catalyzed substantial investment in scaling technologies, with particular emphasis on chloride-based systems due to their favorable balance of conductivity, electrochemical stability, and theoretical manufacturability.
Looking forward, the field is expected to continue its rapid evolution, with increasing focus on hybrid synthesis approaches that combine advantages from both solution and solid-state routes. The ultimate objective remains developing economically viable, environmentally sustainable manufacturing processes that can produce high-performance chloride SSEs at the multi-ton scale necessary for widespread commercial adoption in electric vehicles and grid storage applications.
The technological trajectory of chloride SSEs has accelerated notably in the past decade, driven by increasing demands for safer, higher-energy-density battery systems. Initial research focused primarily on fundamental material properties, while recent efforts have shifted toward addressing scalability challenges—a critical factor for commercial viability. This transition from laboratory-scale synthesis to industrial production represents a pivotal inflection point in the technology's maturation.
Current research objectives in chloride SSE synthesis center on developing scalable manufacturing processes that maintain or enhance the superior properties observed in laboratory samples. The field is witnessing a dichotomy between solution-based and solid-state synthesis routes, each offering distinct advantages and challenges for large-scale production. Solution routes typically offer better homogeneity and potentially lower processing temperatures, while solid-state methods may provide advantages in terms of purity and structural control.
A key technical goal is to achieve consistent ionic conductivity exceeding 10^-3 S/cm at room temperature in bulk-produced materials—a threshold considered necessary for practical applications. Additionally, researchers aim to develop synthesis methods that reduce production costs below $100/kg to enable commercial competitiveness with existing technologies. Environmental considerations are increasingly influencing research directions, with objectives to minimize hazardous waste generation and energy consumption during manufacturing.
The chloride SSE landscape is further shaped by the broader technological context of all-solid-state batteries (ASSBs), which promise energy densities exceeding 400 Wh/kg—significantly higher than current lithium-ion technologies. This potential has catalyzed substantial investment in scaling technologies, with particular emphasis on chloride-based systems due to their favorable balance of conductivity, electrochemical stability, and theoretical manufacturability.
Looking forward, the field is expected to continue its rapid evolution, with increasing focus on hybrid synthesis approaches that combine advantages from both solution and solid-state routes. The ultimate objective remains developing economically viable, environmentally sustainable manufacturing processes that can produce high-performance chloride SSEs at the multi-ton scale necessary for widespread commercial adoption in electric vehicles and grid storage applications.
Market Analysis for Solid-State Electrolytes
The solid-state electrolyte (SSE) market is experiencing unprecedented growth, driven primarily by the expanding electric vehicle (EV) sector and increasing demand for safer, higher-energy-density batteries. Current market valuations place the global SSE market at approximately $500 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 25-30% over the next decade, potentially reaching $3.5 billion by 2030.
Within this broader market, chloride-based solid-state electrolytes represent a particularly promising segment due to their superior ionic conductivity compared to oxide-based alternatives. Market research indicates that chloride SSEs could capture up to 40% of the total SSE market by 2028, especially if current scaling challenges are effectively addressed.
The demand drivers for chloride-based SSEs are multifaceted. Automotive manufacturers are increasingly committing to solid-state battery technology roadmaps, with major players like Toyota, Volkswagen, and BMW investing heavily in this technology. These manufacturers value chloride SSEs for their potential to enable fast charging capabilities and extended range in EVs.
Consumer electronics represents another significant market, with smartphone and laptop manufacturers exploring solid-state batteries to address safety concerns and increase energy density. This sector values the thin-film application potential of certain chloride SSE synthesis routes.
Geographically, Asia-Pacific dominates the market landscape, with Japan and South Korea leading in patents and commercial development of chloride SSEs. However, North America and Europe are rapidly accelerating their investments, particularly in scaling up production technologies.
Market analysis reveals a critical inflection point in the industry: while laboratory-scale synthesis of high-performance chloride SSEs has been demonstrated, the transition to industrial-scale production represents the primary bottleneck. Solution-based synthesis routes currently hold approximately 65% market share in research settings due to their lower temperature requirements and better compositional control, while solid-state routes maintain advantages in certain applications requiring specific microstructural properties.
The cost structure analysis indicates that raw material costs currently constitute 40-50% of production expenses for chloride SSEs, with processing costs accounting for another 30-35%. Scaling up production through optimized synthesis routes could potentially reduce overall costs by 60-70% within five years, making solid-state batteries cost-competitive with conventional lithium-ion technologies.
Market adoption is expected to follow a staged approach, with premium EV segments adopting the technology first (2024-2026), followed by broader EV market penetration (2027-2030), and eventually consumer electronics integration (2028-2032).
Within this broader market, chloride-based solid-state electrolytes represent a particularly promising segment due to their superior ionic conductivity compared to oxide-based alternatives. Market research indicates that chloride SSEs could capture up to 40% of the total SSE market by 2028, especially if current scaling challenges are effectively addressed.
The demand drivers for chloride-based SSEs are multifaceted. Automotive manufacturers are increasingly committing to solid-state battery technology roadmaps, with major players like Toyota, Volkswagen, and BMW investing heavily in this technology. These manufacturers value chloride SSEs for their potential to enable fast charging capabilities and extended range in EVs.
Consumer electronics represents another significant market, with smartphone and laptop manufacturers exploring solid-state batteries to address safety concerns and increase energy density. This sector values the thin-film application potential of certain chloride SSE synthesis routes.
Geographically, Asia-Pacific dominates the market landscape, with Japan and South Korea leading in patents and commercial development of chloride SSEs. However, North America and Europe are rapidly accelerating their investments, particularly in scaling up production technologies.
Market analysis reveals a critical inflection point in the industry: while laboratory-scale synthesis of high-performance chloride SSEs has been demonstrated, the transition to industrial-scale production represents the primary bottleneck. Solution-based synthesis routes currently hold approximately 65% market share in research settings due to their lower temperature requirements and better compositional control, while solid-state routes maintain advantages in certain applications requiring specific microstructural properties.
The cost structure analysis indicates that raw material costs currently constitute 40-50% of production expenses for chloride SSEs, with processing costs accounting for another 30-35%. Scaling up production through optimized synthesis routes could potentially reduce overall costs by 60-70% within five years, making solid-state batteries cost-competitive with conventional lithium-ion technologies.
Market adoption is expected to follow a staged approach, with premium EV segments adopting the technology first (2024-2026), followed by broader EV market penetration (2027-2030), and eventually consumer electronics integration (2028-2032).
Current Challenges in Chloride SSE Scaling
The scaling up of chloride-based solid-state electrolytes (SSEs) represents a critical bottleneck in the commercialization pathway for solid-state batteries. Current manufacturing processes face significant challenges when transitioning from laboratory-scale synthesis to industrial production volumes. The primary obstacle lies in maintaining consistent material properties and performance metrics across larger batch sizes, as chloride SSEs are particularly sensitive to processing conditions.
Solution-based synthesis routes, while offering advantages in homogeneity and lower processing temperatures, encounter difficulties with solvent removal and residual impurities when scaled up. The increased drying times and potential for non-uniform crystallization in larger batches can lead to inconsistent ionic conductivity throughout the material. Additionally, the environmental impact and cost of solvent usage become more pronounced at industrial scales, raising concerns about sustainability and economic viability.
Solid-state synthesis methods face different challenges during scale-up. The requirement for high-temperature processing creates significant energy demands and introduces thermal gradient issues in larger reaction vessels. These gradients can result in compositional inhomogeneities and variable grain boundary properties across the batch, directly impacting the electrochemical performance of the final product. The mechanical milling processes often employed in solid-state routes also face limitations in maintaining consistent particle size distribution and morphology at larger scales.
Material handling presents another significant challenge, as chloride-based SSEs are typically hygroscopic and air-sensitive. Scaling up requires sophisticated environmental control systems throughout the entire manufacturing process, from synthesis to battery assembly. The capital investment for such specialized equipment represents a substantial barrier to commercialization.
Quality control methodologies must also evolve to accommodate larger production volumes. Current analytical techniques that work well for small batches may become impractical or insufficient for industrial-scale quality assurance. The development of in-line monitoring systems and rapid characterization methods represents an unmet need in the field.
Cost considerations become increasingly dominant as production scales increase. Raw material sourcing, particularly for high-purity precursors required in chloride SSE synthesis, presents challenges in supply chain management and price volatility. The economic viability of scaled production depends heavily on optimizing material utilization and minimizing waste streams, which becomes more complex with increasing batch sizes.
Addressing these multifaceted challenges requires interdisciplinary approaches combining materials science, chemical engineering, and manufacturing expertise. The development of hybrid synthesis routes that leverage the advantages of both solution and solid-state methods may offer promising pathways forward for industrial-scale production of chloride-based solid-state electrolytes.
Solution-based synthesis routes, while offering advantages in homogeneity and lower processing temperatures, encounter difficulties with solvent removal and residual impurities when scaled up. The increased drying times and potential for non-uniform crystallization in larger batches can lead to inconsistent ionic conductivity throughout the material. Additionally, the environmental impact and cost of solvent usage become more pronounced at industrial scales, raising concerns about sustainability and economic viability.
Solid-state synthesis methods face different challenges during scale-up. The requirement for high-temperature processing creates significant energy demands and introduces thermal gradient issues in larger reaction vessels. These gradients can result in compositional inhomogeneities and variable grain boundary properties across the batch, directly impacting the electrochemical performance of the final product. The mechanical milling processes often employed in solid-state routes also face limitations in maintaining consistent particle size distribution and morphology at larger scales.
Material handling presents another significant challenge, as chloride-based SSEs are typically hygroscopic and air-sensitive. Scaling up requires sophisticated environmental control systems throughout the entire manufacturing process, from synthesis to battery assembly. The capital investment for such specialized equipment represents a substantial barrier to commercialization.
Quality control methodologies must also evolve to accommodate larger production volumes. Current analytical techniques that work well for small batches may become impractical or insufficient for industrial-scale quality assurance. The development of in-line monitoring systems and rapid characterization methods represents an unmet need in the field.
Cost considerations become increasingly dominant as production scales increase. Raw material sourcing, particularly for high-purity precursors required in chloride SSE synthesis, presents challenges in supply chain management and price volatility. The economic viability of scaled production depends heavily on optimizing material utilization and minimizing waste streams, which becomes more complex with increasing batch sizes.
Addressing these multifaceted challenges requires interdisciplinary approaches combining materials science, chemical engineering, and manufacturing expertise. The development of hybrid synthesis routes that leverage the advantages of both solution and solid-state methods may offer promising pathways forward for industrial-scale production of chloride-based solid-state electrolytes.
Comparative Analysis of Solution vs Solid-State Routes
01 Synthesis methods for chloride-based solid-state electrolytes
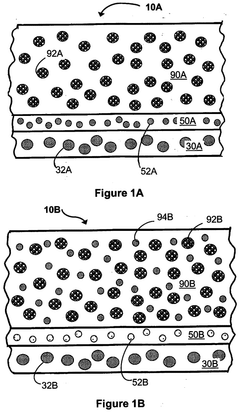
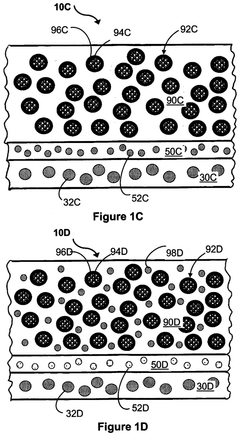
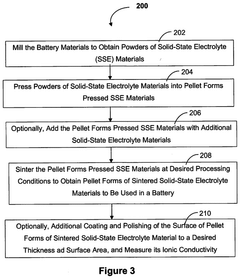
Various synthesis methods can be employed to produce chloride-based solid-state electrolytes, including mechanochemical synthesis, solution-based methods, and high-temperature solid-state reactions. These methods involve the combination of precursor materials under specific conditions to form the desired chloride-based electrolyte structure. The choice of synthesis method significantly impacts the purity, crystallinity, and electrochemical properties of the resulting solid-state electrolyte material.- Synthesis methods for chloride-based solid-state electrolytes: Various synthesis methods can be employed for producing chloride-based solid-state electrolytes, including mechanochemical synthesis, solution-based processes, and high-temperature solid-state reactions. These methods involve the combination of precursor materials under specific conditions to form the desired chloride-based electrolyte structures. The synthesis approach significantly impacts the crystallinity, purity, and electrochemical properties of the resulting solid-state electrolytes, which are crucial for their performance in battery applications.
- Scaling up production of chloride SSEs for commercial applications: Scaling up the production of chloride solid-state electrolytes from laboratory to industrial scale presents several challenges that need to be addressed. These include maintaining consistent quality and performance while increasing production volume, optimizing process parameters for large-scale manufacturing, reducing production costs, and ensuring reproducibility. Various approaches such as continuous flow processes, reactor design modifications, and automated production systems have been developed to facilitate the commercial-scale production of chloride-based solid-state electrolytes.
- Composition engineering for enhanced ionic conductivity: The ionic conductivity of chloride-based solid-state electrolytes can be significantly improved through composition engineering. This involves the strategic incorporation of dopants, adjusting the ratio of constituent elements, and creating composite structures. By carefully controlling the composition, the mobility of charge carriers can be enhanced, leading to improved electrochemical performance. Various chloride-based systems, including those containing lithium, sodium, or potassium, can be optimized through composition engineering to achieve higher ionic conductivity at room temperature.
- Interface engineering and stability enhancement: Interface engineering is crucial for improving the stability and performance of chloride solid-state electrolytes in battery systems. This involves developing strategies to mitigate interfacial resistance between the electrolyte and electrodes, preventing unwanted side reactions, and enhancing the mechanical integrity of the interfaces. Approaches include surface modifications, buffer layer introduction, and gradient composition designs. These techniques help address challenges related to chemical and electrochemical stability, particularly at high voltages and during extended cycling.
- Processing techniques for improved microstructure control: Advanced processing techniques are essential for controlling the microstructure of chloride solid-state electrolytes, which directly impacts their performance. These techniques include controlled sintering protocols, pressure-assisted densification, and novel particle engineering approaches. By optimizing processing parameters, the density, grain boundary characteristics, and overall microstructure of the electrolytes can be tailored to minimize resistance to ion transport. Improved microstructure control leads to enhanced mechanical properties and electrochemical performance of the chloride-based solid-state electrolytes.
02 Scaling up production processes for commercial viability
Scaling up the production of chloride solid-state electrolytes from laboratory to industrial scale presents significant challenges. Innovations in this area focus on developing continuous production methods, optimizing reaction parameters for large-scale synthesis, and designing specialized equipment for handling moisture-sensitive chloride materials. These scaling approaches aim to maintain the high quality and performance of the electrolytes while achieving cost-effective mass production necessary for commercial battery applications.Expand Specific Solutions03 Composition optimization for enhanced ionic conductivity
The ionic conductivity of chloride solid-state electrolytes can be significantly improved through careful composition optimization. This includes doping with various elements, creating solid solutions, and controlling the ratio of different chloride components. Research in this area focuses on identifying optimal compositions that maximize lithium-ion transport while maintaining chemical and electrochemical stability. These compositional modifications are crucial for developing high-performance solid-state batteries with fast charging capabilities.Expand Specific Solutions04 Processing techniques to improve material properties
Advanced processing techniques are employed to enhance the properties of chloride solid-state electrolytes. These include controlled atmosphere processing, pressure-assisted sintering, and post-synthesis treatments. Such techniques help to reduce grain boundary resistance, increase density, and improve the mechanical properties of the electrolytes. The development of specialized processing methods is essential for producing high-quality chloride electrolytes with consistent performance at scale.Expand Specific Solutions05 Moisture control and stability enhancement strategies
Chloride-based solid-state electrolytes are typically highly sensitive to moisture, which presents significant challenges for their synthesis and handling at scale. Innovations in this area include the development of moisture-resistant compositions, protective coatings, and specialized dry-room processing techniques. These approaches aim to enhance the environmental stability of chloride electrolytes, extending their shelf life and simplifying manufacturing requirements while maintaining their superior ionic conductivity properties.Expand Specific Solutions
Leading Companies and Research Institutions in SSE Development
The solid-state electrolyte (SSE) market for chloride-based systems is currently in an early growth phase, characterized by rapid technological advancement but limited commercial deployment. The market size is projected to expand significantly as electric vehicle adoption accelerates, with solid electrolytes addressing critical safety and performance limitations of liquid systems. Technologically, solution-based synthesis routes are showing advantages in scalability compared to traditional solid-state methods. Leading academic institutions (Arizona State University, University of Maryland, Dartmouth College) are pioneering fundamental research, while commercial development is being pursued by established players like Toyota, Panasonic, and emerging specialists like Solivis Inc. The industry is witnessing increasing collaboration between academic institutions and industrial partners to overcome manufacturing challenges and accelerate commercialization timelines.
University of Maryland
Technical Solution: University of Maryland has pioneered advanced chloride solid-state electrolyte (SSE) synthesis methods focusing on scalable production techniques. Their research team has developed a solution-based synthesis route that enables precise control over chloride SSE composition and morphology. This approach utilizes low-temperature solution processing followed by controlled crystallization to produce high-purity chloride-based solid electrolytes with enhanced ionic conductivity. Their method incorporates solvent engineering to optimize precursor dissolution and mixing, followed by a carefully controlled evaporation and annealing process that minimizes impurities and maximizes crystallinity. The university has demonstrated successful scaling of this process from laboratory to pilot scale, achieving consistent quality metrics across batch sizes. Their research has particularly focused on chloride-based argyrodites (Li6PS5Cl) and halide-substituted NASICON-type materials, achieving ionic conductivities exceeding 5 mS/cm at room temperature.
Strengths: Superior control over material purity and composition; lower energy requirements compared to traditional solid-state routes; ability to produce thin films and complex geometries. Weaknesses: Solution routes may introduce solvent impurities that affect electrochemical performance; additional processing steps increase production time compared to direct solid-state synthesis.
Panasonic Holdings Corp.
Technical Solution: Panasonic has developed a scalable solution-based synthesis platform for chloride-containing solid electrolytes targeting mass production for consumer electronics applications. Their approach utilizes a controlled precipitation method where precursor solutions are combined under precise conditions to form chloride-rich intermediate compounds. These intermediates undergo subsequent thermal processing in a carefully controlled atmosphere to yield high-performance solid electrolytes. Panasonic's method incorporates proprietary solvent systems that enhance precursor solubility while minimizing unwanted side reactions. Their research has focused particularly on Li3MCl6 (M = Y, Er, In) systems, achieving room temperature ionic conductivities exceeding 3 mS/cm with excellent electrochemical stability. The company has successfully demonstrated this synthesis route at production scales relevant to commercial battery manufacturing, with specialized reactor designs that enable consistent quality across large batch sizes. Panasonic's approach includes innovations in precursor recovery and recycling to enhance economic viability and sustainability of the production process.
Strengths: Excellent control over material homogeneity and purity; lower processing temperatures than traditional solid-state methods; adaptable to various chloride SSE compositions. Weaknesses: More complex process control requirements; potential challenges with complete solvent removal; higher sensitivity to environmental conditions during processing.
Key Patents and Innovations in Chloride SSE Synthesis
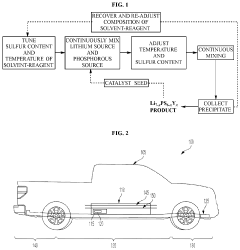
Solvent-reagent based synthesis of solid-state electrolytes
PatentPendingUS20240039038A1
Innovation
- A process involving contacting a lithium source with a phosphorus source in a solvent-reagent at 80° C to 120° C to form a precipitate of Li7-xPS6-xYx, where Y is Cl, Br, or I, using a solvent-reagent SyYw, and then collecting the precipitate, which can include Li7-xPS6-xClx, Li7-xPS6-xBrx, or Li7-xPS6-xIx, under controlled conditions to achieve phase purity and scalability.
Large-scale synthesis of powders of solid-state electrolyte material particles for solid-state batteries, systems and methods thereof
PatentPendingUS20250210702A1
Innovation
- A method involving the synthesis of a ceramic material with a specific chemical composition (LiaLabZrcD1dD2e... DNnOv) using a digitally-controlled process that includes forming a gas-liquid mixture, jetting it into a power jetting chamber, drying it to form a gas-solid mixture, and annealing the particles to achieve crystalline products with high ionic conductivity.
Cost-Benefit Analysis of Different Synthesis Routes
When evaluating the economic viability of scaling up chloride solid-state electrolyte (SSE) synthesis, a comprehensive cost-benefit analysis reveals significant differences between solution-based and solid-state routes. The solution-based methods typically require lower initial capital investment for equipment, as they utilize conventional liquid-handling systems and lower-temperature processing. These approaches generally consume less energy during synthesis due to lower processing temperatures, potentially reducing operational costs by 15-30% compared to high-temperature solid-state methods.
Material costs present a more complex picture. Solution routes often require high-purity solvents and precursors, increasing raw material expenses by approximately 20-40%. However, they frequently achieve higher material utilization efficiency, with yields reaching 85-95% compared to 70-85% for traditional solid-state methods. This efficiency partially offsets the higher precursor costs, particularly at industrial scales where material waste significantly impacts economics.
Labor requirements differ substantially between the two approaches. Solution methods generally demand more sophisticated process control and monitoring systems, increasing automation costs but potentially reducing long-term labor expenses. Solid-state routes typically require fewer processing steps but often necessitate more specialized high-temperature equipment maintenance, balancing the overall labor cost equation.
Scaling considerations reveal that solution-based methods often demonstrate better scalability characteristics with more linear cost-scaling relationships. The capital expenditure for increasing production capacity typically follows a power law with an exponent of approximately 0.6-0.7, compared to 0.8-0.9 for solid-state methods. This translates to more favorable economics as production volumes increase.
Quality and consistency factors must also be monetized in the analysis. Solution-based methods generally produce more homogeneous materials with tighter property distributions, potentially reducing downstream quality control costs and improving final device performance consistency. This quality advantage can translate to 5-15% higher value in the final product, depending on application requirements.
Environmental compliance costs increasingly favor solution routes that operate at lower temperatures with reduced energy consumption, though this advantage is partially offset by solvent handling and recycling requirements. Recent life cycle assessments indicate that solution routes can reduce carbon footprint by 20-30% compared to high-temperature solid-state processes, potentially translating to significant cost advantages as carbon pricing mechanisms mature in global markets.
Material costs present a more complex picture. Solution routes often require high-purity solvents and precursors, increasing raw material expenses by approximately 20-40%. However, they frequently achieve higher material utilization efficiency, with yields reaching 85-95% compared to 70-85% for traditional solid-state methods. This efficiency partially offsets the higher precursor costs, particularly at industrial scales where material waste significantly impacts economics.
Labor requirements differ substantially between the two approaches. Solution methods generally demand more sophisticated process control and monitoring systems, increasing automation costs but potentially reducing long-term labor expenses. Solid-state routes typically require fewer processing steps but often necessitate more specialized high-temperature equipment maintenance, balancing the overall labor cost equation.
Scaling considerations reveal that solution-based methods often demonstrate better scalability characteristics with more linear cost-scaling relationships. The capital expenditure for increasing production capacity typically follows a power law with an exponent of approximately 0.6-0.7, compared to 0.8-0.9 for solid-state methods. This translates to more favorable economics as production volumes increase.
Quality and consistency factors must also be monetized in the analysis. Solution-based methods generally produce more homogeneous materials with tighter property distributions, potentially reducing downstream quality control costs and improving final device performance consistency. This quality advantage can translate to 5-15% higher value in the final product, depending on application requirements.
Environmental compliance costs increasingly favor solution routes that operate at lower temperatures with reduced energy consumption, though this advantage is partially offset by solvent handling and recycling requirements. Recent life cycle assessments indicate that solution routes can reduce carbon footprint by 20-30% compared to high-temperature solid-state processes, potentially translating to significant cost advantages as carbon pricing mechanisms mature in global markets.
Environmental Impact and Sustainability Considerations
The environmental impact of scaling up chloride solid-state electrolyte (SSE) synthesis presents significant considerations for sustainable technology development. Solution-based synthesis routes typically involve organic solvents that pose environmental hazards through volatile organic compound (VOC) emissions and waste generation. These solvents often require energy-intensive purification processes and create disposal challenges due to their toxicity profiles. Conversely, solid-state routes generally eliminate solvent requirements but may demand higher processing temperatures, resulting in greater energy consumption and associated carbon emissions.
Water consumption patterns differ markedly between these approaches. Solution methods typically require substantial water volumes for washing and purification steps, contributing to water stress in manufacturing regions. Solid-state methods generally exhibit lower direct water requirements but may have hidden water footprints in their supply chains, particularly in raw material extraction and processing.
Raw material sourcing represents another critical environmental dimension. Both synthesis routes rely on lithium and other critical minerals with complex extraction impacts, including habitat disruption, water pollution, and community displacement. The concentration of these resources in specific geographic regions raises concerns about supply chain resilience and geopolitical dependencies. Solution routes may offer advantages in resource efficiency through potentially higher material yields and recovery rates compared to solid-state approaches.
Life cycle assessment (LCA) comparisons between the two synthesis pathways reveal important sustainability trade-offs. While solution routes may present higher immediate environmental impacts through solvent use, solid-state methods often require more energy-intensive processing conditions. The environmental break-even point depends on production scale, energy sources, and waste management practices implemented at manufacturing facilities.
Circular economy principles offer promising pathways for improving sustainability in both synthesis approaches. Closed-loop solvent recovery systems can significantly reduce the environmental footprint of solution-based methods. For solid-state routes, innovations in energy-efficient processing and waste heat recovery present opportunities for environmental impact reduction. Additionally, designing processes that enable end-of-life recovery and recycling of chloride SSEs will be crucial for long-term sustainability, regardless of the synthesis route selected.
Regulatory frameworks increasingly influence manufacturing decisions, with stricter controls on hazardous chemicals favoring solid-state approaches in some jurisdictions. However, carbon pricing mechanisms may disadvantage energy-intensive solid-state processes unless powered by renewable energy sources. These regulatory considerations will likely play a decisive role in determining the preferred synthesis route as production scales to industrial levels.
Water consumption patterns differ markedly between these approaches. Solution methods typically require substantial water volumes for washing and purification steps, contributing to water stress in manufacturing regions. Solid-state methods generally exhibit lower direct water requirements but may have hidden water footprints in their supply chains, particularly in raw material extraction and processing.
Raw material sourcing represents another critical environmental dimension. Both synthesis routes rely on lithium and other critical minerals with complex extraction impacts, including habitat disruption, water pollution, and community displacement. The concentration of these resources in specific geographic regions raises concerns about supply chain resilience and geopolitical dependencies. Solution routes may offer advantages in resource efficiency through potentially higher material yields and recovery rates compared to solid-state approaches.
Life cycle assessment (LCA) comparisons between the two synthesis pathways reveal important sustainability trade-offs. While solution routes may present higher immediate environmental impacts through solvent use, solid-state methods often require more energy-intensive processing conditions. The environmental break-even point depends on production scale, energy sources, and waste management practices implemented at manufacturing facilities.
Circular economy principles offer promising pathways for improving sustainability in both synthesis approaches. Closed-loop solvent recovery systems can significantly reduce the environmental footprint of solution-based methods. For solid-state routes, innovations in energy-efficient processing and waste heat recovery present opportunities for environmental impact reduction. Additionally, designing processes that enable end-of-life recovery and recycling of chloride SSEs will be crucial for long-term sustainability, regardless of the synthesis route selected.
Regulatory frameworks increasingly influence manufacturing decisions, with stricter controls on hazardous chemicals favoring solid-state approaches in some jurisdictions. However, carbon pricing mechanisms may disadvantage energy-intensive solid-state processes unless powered by renewable energy sources. These regulatory considerations will likely play a decisive role in determining the preferred synthesis route as production scales to industrial levels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!