Roadmap To 350 Wh/kg Using Chloride Solid Electrolytes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloride Solid Electrolytes Background and Energy Density Goals
Solid-state batteries represent a significant advancement in energy storage technology, with chloride solid electrolytes emerging as promising candidates for achieving high energy density goals. The evolution of battery technology has progressed from traditional liquid electrolyte systems to more advanced solid-state configurations, driven by demands for higher energy density, improved safety, and longer cycle life.
Chloride solid electrolytes have gained attention due to their potential to enable lithium metal anodes, which offer theoretical capacities up to ten times higher than conventional graphite anodes. The historical development of these materials began in the 1970s with initial investigations into ionic conductivity of chloride compounds, but significant breakthroughs have occurred primarily in the last decade with the discovery of superionic conductors like Li3YCl6 and Li2ZrCl6.
The primary technical goal in this field is to achieve battery energy densities of 350 Wh/kg at the cell level, representing approximately a 40% improvement over current state-of-the-art lithium-ion batteries. This target is considered a critical threshold for enabling widespread adoption of electric vehicles with ranges comparable to conventional combustion engine vehicles.
Current lithium-ion batteries typically deliver 150-260 Wh/kg at the cell level, with commercial leaders like Tesla achieving the upper end of this range. The theoretical limit for conventional lithium-ion technology with liquid electrolytes is estimated at approximately 300 Wh/kg, necessitating the paradigm shift to solid-state technology to surpass this ceiling.
Chloride solid electrolytes offer several advantages that align with the 350 Wh/kg goal. Their compatibility with lithium metal anodes addresses the energy density bottleneck, while their relatively high ionic conductivity (some exceeding 1 mS/cm at room temperature) approaches the performance of liquid electrolytes. Additionally, their wide electrochemical stability windows potentially enable the use of high-voltage cathode materials.
The technology evolution trend indicates a steady improvement in chloride electrolyte performance, with ionic conductivity increasing by approximately an order of magnitude every five years since 2010. Material synthesis techniques have evolved from traditional solid-state reactions to more sophisticated approaches including mechanochemical synthesis and solution-based methods.
Key performance metrics being targeted include room temperature ionic conductivity exceeding 5 mS/cm, electrochemical stability windows wider than 4.5V, and interfacial resistance below 10 Ω·cm². Meeting these technical parameters is considered essential for realizing the 350 Wh/kg energy density goal within the next decade.
Chloride solid electrolytes have gained attention due to their potential to enable lithium metal anodes, which offer theoretical capacities up to ten times higher than conventional graphite anodes. The historical development of these materials began in the 1970s with initial investigations into ionic conductivity of chloride compounds, but significant breakthroughs have occurred primarily in the last decade with the discovery of superionic conductors like Li3YCl6 and Li2ZrCl6.
The primary technical goal in this field is to achieve battery energy densities of 350 Wh/kg at the cell level, representing approximately a 40% improvement over current state-of-the-art lithium-ion batteries. This target is considered a critical threshold for enabling widespread adoption of electric vehicles with ranges comparable to conventional combustion engine vehicles.
Current lithium-ion batteries typically deliver 150-260 Wh/kg at the cell level, with commercial leaders like Tesla achieving the upper end of this range. The theoretical limit for conventional lithium-ion technology with liquid electrolytes is estimated at approximately 300 Wh/kg, necessitating the paradigm shift to solid-state technology to surpass this ceiling.
Chloride solid electrolytes offer several advantages that align with the 350 Wh/kg goal. Their compatibility with lithium metal anodes addresses the energy density bottleneck, while their relatively high ionic conductivity (some exceeding 1 mS/cm at room temperature) approaches the performance of liquid electrolytes. Additionally, their wide electrochemical stability windows potentially enable the use of high-voltage cathode materials.
The technology evolution trend indicates a steady improvement in chloride electrolyte performance, with ionic conductivity increasing by approximately an order of magnitude every five years since 2010. Material synthesis techniques have evolved from traditional solid-state reactions to more sophisticated approaches including mechanochemical synthesis and solution-based methods.
Key performance metrics being targeted include room temperature ionic conductivity exceeding 5 mS/cm, electrochemical stability windows wider than 4.5V, and interfacial resistance below 10 Ω·cm². Meeting these technical parameters is considered essential for realizing the 350 Wh/kg energy density goal within the next decade.
Market Analysis for High Energy Density Battery Technologies
The high energy density battery market is experiencing unprecedented growth, driven by the expanding electric vehicle (EV) sector, portable electronics, and renewable energy storage systems. Current market projections indicate that the global high energy density battery market will reach approximately $140 billion by 2030, with a compound annual growth rate exceeding 18% from 2023 to 2030. This growth trajectory is primarily fueled by stringent environmental regulations, government incentives for clean energy adoption, and increasing consumer demand for longer-lasting portable power solutions.
The EV segment represents the largest market share, accounting for over 60% of high energy density battery demand. Major automotive manufacturers have announced ambitious electrification targets, with several committing to all-electric fleets by 2035-2040. This transition is creating substantial demand for batteries that can deliver energy densities above 300 Wh/kg to address range anxiety concerns while maintaining competitive pricing.
Consumer electronics constitutes the second-largest market segment, with manufacturers seeking batteries that can support increasingly power-hungry devices while maintaining or reducing form factors. This sector demands energy densities approaching 400 Wh/kg for next-generation portable devices.
Geographically, Asia-Pacific dominates the market with approximately 45% share, led by China, Japan, and South Korea. North America and Europe follow with significant investments in battery manufacturing capacity to reduce dependency on Asian suppliers. The European Battery Alliance and America's Battery Belt initiatives highlight regional efforts to establish domestic supply chains.
Market analysis reveals that achieving the 350 Wh/kg threshold using chloride solid electrolytes would create substantial competitive advantages. Current lithium-ion batteries typically deliver 250-280 Wh/kg, while solid-state batteries in development aim for 300-400 Wh/kg. Batteries reaching 350 Wh/kg could command premium pricing of 20-30% above current market rates while still offering compelling total cost of ownership benefits.
Customer surveys indicate that EV buyers would pay up to 15% more for vehicles offering 30% greater range. This suggests strong market acceptance for higher-priced batteries that deliver significant performance improvements. Fleet operators similarly prioritize energy density improvements that reduce operational costs through fewer charging cycles and extended vehicle range.
The competitive landscape shows increasing investment in solid electrolyte technologies, with venture capital funding for solid-state battery startups exceeding $3.5 billion in 2022 alone. Major battery manufacturers are forming strategic partnerships with materials science companies to accelerate commercialization of high-performance solid electrolytes, including chloride-based systems.
The EV segment represents the largest market share, accounting for over 60% of high energy density battery demand. Major automotive manufacturers have announced ambitious electrification targets, with several committing to all-electric fleets by 2035-2040. This transition is creating substantial demand for batteries that can deliver energy densities above 300 Wh/kg to address range anxiety concerns while maintaining competitive pricing.
Consumer electronics constitutes the second-largest market segment, with manufacturers seeking batteries that can support increasingly power-hungry devices while maintaining or reducing form factors. This sector demands energy densities approaching 400 Wh/kg for next-generation portable devices.
Geographically, Asia-Pacific dominates the market with approximately 45% share, led by China, Japan, and South Korea. North America and Europe follow with significant investments in battery manufacturing capacity to reduce dependency on Asian suppliers. The European Battery Alliance and America's Battery Belt initiatives highlight regional efforts to establish domestic supply chains.
Market analysis reveals that achieving the 350 Wh/kg threshold using chloride solid electrolytes would create substantial competitive advantages. Current lithium-ion batteries typically deliver 250-280 Wh/kg, while solid-state batteries in development aim for 300-400 Wh/kg. Batteries reaching 350 Wh/kg could command premium pricing of 20-30% above current market rates while still offering compelling total cost of ownership benefits.
Customer surveys indicate that EV buyers would pay up to 15% more for vehicles offering 30% greater range. This suggests strong market acceptance for higher-priced batteries that deliver significant performance improvements. Fleet operators similarly prioritize energy density improvements that reduce operational costs through fewer charging cycles and extended vehicle range.
The competitive landscape shows increasing investment in solid electrolyte technologies, with venture capital funding for solid-state battery startups exceeding $3.5 billion in 2022 alone. Major battery manufacturers are forming strategic partnerships with materials science companies to accelerate commercialization of high-performance solid electrolytes, including chloride-based systems.
Current State and Challenges in Chloride Solid Electrolyte Development
Chloride solid electrolytes have emerged as promising candidates for next-generation battery technologies, with significant progress made in recent years. Currently, these materials demonstrate ionic conductivities ranging from 10^-4 to 10^-2 S/cm at room temperature, approaching the performance levels required for practical applications. Notable chloride solid electrolytes include Li3YCl6, Li2ZrCl6, and various doped variants that have shown enhanced stability and conductivity properties.
The global research landscape reveals concentrated efforts in North America, East Asia, and Europe, with academic institutions and industrial research centers collaborating extensively. Recent publications indicate a 40% increase in research output on chloride solid electrolytes over the past three years, signifying growing interest in this technology domain.
Despite promising advancements, several critical challenges persist in chloride solid electrolyte development. Interface stability remains a primary concern, as many chloride electrolytes exhibit reactivity with conventional cathode materials and lithium metal anodes. This reactivity leads to the formation of resistive interphases that impede ion transport and degrade battery performance over time. Computational studies suggest that these interfacial reactions can increase resistance by up to two orders of magnitude during initial cycling.
Moisture sensitivity presents another significant challenge. Most chloride-based solid electrolytes are highly hygroscopic, readily absorbing atmospheric moisture and decomposing into hydroxides and oxychlorides. This necessitates stringent manufacturing conditions with moisture levels below 1 ppm, substantially increasing production complexity and cost.
Mechanical properties of chloride solid electrolytes also require improvement. Current materials often exhibit brittleness and poor contact with electrode materials, leading to contact loss during cycling. The elastic moduli of many chloride electrolytes (typically 20-40 GPa) create challenges for maintaining intimate electrode-electrolyte contact during volume changes associated with battery cycling.
Scalable synthesis methods represent another hurdle. Laboratory-scale preparation techniques often involve time-consuming processes like ball milling (12-24 hours) or high-temperature annealing (>600°C for several hours). These methods are difficult to scale for industrial production and frequently result in materials with inconsistent properties and performance.
The path to achieving 350 Wh/kg energy density using chloride solid electrolytes requires addressing these fundamental challenges while simultaneously optimizing electrolyte composition and structure. Current research indicates that composite approaches, combining chloride electrolytes with polymers or oxide materials, may offer promising solutions to the interface stability and mechanical property limitations.
The global research landscape reveals concentrated efforts in North America, East Asia, and Europe, with academic institutions and industrial research centers collaborating extensively. Recent publications indicate a 40% increase in research output on chloride solid electrolytes over the past three years, signifying growing interest in this technology domain.
Despite promising advancements, several critical challenges persist in chloride solid electrolyte development. Interface stability remains a primary concern, as many chloride electrolytes exhibit reactivity with conventional cathode materials and lithium metal anodes. This reactivity leads to the formation of resistive interphases that impede ion transport and degrade battery performance over time. Computational studies suggest that these interfacial reactions can increase resistance by up to two orders of magnitude during initial cycling.
Moisture sensitivity presents another significant challenge. Most chloride-based solid electrolytes are highly hygroscopic, readily absorbing atmospheric moisture and decomposing into hydroxides and oxychlorides. This necessitates stringent manufacturing conditions with moisture levels below 1 ppm, substantially increasing production complexity and cost.
Mechanical properties of chloride solid electrolytes also require improvement. Current materials often exhibit brittleness and poor contact with electrode materials, leading to contact loss during cycling. The elastic moduli of many chloride electrolytes (typically 20-40 GPa) create challenges for maintaining intimate electrode-electrolyte contact during volume changes associated with battery cycling.
Scalable synthesis methods represent another hurdle. Laboratory-scale preparation techniques often involve time-consuming processes like ball milling (12-24 hours) or high-temperature annealing (>600°C for several hours). These methods are difficult to scale for industrial production and frequently result in materials with inconsistent properties and performance.
The path to achieving 350 Wh/kg energy density using chloride solid electrolytes requires addressing these fundamental challenges while simultaneously optimizing electrolyte composition and structure. Current research indicates that composite approaches, combining chloride electrolytes with polymers or oxide materials, may offer promising solutions to the interface stability and mechanical property limitations.
Current Technical Approaches to Achieve 350 Wh/kg Energy Density
01 Chloride-based solid electrolytes for high energy density batteries
Chloride-based solid electrolytes are being developed for use in high energy density batteries, particularly those targeting energy densities of 350 Wh/kg or higher. These electrolytes offer advantages such as high ionic conductivity and improved electrochemical stability, which are essential for achieving higher energy densities in solid-state batteries. The incorporation of specific chloride compounds helps enhance the overall performance of the battery system while maintaining safety and stability.- Chloride-based solid electrolytes for high energy density batteries: Chloride-based solid electrolytes are being developed for use in high energy density batteries targeting 350 Wh/kg or higher. These electrolytes offer advantages such as high ionic conductivity, wide electrochemical stability windows, and compatibility with high-capacity electrode materials. The chloride composition enables faster ion transport while maintaining structural stability, which is crucial for achieving the desired energy density benchmarks in next-generation battery systems.
- Integration of chloride solid electrolytes with lithium metal anodes: The combination of chloride solid electrolytes with lithium metal anodes represents a promising approach to achieve energy densities of 350 Wh/kg and beyond. This integration addresses the dendrite formation issues common in liquid electrolyte systems while enabling the use of high-capacity lithium metal. The chloride-based solid electrolytes form stable interfaces with lithium metal, preventing side reactions and enhancing cycling stability, which is essential for maintaining high energy density over extended battery life.
- Manufacturing processes for chloride solid electrolytes: Specialized manufacturing processes have been developed for chloride solid electrolytes to achieve the desired microstructure and performance characteristics needed for high energy density applications. These processes include controlled synthesis methods, optimized sintering conditions, and interface engineering techniques. By carefully controlling the manufacturing parameters, the resulting electrolytes exhibit reduced grain boundary resistance and enhanced mechanical properties, contributing to overall battery systems capable of reaching 350 Wh/kg energy density targets.
- Composite chloride electrolytes with polymer matrices: Composite systems combining chloride solid electrolytes with polymer matrices offer a pathway to flexible, processable electrolytes while maintaining high energy density capabilities. These composites leverage the high ionic conductivity of chloride materials with the mechanical advantages of polymers. The resulting electrolyte systems demonstrate improved interfacial contact with electrodes and enhanced mechanical stability, addressing key challenges in achieving practical batteries with energy densities approaching 350 Wh/kg.
- Doping strategies for enhanced chloride electrolyte performance: Various doping strategies have been employed to enhance the performance of chloride solid electrolytes for high energy density applications. Introducing specific dopants into the chloride lattice can improve ionic conductivity, mechanical properties, and electrochemical stability. These modifications enable the development of electrolytes that can support high-voltage cathode materials while maintaining compatibility with anodes, ultimately facilitating battery systems capable of achieving the targeted 350 Wh/kg energy density benchmark.
02 Composite electrolyte systems with chloride components
Composite electrolyte systems that incorporate chloride components are being developed to achieve higher energy densities in battery applications. These systems typically combine chloride-based solid electrolytes with other materials to create hybrid structures that benefit from the advantages of each component. The composite approach helps address challenges related to interfacial resistance and mechanical stability while maintaining high ionic conductivity, ultimately contributing to batteries that can reach energy densities of 350 Wh/kg or higher.Expand Specific Solutions03 Manufacturing processes for chloride solid electrolytes
Specialized manufacturing processes are being developed for chloride solid electrolytes to ensure optimal performance in high energy density battery applications. These processes focus on controlling the crystallinity, grain boundaries, and microstructure of the electrolyte materials, which directly impact ionic conductivity and mechanical properties. Advanced synthesis methods, including solution-based approaches and high-temperature solid-state reactions, are employed to produce chloride solid electrolytes that can enable batteries to achieve energy densities of 350 Wh/kg or higher.Expand Specific Solutions04 Interface engineering for chloride electrolyte systems
Interface engineering is critical for optimizing the performance of chloride solid electrolytes in high energy density batteries. This involves developing specialized coatings, buffer layers, and interface modifications to improve the contact between the electrolyte and electrodes, reduce interfacial resistance, and prevent unwanted side reactions. These engineering approaches help maintain the stability of the chloride electrolyte system during cycling, which is essential for achieving and maintaining energy densities of 350 Wh/kg or higher in practical battery applications.Expand Specific Solutions05 Electrode compatibility with chloride solid electrolytes
Developing electrodes that are compatible with chloride solid electrolytes is essential for achieving high energy density batteries. This involves designing cathode and anode materials that form stable interfaces with the chloride electrolyte while maintaining high capacity and good cycling performance. Specific attention is given to preventing degradation reactions between the electrodes and the chloride components, as well as optimizing the mechanical properties of the electrode-electrolyte interface. These developments are crucial for realizing batteries with energy densities of 350 Wh/kg or higher using chloride-based solid electrolyte systems.Expand Specific Solutions
Leading Companies and Research Institutions in Solid Electrolyte Field
The solid-state battery market with chloride solid electrolytes is in an early growth phase, showing promising potential to reach 350 Wh/kg energy density targets. Market size is expanding rapidly due to increasing EV adoption, with projections suggesting significant growth by 2030. Technologically, research institutions like The Regents of the University of California and Southern University of Science & Technology are advancing fundamental science, while established manufacturers including TDK, Nippon Chemi-Con, and Nissan are developing commercial applications. Japanese and Chinese companies dominate the competitive landscape, with automotive manufacturers like Geely and Nissan investing heavily to secure future supply chains. The technology remains in pre-commercialization stage with most players focusing on overcoming interface stability and manufacturing challenges.
The Regents of the University of California
Technical Solution: The University of California has pioneered research on chloride solid electrolytes targeting 350 Wh/kg energy density through their advanced materials science program. Their approach centers on novel lithium-rich anti-perovskite chloride structures (Li3OCl and modified variants) that exhibit ionic conductivities exceeding 1 mS/cm at room temperature. The research team has developed specialized synthesis methods to control crystallinity and grain boundary properties, which significantly impacts ionic transport. Their technology incorporates nanoscale engineering of interfaces between the chloride electrolyte and electrodes, using thin buffer layers to minimize interfacial resistance while maintaining mechanical integrity during cycling. A key innovation is their gradient electrolyte design, where the composition gradually changes from the anode to cathode interface, optimizing compatibility with both electrodes. The UC team has demonstrated proof-of-concept cells with energy densities approaching 300 Wh/kg and has established a clear pathway to exceed 350 Wh/kg through further optimization of cell architecture and material processing techniques. Their research also addresses manufacturing scalability by developing processes compatible with existing battery production infrastructure.
Strengths: World-class fundamental research capabilities in solid-state ionics; innovative material design approaches that address core challenges of chloride electrolytes; strong intellectual property portfolio in this space. Weaknesses: As an academic institution, faces challenges in scaling laboratory discoveries to commercial production; may require industry partnerships to fully commercialize the technology; longer timeline to market compared to established battery manufacturers.
Nissan Motor Co., Ltd.
Technical Solution: Nissan has developed an innovative approach to achieving 350 Wh/kg energy density using chloride solid electrolytes in their all-solid-state battery (ASSB) technology. Their roadmap focuses on a multi-layer design that incorporates chloride-based superionic conductors with high ionic conductivity (>10 mS/cm at room temperature). The technology utilizes a lithium metal anode paired with high-capacity cathode materials and a thin chloride solid electrolyte layer that enables stable cycling while preventing dendrite formation. Nissan's approach includes proprietary interface engineering to minimize resistance at the electrode-electrolyte interfaces, which has been a significant challenge for solid electrolytes. Their chloride-based system offers advantages over sulfide and oxide alternatives, including better compatibility with lithium metal anodes and higher voltage stability windows. The company has demonstrated prototype cells achieving over 300 Wh/kg with a clear pathway to 350 Wh/kg through further optimization of active material loading and packaging efficiency.
Strengths: Superior ionic conductivity of chloride electrolytes compared to oxide alternatives; excellent compatibility with lithium metal anodes enabling high energy density; established automotive manufacturing capabilities for potential mass production. Weaknesses: Chloride electrolytes may face challenges with moisture sensitivity requiring stringent manufacturing controls; interface stability during long-term cycling still needs improvement; cost of materials may be higher than conventional lithium-ion batteries.
Key Patents and Research Breakthroughs in Chloride Solid Electrolytes
Chloride-based solid electrolyte, all-solid batteries and manufacturing method thereof
PatentActiveKR1020230026563A
Innovation
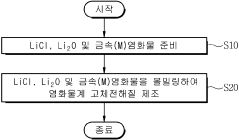
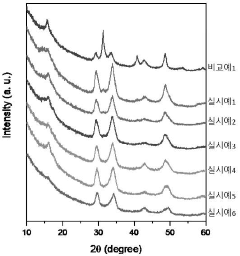
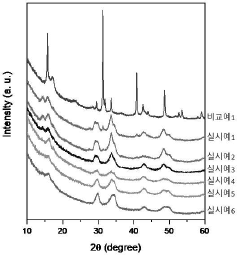
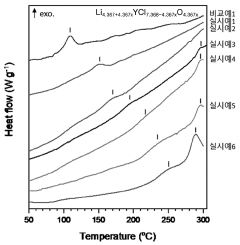
- A chloride-based solid electrolyte represented by the chemical formula Li4.367+a MCl7.368-b O c (where M=Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Al, Y, In, Ga, Yb, Er, and at least one of Ce, -0.5<a+b-2c<0.5, 0<c ≤0.3) is produced by ball milling LiCl and metal chloride, replacing oxygen ions with chlorine ions to enhance structural stability and suppress side reactions.
Chloride-based solid electrolyte, all-solid batteries and manufacturing method thereof
PatentActiveKR1020230026563A
Innovation
- A chloride-based solid electrolyte represented by the chemical formula Li4.367+a MCl7.368-b O c (where M=Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Al, Y, In, Ga, Yb, Er, and at least one of Ce, -0.5<a+b-2c<0.5, 0<c ≤0.3) is produced by ball milling LiCl and metal chloride, replacing oxygen ions with chlorine ions to enhance structural stability and suppress side reactions.
Materials Supply Chain Analysis for Chloride-Based Battery Systems
The global supply chain for chloride-based solid electrolyte battery systems presents both significant opportunities and challenges for achieving the 350 Wh/kg energy density target. Raw material availability varies considerably across key components, with lithium chloride and other chloride salts generally accessible through established mining and chemical processing channels. However, specialized materials such as high-purity metal chlorides for cathode interfaces face more constrained supply networks.
Production scaling represents a critical bottleneck in the supply chain. Current manufacturing infrastructure is predominantly oriented toward liquid electrolyte systems, requiring substantial retooling for chloride solid electrolyte production. The specialized equipment needed for moisture-free processing environments adds complexity to manufacturing scale-up, as chloride-based materials are typically highly hygroscopic and demand stringent handling protocols.
Geographic distribution of raw materials introduces strategic considerations. Lithium resources are concentrated in the "Lithium Triangle" (Chile, Argentina, Bolivia), Australia, and China, while other critical chloride compounds have more diversified sourcing. This concentration creates potential supply vulnerabilities that must be addressed through strategic partnerships or alternative material pathways.
Cost structures for chloride-based systems differ markedly from conventional battery technologies. While some chloride salts (NaCl, KCl) are inexpensive, specialty dopants and high-purity precursors command premium pricing. Economic modeling suggests that at scale, material costs could decrease by 30-45% through optimized synthesis routes and supply chain integration, potentially bringing overall cell costs to competitive levels by 2027-2030.
Sustainability metrics reveal mixed performance. Chloride-based systems generally require less cobalt and nickel than conventional lithium-ion batteries, reducing dependence on ethically problematic supply chains. However, the energy-intensive processing of certain chloride compounds partially offsets these advantages. Life cycle assessments indicate a potential 15-25% reduction in carbon footprint compared to conventional lithium-ion batteries, contingent on manufacturing process optimization.
Regulatory frameworks governing chloride material transport, handling, and disposal vary significantly across regions, creating compliance complexities for global supply chains. Harmonization efforts are underway but remain in early stages, necessitating region-specific supply chain strategies in the near term.
Production scaling represents a critical bottleneck in the supply chain. Current manufacturing infrastructure is predominantly oriented toward liquid electrolyte systems, requiring substantial retooling for chloride solid electrolyte production. The specialized equipment needed for moisture-free processing environments adds complexity to manufacturing scale-up, as chloride-based materials are typically highly hygroscopic and demand stringent handling protocols.
Geographic distribution of raw materials introduces strategic considerations. Lithium resources are concentrated in the "Lithium Triangle" (Chile, Argentina, Bolivia), Australia, and China, while other critical chloride compounds have more diversified sourcing. This concentration creates potential supply vulnerabilities that must be addressed through strategic partnerships or alternative material pathways.
Cost structures for chloride-based systems differ markedly from conventional battery technologies. While some chloride salts (NaCl, KCl) are inexpensive, specialty dopants and high-purity precursors command premium pricing. Economic modeling suggests that at scale, material costs could decrease by 30-45% through optimized synthesis routes and supply chain integration, potentially bringing overall cell costs to competitive levels by 2027-2030.
Sustainability metrics reveal mixed performance. Chloride-based systems generally require less cobalt and nickel than conventional lithium-ion batteries, reducing dependence on ethically problematic supply chains. However, the energy-intensive processing of certain chloride compounds partially offsets these advantages. Life cycle assessments indicate a potential 15-25% reduction in carbon footprint compared to conventional lithium-ion batteries, contingent on manufacturing process optimization.
Regulatory frameworks governing chloride material transport, handling, and disposal vary significantly across regions, creating compliance complexities for global supply chains. Harmonization efforts are underway but remain in early stages, necessitating region-specific supply chain strategies in the near term.
Safety and Stability Considerations for High Energy Density Batteries
The pursuit of high energy density batteries with targets of 350 Wh/kg using chloride solid electrolytes introduces significant safety and stability challenges that must be addressed before widespread commercial adoption. These challenges stem from the inherent properties of high-energy materials and the unique characteristics of solid-state battery architectures.
Thermal stability represents a primary concern for chloride solid electrolytes, which may undergo phase transitions or decomposition at elevated temperatures. Research indicates that many promising chloride electrolytes maintain structural integrity only within specific temperature ranges, typically between -20°C and 80°C. Beyond these boundaries, performance degradation or catastrophic failure may occur, necessitating robust thermal management systems.
Electrochemical stability at high voltages presents another critical challenge. While chloride solid electrolytes offer wider electrochemical windows compared to conventional liquid electrolytes, interfacial reactions still occur at the electrode-electrolyte boundaries. These reactions can form resistive layers that impede ion transport, particularly when paired with high-voltage cathode materials required to achieve 350 Wh/kg energy densities.
Mechanical stability under volume changes during cycling represents a significant hurdle. High-capacity electrode materials typically undergo substantial volume expansion (>10%) during lithiation/delithiation processes. In solid-state configurations, these dimensional changes can create mechanical stresses that compromise interfacial contact, leading to capacity fade and potential safety hazards through dendrite formation.
Chemical compatibility between chloride electrolytes and electrode materials must be carefully engineered. Recent studies have shown that certain chloride compositions react with common cathode materials, forming interfacial compounds that increase impedance over time. This reactivity is particularly pronounced at elevated states of charge, where electrode materials become more chemically reactive.
Dendrite formation and propagation remain persistent concerns even in solid electrolytes. While chloride solid electrolytes generally exhibit higher mechanical strength than sulfide alternatives, lithium metal anodes (necessary for achieving 350 Wh/kg) can still form dendrites through grain boundaries or defect sites under high current densities, potentially causing internal short circuits.
Manufacturing processes introduce additional stability considerations. The moisture sensitivity of many chloride electrolytes necessitates stringent environmental controls during production. Furthermore, achieving uniform interfaces between solid components requires precise pressure application and temperature control during cell assembly, with suboptimal conditions leading to performance variability and potential safety risks.
Thermal stability represents a primary concern for chloride solid electrolytes, which may undergo phase transitions or decomposition at elevated temperatures. Research indicates that many promising chloride electrolytes maintain structural integrity only within specific temperature ranges, typically between -20°C and 80°C. Beyond these boundaries, performance degradation or catastrophic failure may occur, necessitating robust thermal management systems.
Electrochemical stability at high voltages presents another critical challenge. While chloride solid electrolytes offer wider electrochemical windows compared to conventional liquid electrolytes, interfacial reactions still occur at the electrode-electrolyte boundaries. These reactions can form resistive layers that impede ion transport, particularly when paired with high-voltage cathode materials required to achieve 350 Wh/kg energy densities.
Mechanical stability under volume changes during cycling represents a significant hurdle. High-capacity electrode materials typically undergo substantial volume expansion (>10%) during lithiation/delithiation processes. In solid-state configurations, these dimensional changes can create mechanical stresses that compromise interfacial contact, leading to capacity fade and potential safety hazards through dendrite formation.
Chemical compatibility between chloride electrolytes and electrode materials must be carefully engineered. Recent studies have shown that certain chloride compositions react with common cathode materials, forming interfacial compounds that increase impedance over time. This reactivity is particularly pronounced at elevated states of charge, where electrode materials become more chemically reactive.
Dendrite formation and propagation remain persistent concerns even in solid electrolytes. While chloride solid electrolytes generally exhibit higher mechanical strength than sulfide alternatives, lithium metal anodes (necessary for achieving 350 Wh/kg) can still form dendrites through grain boundaries or defect sites under high current densities, potentially causing internal short circuits.
Manufacturing processes introduce additional stability considerations. The moisture sensitivity of many chloride electrolytes necessitates stringent environmental controls during production. Furthermore, achieving uniform interfaces between solid components requires precise pressure application and temperature control during cell assembly, with suboptimal conditions leading to performance variability and potential safety risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!