Comparative Analysis of Sodium Percarbonate and Hydrogen Peroxide
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Background and Objectives
Sodium percarbonate and hydrogen peroxide have been integral components in the cleaning and bleaching industry for decades. These compounds have garnered significant attention due to their effectiveness in oxidizing and removing stains, as well as their environmentally friendly nature compared to traditional chlorine-based bleaches. The evolution of these technologies has been driven by the increasing demand for safer, more sustainable cleaning solutions in both household and industrial applications.
The primary objective of this comparative analysis is to provide a comprehensive understanding of the similarities, differences, and relative advantages of sodium percarbonate and hydrogen peroxide. This study aims to explore their chemical properties, production methods, stability, and efficacy in various applications. By examining these aspects, we seek to identify the most suitable contexts for each compound and potential areas for further innovation.
In recent years, there has been a notable shift towards eco-friendly and biodegradable cleaning agents, propelling the growth of the sodium percarbonate and hydrogen peroxide markets. This trend is further reinforced by stringent environmental regulations and growing consumer awareness about the impact of household chemicals on the environment. As a result, manufacturers and researchers are continuously exploring ways to enhance the stability, effectiveness, and versatility of these compounds.
The analysis will delve into the current state of technology for both sodium percarbonate and hydrogen peroxide, including their production processes, storage requirements, and handling considerations. We will examine the latest advancements in formulation techniques that have improved their performance and expanded their applications across various industries, including textiles, paper and pulp, water treatment, and personal care.
Furthermore, this study will investigate the ongoing research and development efforts aimed at overcoming the limitations of these compounds, such as improving their stability in aqueous solutions and enhancing their efficacy at lower temperatures. By identifying these technological challenges and potential solutions, we aim to provide insights into future research directions and innovation opportunities in this field.
Ultimately, this comparative analysis seeks to equip stakeholders with a comprehensive understanding of sodium percarbonate and hydrogen peroxide technologies. This knowledge will be crucial for informed decision-making in product development, market strategies, and research initiatives, thereby contributing to the advancement of safer and more efficient cleaning and bleaching solutions.
The primary objective of this comparative analysis is to provide a comprehensive understanding of the similarities, differences, and relative advantages of sodium percarbonate and hydrogen peroxide. This study aims to explore their chemical properties, production methods, stability, and efficacy in various applications. By examining these aspects, we seek to identify the most suitable contexts for each compound and potential areas for further innovation.
In recent years, there has been a notable shift towards eco-friendly and biodegradable cleaning agents, propelling the growth of the sodium percarbonate and hydrogen peroxide markets. This trend is further reinforced by stringent environmental regulations and growing consumer awareness about the impact of household chemicals on the environment. As a result, manufacturers and researchers are continuously exploring ways to enhance the stability, effectiveness, and versatility of these compounds.
The analysis will delve into the current state of technology for both sodium percarbonate and hydrogen peroxide, including their production processes, storage requirements, and handling considerations. We will examine the latest advancements in formulation techniques that have improved their performance and expanded their applications across various industries, including textiles, paper and pulp, water treatment, and personal care.
Furthermore, this study will investigate the ongoing research and development efforts aimed at overcoming the limitations of these compounds, such as improving their stability in aqueous solutions and enhancing their efficacy at lower temperatures. By identifying these technological challenges and potential solutions, we aim to provide insights into future research directions and innovation opportunities in this field.
Ultimately, this comparative analysis seeks to equip stakeholders with a comprehensive understanding of sodium percarbonate and hydrogen peroxide technologies. This knowledge will be crucial for informed decision-making in product development, market strategies, and research initiatives, thereby contributing to the advancement of safer and more efficient cleaning and bleaching solutions.
Market Demand Analysis
The market demand for sodium percarbonate and hydrogen peroxide has been steadily growing due to their versatile applications in various industries. Both compounds are widely used as bleaching agents and oxidizers, with sodium percarbonate gaining popularity as an eco-friendly alternative to traditional chlorine-based bleaches.
In the household cleaning sector, sodium percarbonate has seen a significant increase in demand. Consumers are increasingly seeking environmentally friendly cleaning products, driving the growth of oxygen-based bleaches. Sodium percarbonate, often marketed as "oxygen bleach," has become a key ingredient in many laundry detergents and stain removers. Its ability to release hydrogen peroxide when dissolved in water makes it an effective yet gentle cleaning agent, appealing to environmentally conscious consumers.
The industrial cleaning sector also contributes to the rising demand for both compounds. Sodium percarbonate and hydrogen peroxide are used in commercial laundry operations, industrial equipment cleaning, and water treatment processes. The food and beverage industry, in particular, has shown increased interest in these compounds for sanitization purposes, as they leave no harmful residues.
In the personal care and cosmetics industry, hydrogen peroxide continues to be a staple ingredient in hair bleaching products and teeth whitening solutions. The growing trend of at-home beauty treatments has further boosted the demand for hydrogen peroxide-based products in this sector.
The healthcare industry represents another significant market for both compounds. Hydrogen peroxide is widely used as a disinfectant and sterilizing agent in medical facilities. The COVID-19 pandemic has further amplified the demand for disinfectants, benefiting both hydrogen peroxide and sodium percarbonate markets.
The pulp and paper industry is a major consumer of hydrogen peroxide, using it as a bleaching agent for paper products. As the demand for paper and packaging materials continues to grow, particularly with the rise of e-commerce, this sector contributes significantly to the overall market demand for hydrogen peroxide.
Environmental applications present a growing market for both compounds. Hydrogen peroxide is increasingly used in wastewater treatment and soil remediation processes. Sodium percarbonate, with its oxygen-releasing properties, is finding applications in aquaculture and pond treatment.
The textile industry also contributes to the demand, using both compounds in fabric bleaching processes. The trend towards more sustainable textile production methods has led to increased interest in oxygen-based bleaches like sodium percarbonate.
Overall, the market demand for sodium percarbonate and hydrogen peroxide is expected to continue its upward trajectory. Factors such as increasing environmental awareness, stringent regulations on chlorine-based products, and the growing emphasis on hygiene and sanitation across various industries are likely to drive further growth in the coming years.
In the household cleaning sector, sodium percarbonate has seen a significant increase in demand. Consumers are increasingly seeking environmentally friendly cleaning products, driving the growth of oxygen-based bleaches. Sodium percarbonate, often marketed as "oxygen bleach," has become a key ingredient in many laundry detergents and stain removers. Its ability to release hydrogen peroxide when dissolved in water makes it an effective yet gentle cleaning agent, appealing to environmentally conscious consumers.
The industrial cleaning sector also contributes to the rising demand for both compounds. Sodium percarbonate and hydrogen peroxide are used in commercial laundry operations, industrial equipment cleaning, and water treatment processes. The food and beverage industry, in particular, has shown increased interest in these compounds for sanitization purposes, as they leave no harmful residues.
In the personal care and cosmetics industry, hydrogen peroxide continues to be a staple ingredient in hair bleaching products and teeth whitening solutions. The growing trend of at-home beauty treatments has further boosted the demand for hydrogen peroxide-based products in this sector.
The healthcare industry represents another significant market for both compounds. Hydrogen peroxide is widely used as a disinfectant and sterilizing agent in medical facilities. The COVID-19 pandemic has further amplified the demand for disinfectants, benefiting both hydrogen peroxide and sodium percarbonate markets.
The pulp and paper industry is a major consumer of hydrogen peroxide, using it as a bleaching agent for paper products. As the demand for paper and packaging materials continues to grow, particularly with the rise of e-commerce, this sector contributes significantly to the overall market demand for hydrogen peroxide.
Environmental applications present a growing market for both compounds. Hydrogen peroxide is increasingly used in wastewater treatment and soil remediation processes. Sodium percarbonate, with its oxygen-releasing properties, is finding applications in aquaculture and pond treatment.
The textile industry also contributes to the demand, using both compounds in fabric bleaching processes. The trend towards more sustainable textile production methods has led to increased interest in oxygen-based bleaches like sodium percarbonate.
Overall, the market demand for sodium percarbonate and hydrogen peroxide is expected to continue its upward trajectory. Factors such as increasing environmental awareness, stringent regulations on chlorine-based products, and the growing emphasis on hygiene and sanitation across various industries are likely to drive further growth in the coming years.
Technical Challenges
The comparative analysis of sodium percarbonate and hydrogen peroxide reveals several technical challenges that researchers and industry professionals face when working with these compounds. One of the primary challenges is the stability of sodium percarbonate compared to hydrogen peroxide. Sodium percarbonate, while more stable in its solid form, can decompose when exposed to moisture or high temperatures, potentially reducing its effectiveness over time. This necessitates careful storage and handling procedures to maintain its potency.
Hydrogen peroxide, on the other hand, is inherently unstable and prone to decomposition, especially when exposed to light, heat, or certain contaminants. This instability poses significant challenges in terms of storage, transportation, and long-term use. Manufacturers and end-users must implement stringent control measures to prevent premature decomposition and ensure consistent performance.
The concentration and release mechanisms of active oxygen present another technical hurdle. Sodium percarbonate releases hydrogen peroxide gradually when dissolved in water, which can be advantageous for certain applications but may limit its effectiveness in situations requiring rapid action. Conversely, hydrogen peroxide is available in various concentrations, but achieving the desired concentration for specific applications while maintaining safety and efficacy can be challenging.
Compatibility with other materials and substances is a critical consideration. Sodium percarbonate, being alkaline, may not be suitable for use with acid-sensitive materials or in acidic environments. Hydrogen peroxide, particularly at higher concentrations, can be corrosive to certain metals and materials, necessitating careful selection of storage containers and application equipment.
The environmental impact and safety concerns associated with both compounds pose additional technical challenges. While both are considered environmentally friendly oxidizing agents, their production, use, and disposal must be carefully managed to minimize potential ecological effects. Ensuring worker safety during handling and application, especially for higher concentrations of hydrogen peroxide, requires robust safety protocols and protective measures.
Formulation challenges arise when incorporating these compounds into various products. Sodium percarbonate's solid form can be advantageous for certain applications but may present difficulties in achieving uniform distribution or controlled release in liquid formulations. Hydrogen peroxide's liquid form offers versatility but can introduce stability issues in complex formulations.
Lastly, the cost-effectiveness and scalability of production processes for both compounds present ongoing technical challenges. Optimizing production methods to reduce costs while maintaining quality and purity is crucial for widespread adoption in various industries. Additionally, developing innovative applications that leverage the unique properties of each compound while addressing their limitations remains an area of active research and development.
Hydrogen peroxide, on the other hand, is inherently unstable and prone to decomposition, especially when exposed to light, heat, or certain contaminants. This instability poses significant challenges in terms of storage, transportation, and long-term use. Manufacturers and end-users must implement stringent control measures to prevent premature decomposition and ensure consistent performance.
The concentration and release mechanisms of active oxygen present another technical hurdle. Sodium percarbonate releases hydrogen peroxide gradually when dissolved in water, which can be advantageous for certain applications but may limit its effectiveness in situations requiring rapid action. Conversely, hydrogen peroxide is available in various concentrations, but achieving the desired concentration for specific applications while maintaining safety and efficacy can be challenging.
Compatibility with other materials and substances is a critical consideration. Sodium percarbonate, being alkaline, may not be suitable for use with acid-sensitive materials or in acidic environments. Hydrogen peroxide, particularly at higher concentrations, can be corrosive to certain metals and materials, necessitating careful selection of storage containers and application equipment.
The environmental impact and safety concerns associated with both compounds pose additional technical challenges. While both are considered environmentally friendly oxidizing agents, their production, use, and disposal must be carefully managed to minimize potential ecological effects. Ensuring worker safety during handling and application, especially for higher concentrations of hydrogen peroxide, requires robust safety protocols and protective measures.
Formulation challenges arise when incorporating these compounds into various products. Sodium percarbonate's solid form can be advantageous for certain applications but may present difficulties in achieving uniform distribution or controlled release in liquid formulations. Hydrogen peroxide's liquid form offers versatility but can introduce stability issues in complex formulations.
Lastly, the cost-effectiveness and scalability of production processes for both compounds present ongoing technical challenges. Optimizing production methods to reduce costs while maintaining quality and purity is crucial for widespread adoption in various industries. Additionally, developing innovative applications that leverage the unique properties of each compound while addressing their limitations remains an area of active research and development.
Current Comparison Methods
01 Synthesis and production of sodium percarbonate
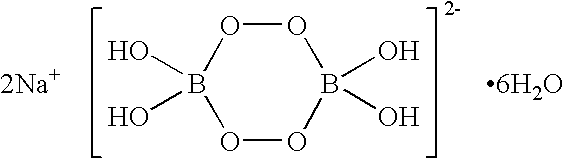
Sodium percarbonate is synthesized by reacting sodium carbonate with hydrogen peroxide. The process involves careful control of reaction conditions, including temperature, concentration, and pH, to optimize yield and product stability. Various methods, such as spray drying or fluidized bed techniques, can be employed to produce sodium percarbonate in granular or powder form.- Production of sodium percarbonate: Sodium percarbonate is produced by reacting sodium carbonate with hydrogen peroxide. The process often involves crystallization and stabilization techniques to ensure the quality and stability of the final product. Various methods and conditions can be employed to optimize the production process and improve the characteristics of the sodium percarbonate.
- Stabilization of sodium percarbonate: Stabilizers are added to sodium percarbonate to improve its storage stability and prevent decomposition. Common stabilizers include silicates, phosphates, and organic compounds. The stabilization process may involve coating the sodium percarbonate particles or incorporating the stabilizers during the production process.
- Applications in cleaning and bleaching: Sodium percarbonate is widely used in cleaning and bleaching applications due to its ability to release hydrogen peroxide when dissolved in water. It is incorporated into laundry detergents, dishwashing products, and other household cleaning agents. The controlled release of hydrogen peroxide provides effective stain removal and disinfection properties.
- Hydrogen peroxide generation from sodium percarbonate: When sodium percarbonate dissolves in water, it releases hydrogen peroxide. This property is utilized in various applications where a controlled release of hydrogen peroxide is desired. The generation of hydrogen peroxide from sodium percarbonate can be influenced by factors such as temperature, pH, and the presence of catalysts or stabilizers.
- Environmental and safety considerations: Sodium percarbonate is considered an environmentally friendly alternative to other bleaching agents, as it decomposes into harmless byproducts. However, proper handling and storage are essential to ensure safety and maintain product efficacy. Considerations include moisture control, temperature regulation, and prevention of contamination with incompatible materials.
02 Stabilization of sodium percarbonate
Stabilizers are added to sodium percarbonate to improve its shelf life and maintain its active oxygen content during storage. Common stabilizers include silicates, phosphates, and organic compounds. These additives help prevent the decomposition of sodium percarbonate and maintain its effectiveness as a bleaching and cleaning agent.Expand Specific Solutions03 Applications in cleaning and laundry products
Sodium percarbonate is widely used in cleaning and laundry products due to its ability to release hydrogen peroxide when dissolved in water. It serves as an effective bleaching agent and stain remover in detergents, laundry boosters, and all-purpose cleaners. The controlled release of hydrogen peroxide provides both cleaning and disinfecting properties.Expand Specific Solutions04 Comparison of sodium percarbonate and hydrogen peroxide
While both sodium percarbonate and hydrogen peroxide are oxidizing agents, sodium percarbonate offers advantages in terms of stability and ease of handling. Sodium percarbonate is a solid, making it easier to store and transport compared to liquid hydrogen peroxide. It also provides a more controlled release of hydrogen peroxide when activated by water.Expand Specific Solutions05 Environmental and safety considerations
Sodium percarbonate is considered an environmentally friendly alternative to chlorine-based bleaches. It decomposes into harmless byproducts of water, oxygen, and sodium carbonate. However, proper handling and storage are essential to prevent accidental exposure and ensure product efficacy. Safety measures include keeping the product dry and away from heat sources.Expand Specific Solutions
Key Industry Players
The comparative analysis of sodium percarbonate and hydrogen peroxide reveals a competitive landscape in a mature market with established players. The global market for these oxidizing agents is substantial, driven by their widespread use in cleaning products, water treatment, and industrial applications. Key players like Henkel, Solvay, and BASF dominate the industry, leveraging their extensive R&D capabilities and global distribution networks. The technology is well-established, with companies focusing on product improvements and sustainable formulations. Emerging trends include the development of eco-friendly alternatives and increased applications in wastewater treatment and healthcare sectors, presenting opportunities for innovation and market expansion.
Henkel AG & Co. KGaA
Technical Solution: Henkel has conducted comprehensive comparative analyses of sodium percarbonate and hydrogen peroxide, focusing on their applications in household and industrial cleaning products. Their research has led to the development of innovative formulations that leverage the strengths of both compounds. For sodium percarbonate, Henkel has created a proprietary coating technology that enhances its stability in detergent formulations, allowing for longer shelf life and improved performance in cold water washing[9]. In the realm of hydrogen peroxide, Henkel has developed stabilized formulations that maintain efficacy in a wide range of pH conditions, making them suitable for various cleaning applications. Additionally, Henkel's research has explored the synergistic effects of combining sodium percarbonate and hydrogen peroxide in specific ratios, resulting in enhanced stain removal and disinfection properties[10].
Strengths: Versatile applications in household and industrial cleaning, improved stability and performance of both compounds. Weaknesses: May require more complex formulations, potentially higher production costs for specialized products.
Solvay SA
Technical Solution: Solvay SA has developed advanced formulations combining sodium percarbonate and hydrogen peroxide for enhanced cleaning and bleaching applications. Their patented technology involves stabilizing sodium percarbonate particles with a protective coating, which improves storage stability and prolongs shelf life[1]. This allows for a controlled release of active oxygen when the product comes into contact with water, providing sustained cleaning power. Solvay's research has also focused on optimizing the synergistic effects between sodium percarbonate and hydrogen peroxide, resulting in formulations that offer superior stain removal and disinfection properties compared to using either compound alone[2].
Strengths: Improved stability and shelf life of sodium percarbonate, controlled release of active oxygen, enhanced cleaning power. Weaknesses: Potentially higher production costs due to specialized coating process, may require specific packaging to maintain efficacy.
Core Research Findings
Pouches for collecting matter excreted by the body
PatentInactiveUS6852100B1
Innovation
- Adhering a layer of MCA onto the pouch wall or using a carrier with absorbent properties attached to the pouch wall, ensuring MCA release near the opening to effectively counteract malodours and minimizing the risk of loose particles during handling.
A Method of Producing Sodium Percarbonate
PatentInactiveGB1195641A
Innovation
- A method involving the reaction of sodium carbonate with hydrogen peroxide, using benzoic acid and inorganic silicate compounds as stabilizers to enhance the stability of sodium percarbonate, effectively sequestering harmful impurities and improving product stability.
Environmental Impact
The environmental impact of sodium percarbonate and hydrogen peroxide is a crucial consideration in their comparative analysis. Both compounds are widely used in various industries, including cleaning products, water treatment, and textile processing. However, their effects on the environment differ in several aspects.
Sodium percarbonate, when dissolved in water, breaks down into hydrogen peroxide and sodium carbonate. This decomposition process is generally considered environmentally friendly, as it produces oxygen, water, and soda ash. The oxygen released can help in oxidizing organic matter, while soda ash can act as a pH buffer in aquatic environments. However, the release of sodium ions may contribute to increased salinity in water bodies, potentially affecting sensitive aquatic ecosystems.
Hydrogen peroxide, on the other hand, decomposes into water and oxygen, leaving no harmful residues. This characteristic makes it an attractive option for environmentally conscious applications. Its rapid breakdown in the environment reduces the risk of long-term ecological impacts. However, high concentrations of hydrogen peroxide can be harmful to aquatic life if released directly into water bodies without proper dilution or treatment.
In terms of production, sodium percarbonate manufacturing typically involves the reaction of sodium carbonate with hydrogen peroxide. This process may have a higher energy requirement and potentially greater carbon footprint compared to the direct production of hydrogen peroxide. The environmental impact of transportation and packaging should also be considered, as sodium percarbonate is often sold in solid form, while hydrogen peroxide is typically distributed as a liquid solution.
Both compounds have applications in wastewater treatment and environmental remediation. Sodium percarbonate's ability to release hydrogen peroxide in a controlled manner can be advantageous in certain scenarios, such as soil remediation or groundwater treatment. Hydrogen peroxide, with its strong oxidizing properties, is effective in breaking down organic pollutants and treating industrial effluents.
The choice between sodium percarbonate and hydrogen peroxide in various applications should consider not only their immediate environmental impact but also their lifecycle assessment. Factors such as raw material sourcing, energy consumption during production, transportation requirements, and end-of-life disposal all contribute to the overall environmental footprint of these compounds.
In conclusion, while both sodium percarbonate and hydrogen peroxide offer environmentally friendly alternatives to more harmful chemicals in many applications, their specific environmental impacts differ. The selection between the two should be based on the particular application, local environmental conditions, and a comprehensive assessment of their lifecycle environmental impacts.
Sodium percarbonate, when dissolved in water, breaks down into hydrogen peroxide and sodium carbonate. This decomposition process is generally considered environmentally friendly, as it produces oxygen, water, and soda ash. The oxygen released can help in oxidizing organic matter, while soda ash can act as a pH buffer in aquatic environments. However, the release of sodium ions may contribute to increased salinity in water bodies, potentially affecting sensitive aquatic ecosystems.
Hydrogen peroxide, on the other hand, decomposes into water and oxygen, leaving no harmful residues. This characteristic makes it an attractive option for environmentally conscious applications. Its rapid breakdown in the environment reduces the risk of long-term ecological impacts. However, high concentrations of hydrogen peroxide can be harmful to aquatic life if released directly into water bodies without proper dilution or treatment.
In terms of production, sodium percarbonate manufacturing typically involves the reaction of sodium carbonate with hydrogen peroxide. This process may have a higher energy requirement and potentially greater carbon footprint compared to the direct production of hydrogen peroxide. The environmental impact of transportation and packaging should also be considered, as sodium percarbonate is often sold in solid form, while hydrogen peroxide is typically distributed as a liquid solution.
Both compounds have applications in wastewater treatment and environmental remediation. Sodium percarbonate's ability to release hydrogen peroxide in a controlled manner can be advantageous in certain scenarios, such as soil remediation or groundwater treatment. Hydrogen peroxide, with its strong oxidizing properties, is effective in breaking down organic pollutants and treating industrial effluents.
The choice between sodium percarbonate and hydrogen peroxide in various applications should consider not only their immediate environmental impact but also their lifecycle assessment. Factors such as raw material sourcing, energy consumption during production, transportation requirements, and end-of-life disposal all contribute to the overall environmental footprint of these compounds.
In conclusion, while both sodium percarbonate and hydrogen peroxide offer environmentally friendly alternatives to more harmful chemicals in many applications, their specific environmental impacts differ. The selection between the two should be based on the particular application, local environmental conditions, and a comprehensive assessment of their lifecycle environmental impacts.
Safety Regulations
Safety regulations play a crucial role in the handling, storage, and use of both sodium percarbonate and hydrogen peroxide. These chemicals, while essential in various industries, pose potential risks that necessitate stringent safety measures.
For sodium percarbonate, regulations typically focus on its classification as an oxidizing solid. The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) categorizes it under Category 3 for oxidizing solids. This classification requires specific labeling, including hazard pictograms, signal words, and hazard statements. Storage regulations often mandate keeping sodium percarbonate in a cool, dry place away from combustible materials and sources of heat.
Hydrogen peroxide, being a more reactive substance, is subject to more stringent safety regulations. Its concentration levels significantly influence the applicable safety measures. For industrial-grade hydrogen peroxide (typically 35% or higher), regulations often require specialized storage tanks with venting systems to prevent pressure build-up. The U.S. Occupational Safety and Health Administration (OSHA) has established specific exposure limits for hydrogen peroxide in workplace environments.
Transportation of both chemicals is governed by international and national regulations. For instance, the International Air Transport Association (IATA) classifies hydrogen peroxide solutions above certain concentrations as dangerous goods, requiring special packaging and handling procedures. Similar regulations apply to sodium percarbonate, particularly for bulk transportation.
Personal protective equipment (PPE) requirements are another critical aspect of safety regulations for both chemicals. For hydrogen peroxide, especially at higher concentrations, impervious gloves, protective eyewear, and sometimes full-body protection are mandated. Sodium percarbonate handling typically requires less stringent PPE, but eye protection and gloves are still recommended.
Emergency response procedures are also regulated. Facilities storing or using these chemicals must have appropriate spill containment and neutralization protocols in place. For hydrogen peroxide, this often includes specialized absorbent materials and neutralizing agents. Sodium percarbonate spills generally require less intensive measures but still necessitate proper cleanup procedures.
Environmental regulations also apply to both chemicals. Discharge of high concentrations of either substance into water bodies is typically restricted due to their potential impact on aquatic life. Waste disposal regulations often classify spent solutions or contaminated materials as hazardous waste, requiring specialized disposal methods.
In conclusion, while both sodium percarbonate and hydrogen peroxide are subject to safety regulations, the more reactive nature of hydrogen peroxide generally results in more stringent controls. Companies working with these chemicals must stay abreast of evolving regulations and ensure compliance to maintain safe working environments and protect public health.
For sodium percarbonate, regulations typically focus on its classification as an oxidizing solid. The Globally Harmonized System of Classification and Labelling of Chemicals (GHS) categorizes it under Category 3 for oxidizing solids. This classification requires specific labeling, including hazard pictograms, signal words, and hazard statements. Storage regulations often mandate keeping sodium percarbonate in a cool, dry place away from combustible materials and sources of heat.
Hydrogen peroxide, being a more reactive substance, is subject to more stringent safety regulations. Its concentration levels significantly influence the applicable safety measures. For industrial-grade hydrogen peroxide (typically 35% or higher), regulations often require specialized storage tanks with venting systems to prevent pressure build-up. The U.S. Occupational Safety and Health Administration (OSHA) has established specific exposure limits for hydrogen peroxide in workplace environments.
Transportation of both chemicals is governed by international and national regulations. For instance, the International Air Transport Association (IATA) classifies hydrogen peroxide solutions above certain concentrations as dangerous goods, requiring special packaging and handling procedures. Similar regulations apply to sodium percarbonate, particularly for bulk transportation.
Personal protective equipment (PPE) requirements are another critical aspect of safety regulations for both chemicals. For hydrogen peroxide, especially at higher concentrations, impervious gloves, protective eyewear, and sometimes full-body protection are mandated. Sodium percarbonate handling typically requires less stringent PPE, but eye protection and gloves are still recommended.
Emergency response procedures are also regulated. Facilities storing or using these chemicals must have appropriate spill containment and neutralization protocols in place. For hydrogen peroxide, this often includes specialized absorbent materials and neutralizing agents. Sodium percarbonate spills generally require less intensive measures but still necessitate proper cleanup procedures.
Environmental regulations also apply to both chemicals. Discharge of high concentrations of either substance into water bodies is typically restricted due to their potential impact on aquatic life. Waste disposal regulations often classify spent solutions or contaminated materials as hazardous waste, requiring specialized disposal methods.
In conclusion, while both sodium percarbonate and hydrogen peroxide are subject to safety regulations, the more reactive nature of hydrogen peroxide generally results in more stringent controls. Companies working with these chemicals must stay abreast of evolving regulations and ensure compliance to maintain safe working environments and protect public health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!