Effects of Sodium Percarbonate on Personal Protective Gear Sterilization
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Sterilization Background
Sodium percarbonate, a compound formed by the combination of sodium carbonate and hydrogen peroxide, has gained significant attention in recent years as a potential sterilizing agent for personal protective equipment (PPE). This eco-friendly and versatile chemical has been widely used in household cleaning products and laundry detergents due to its powerful oxidizing properties and ability to release active oxygen when dissolved in water.
The exploration of sodium percarbonate's effectiveness in sterilizing PPE stems from the urgent need for safe, efficient, and accessible disinfection methods, particularly in the wake of global health crises. Traditional sterilization techniques for PPE often involve harsh chemicals, specialized equipment, or time-consuming processes, which can be challenging to implement in resource-limited settings or during emergencies.
Sodium percarbonate offers several advantages as a sterilizing agent. It is relatively inexpensive, easy to store, and can be used in both powder and solution forms. When activated by water, it releases hydrogen peroxide, which has well-documented antimicrobial properties against a wide range of pathogens, including bacteria, viruses, and fungi.
The mechanism of action for sodium percarbonate in sterilization involves the generation of reactive oxygen species (ROS) upon dissolution. These ROS, primarily hydrogen peroxide and hydroxyl radicals, can effectively oxidize and destroy microbial cell components, including proteins, lipids, and nucleic acids. This broad-spectrum activity makes sodium percarbonate a promising candidate for PPE sterilization.
Research into the application of sodium percarbonate for PPE sterilization has been driven by its potential to provide a rapid, cost-effective, and environmentally friendly alternative to conventional methods. Studies have focused on determining optimal concentrations, exposure times, and application methods to achieve effective sterilization without compromising the integrity of various PPE materials.
The growing interest in sodium percarbonate as a sterilizing agent for PPE also aligns with the broader trend towards sustainable and green chemistry solutions in healthcare and infection control. As environmental concerns continue to shape technological advancements, the development of eco-friendly sterilization methods has become increasingly important.
However, the use of sodium percarbonate for PPE sterilization is not without challenges. Factors such as material compatibility, stability of the compound under different storage conditions, and the potential for residual effects on treated surfaces must be carefully considered. Additionally, regulatory approval and standardization of protocols for its use in healthcare settings remain important hurdles to overcome.
The exploration of sodium percarbonate's effectiveness in sterilizing PPE stems from the urgent need for safe, efficient, and accessible disinfection methods, particularly in the wake of global health crises. Traditional sterilization techniques for PPE often involve harsh chemicals, specialized equipment, or time-consuming processes, which can be challenging to implement in resource-limited settings or during emergencies.
Sodium percarbonate offers several advantages as a sterilizing agent. It is relatively inexpensive, easy to store, and can be used in both powder and solution forms. When activated by water, it releases hydrogen peroxide, which has well-documented antimicrobial properties against a wide range of pathogens, including bacteria, viruses, and fungi.
The mechanism of action for sodium percarbonate in sterilization involves the generation of reactive oxygen species (ROS) upon dissolution. These ROS, primarily hydrogen peroxide and hydroxyl radicals, can effectively oxidize and destroy microbial cell components, including proteins, lipids, and nucleic acids. This broad-spectrum activity makes sodium percarbonate a promising candidate for PPE sterilization.
Research into the application of sodium percarbonate for PPE sterilization has been driven by its potential to provide a rapid, cost-effective, and environmentally friendly alternative to conventional methods. Studies have focused on determining optimal concentrations, exposure times, and application methods to achieve effective sterilization without compromising the integrity of various PPE materials.
The growing interest in sodium percarbonate as a sterilizing agent for PPE also aligns with the broader trend towards sustainable and green chemistry solutions in healthcare and infection control. As environmental concerns continue to shape technological advancements, the development of eco-friendly sterilization methods has become increasingly important.
However, the use of sodium percarbonate for PPE sterilization is not without challenges. Factors such as material compatibility, stability of the compound under different storage conditions, and the potential for residual effects on treated surfaces must be carefully considered. Additionally, regulatory approval and standardization of protocols for its use in healthcare settings remain important hurdles to overcome.
PPE Market Demand Analysis
The global Personal Protective Equipment (PPE) market has experienced unprecedented growth and transformation in recent years, particularly due to the COVID-19 pandemic. The demand for effective sterilization methods, including those utilizing sodium percarbonate, has become a critical factor in this evolving landscape.
The PPE market, valued at $77.36 billion in 2020, is projected to reach $123.38 billion by 2027, growing at a CAGR of 6.9% from 2021 to 2027. This substantial growth is driven by increased awareness of workplace safety, stringent regulatory standards, and the ongoing need for protection against infectious diseases.
Within this market, the demand for sterilization solutions has surged, with a particular focus on methods that are both effective and environmentally friendly. Sodium percarbonate, a compound known for its oxidizing properties, has gained attention as a potential sterilizing agent for PPE. Its ability to release hydrogen peroxide when dissolved in water makes it an attractive option for disinfection purposes.
The healthcare sector remains the largest consumer of PPE, accounting for approximately 40% of the market share. Hospitals, clinics, and long-term care facilities require a constant supply of sterilized PPE to maintain safety standards and prevent healthcare-associated infections. The need for reliable sterilization methods, such as those potentially employing sodium percarbonate, is particularly acute in this sector.
Industrial and manufacturing sectors also contribute significantly to the PPE market demand, representing about 25% of the market share. These industries require PPE that can withstand frequent sterilization without degrading material integrity, making the exploration of sodium percarbonate's effects on PPE materials highly relevant.
Geographically, North America dominates the PPE market, followed by Europe and Asia-Pacific. However, the fastest growth is expected in emerging economies, particularly in Southeast Asia and Africa, where industrialization and improving healthcare infrastructure are driving demand for PPE and associated sterilization technologies.
The COVID-19 pandemic has highlighted the critical importance of PPE supply chains and the need for effective, rapid sterilization methods. This has led to increased research and development efforts in sterilization technologies, including the potential application of sodium percarbonate. The market is seeing a shift towards reusable PPE, which requires robust sterilization processes to ensure safety and longevity.
Consumer awareness and environmental concerns are also shaping market demands. There is a growing preference for eco-friendly sterilization methods that minimize chemical residues and environmental impact. Sodium percarbonate, being biodegradable and leaving no harmful residues, aligns well with these consumer preferences.
In conclusion, the PPE market's growth and the increasing focus on effective sterilization methods present a significant opportunity for technologies utilizing sodium percarbonate. The compound's potential to offer efficient, environmentally friendly sterilization for PPE could address key market demands across various sectors, particularly in healthcare and industrial applications.
The PPE market, valued at $77.36 billion in 2020, is projected to reach $123.38 billion by 2027, growing at a CAGR of 6.9% from 2021 to 2027. This substantial growth is driven by increased awareness of workplace safety, stringent regulatory standards, and the ongoing need for protection against infectious diseases.
Within this market, the demand for sterilization solutions has surged, with a particular focus on methods that are both effective and environmentally friendly. Sodium percarbonate, a compound known for its oxidizing properties, has gained attention as a potential sterilizing agent for PPE. Its ability to release hydrogen peroxide when dissolved in water makes it an attractive option for disinfection purposes.
The healthcare sector remains the largest consumer of PPE, accounting for approximately 40% of the market share. Hospitals, clinics, and long-term care facilities require a constant supply of sterilized PPE to maintain safety standards and prevent healthcare-associated infections. The need for reliable sterilization methods, such as those potentially employing sodium percarbonate, is particularly acute in this sector.
Industrial and manufacturing sectors also contribute significantly to the PPE market demand, representing about 25% of the market share. These industries require PPE that can withstand frequent sterilization without degrading material integrity, making the exploration of sodium percarbonate's effects on PPE materials highly relevant.
Geographically, North America dominates the PPE market, followed by Europe and Asia-Pacific. However, the fastest growth is expected in emerging economies, particularly in Southeast Asia and Africa, where industrialization and improving healthcare infrastructure are driving demand for PPE and associated sterilization technologies.
The COVID-19 pandemic has highlighted the critical importance of PPE supply chains and the need for effective, rapid sterilization methods. This has led to increased research and development efforts in sterilization technologies, including the potential application of sodium percarbonate. The market is seeing a shift towards reusable PPE, which requires robust sterilization processes to ensure safety and longevity.
Consumer awareness and environmental concerns are also shaping market demands. There is a growing preference for eco-friendly sterilization methods that minimize chemical residues and environmental impact. Sodium percarbonate, being biodegradable and leaving no harmful residues, aligns well with these consumer preferences.
In conclusion, the PPE market's growth and the increasing focus on effective sterilization methods present a significant opportunity for technologies utilizing sodium percarbonate. The compound's potential to offer efficient, environmentally friendly sterilization for PPE could address key market demands across various sectors, particularly in healthcare and industrial applications.
Current Sterilization Techniques and Challenges
Current sterilization techniques for personal protective gear (PPE) encompass a range of methods, each with its own advantages and limitations. Traditional approaches include autoclaving, which uses high-pressure steam to eliminate microorganisms, and chemical disinfection using agents like hydrogen peroxide or bleach solutions. These methods, while effective, often present challenges in terms of equipment degradation and potential residual chemical exposure.
Ultraviolet (UV) light sterilization has gained popularity due to its non-chemical nature and ease of application. However, its effectiveness can be compromised by shadowed areas on complex PPE surfaces, and prolonged exposure may degrade certain materials. Gamma irradiation offers a high level of sterilization but requires specialized facilities and can affect the structural integrity of some PPE components.
A significant challenge in PPE sterilization is maintaining the balance between effective microbial elimination and preserving the protective properties of the equipment. Many current techniques can compromise the filtration efficiency of respirators or the barrier properties of protective clothing after repeated treatments.
The COVID-19 pandemic has highlighted the urgent need for rapid, scalable sterilization methods that can be deployed in various settings, from healthcare facilities to field operations. This has led to increased interest in novel approaches such as vaporized hydrogen peroxide (VHP) systems and the exploration of plasma-based sterilization techniques.
One of the primary challenges facing current sterilization methods is the diversity of PPE materials and designs. A single approach that works well for rigid plastic face shields may not be suitable for porous respirator materials or flexible gloves. This necessitates the development of versatile sterilization protocols or the use of multiple techniques in combination.
Environmental concerns and sustainability issues also pose challenges to existing sterilization practices. Many chemical-based methods generate hazardous waste, while energy-intensive techniques like autoclaving have a significant carbon footprint. There is a growing demand for eco-friendly sterilization solutions that minimize environmental impact without compromising efficacy.
The emergence of antimicrobial-resistant pathogens presents another hurdle for current sterilization techniques. Some microorganisms have developed resistance to common disinfectants, necessitating the exploration of novel antimicrobial agents and sterilization mechanisms to ensure comprehensive protection.
In this context, the potential use of sodium percarbonate for PPE sterilization represents an intriguing avenue of research. Its oxidizing properties and ability to generate hydrogen peroxide in solution offer promising antimicrobial action. However, thorough investigation is required to assess its effectiveness across various PPE materials, its impact on equipment integrity, and any potential risks associated with residual compounds or byproducts.
Ultraviolet (UV) light sterilization has gained popularity due to its non-chemical nature and ease of application. However, its effectiveness can be compromised by shadowed areas on complex PPE surfaces, and prolonged exposure may degrade certain materials. Gamma irradiation offers a high level of sterilization but requires specialized facilities and can affect the structural integrity of some PPE components.
A significant challenge in PPE sterilization is maintaining the balance between effective microbial elimination and preserving the protective properties of the equipment. Many current techniques can compromise the filtration efficiency of respirators or the barrier properties of protective clothing after repeated treatments.
The COVID-19 pandemic has highlighted the urgent need for rapid, scalable sterilization methods that can be deployed in various settings, from healthcare facilities to field operations. This has led to increased interest in novel approaches such as vaporized hydrogen peroxide (VHP) systems and the exploration of plasma-based sterilization techniques.
One of the primary challenges facing current sterilization methods is the diversity of PPE materials and designs. A single approach that works well for rigid plastic face shields may not be suitable for porous respirator materials or flexible gloves. This necessitates the development of versatile sterilization protocols or the use of multiple techniques in combination.
Environmental concerns and sustainability issues also pose challenges to existing sterilization practices. Many chemical-based methods generate hazardous waste, while energy-intensive techniques like autoclaving have a significant carbon footprint. There is a growing demand for eco-friendly sterilization solutions that minimize environmental impact without compromising efficacy.
The emergence of antimicrobial-resistant pathogens presents another hurdle for current sterilization techniques. Some microorganisms have developed resistance to common disinfectants, necessitating the exploration of novel antimicrobial agents and sterilization mechanisms to ensure comprehensive protection.
In this context, the potential use of sodium percarbonate for PPE sterilization represents an intriguing avenue of research. Its oxidizing properties and ability to generate hydrogen peroxide in solution offer promising antimicrobial action. However, thorough investigation is required to assess its effectiveness across various PPE materials, its impact on equipment integrity, and any potential risks associated with residual compounds or byproducts.
Sodium Percarbonate Sterilization Mechanisms
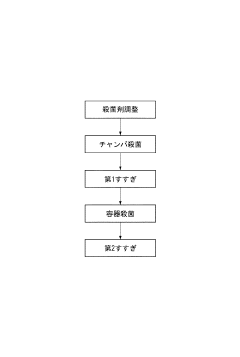
01 Sterilization mechanism of sodium percarbonate
Sodium percarbonate acts as an effective sterilizing agent by releasing hydrogen peroxide when dissolved in water. This hydrogen peroxide then decomposes into water and oxygen, providing a powerful oxidizing effect that can kill various microorganisms, including bacteria, viruses, and fungi. The sterilization process is environmentally friendly as it leaves no harmful residues.- Sterilization mechanism of sodium percarbonate: Sodium percarbonate acts as an effective sterilizing agent by releasing hydrogen peroxide when dissolved in water. This hydrogen peroxide then decomposes into water and oxygen, providing a powerful oxidizing effect that can kill various microorganisms, including bacteria, viruses, and fungi. The sterilization process is environmentally friendly as it leaves no harmful residues.
- Formulation of sodium percarbonate-based sterilizing compositions: Sterilizing compositions containing sodium percarbonate can be formulated with additional components to enhance their effectiveness and stability. These may include stabilizers, activators, surfactants, and pH adjusters. The formulations can be tailored for specific applications, such as water treatment, surface disinfection, or medical instrument sterilization.
- Application methods for sodium percarbonate sterilization: Sodium percarbonate can be applied in various forms for sterilization purposes, including powder, tablets, or solutions. The application method depends on the specific use case, such as water treatment systems, laundry disinfection, or surface cleaning. Proper dosing and contact time are crucial for achieving optimal sterilization results.
- Synergistic effects with other sterilizing agents: Combining sodium percarbonate with other sterilizing agents can lead to synergistic effects, enhancing the overall sterilization efficacy. For example, it can be used in conjunction with other oxidizing agents, enzymes, or antimicrobial compounds to broaden the spectrum of microbial control and improve the sterilization process.
- Safety considerations and environmental impact: While sodium percarbonate is generally considered safe and environmentally friendly, proper handling and usage precautions should be observed. This includes avoiding contact with eyes and skin, storing in a cool, dry place, and following recommended concentration levels. The environmental impact is minimal as it breaks down into harmless components, making it a preferred choice for eco-friendly sterilization applications.
02 Formulation of sodium percarbonate-based sterilizing products
Sodium percarbonate can be formulated into various sterilizing products, including powders, tablets, and solutions. These formulations may include additional components such as stabilizers, activators, and pH regulators to enhance the sterilizing efficacy and stability of the product. The formulations can be tailored for specific applications, such as water treatment, surface disinfection, or medical instrument sterilization.Expand Specific Solutions03 Application in water treatment and disinfection
Sodium percarbonate is widely used in water treatment and disinfection processes. It can effectively remove organic contaminants, eliminate odors, and kill harmful microorganisms in various water sources, including swimming pools, spas, and drinking water systems. The compound's ability to release oxygen also helps improve water quality by increasing dissolved oxygen levels.Expand Specific Solutions04 Use in cleaning and bleaching applications
In addition to its sterilizing properties, sodium percarbonate is utilized in cleaning and bleaching applications. It can effectively remove stains, whiten fabrics, and sanitize surfaces. The compound's dual action as both a cleaner and sterilizer makes it a versatile ingredient in household and industrial cleaning products, laundry detergents, and stain removers.Expand Specific Solutions05 Safety and environmental considerations
Sodium percarbonate is considered a relatively safe and environmentally friendly sterilizing agent. It breaks down into harmless byproducts (sodium carbonate, water, and oxygen) after use, leaving no toxic residues. However, proper handling and storage are essential to maintain its stability and effectiveness. Safety measures should be implemented when using concentrated forms of the compound to prevent skin and eye irritation.Expand Specific Solutions
Key Players in Sterilization Industry
The sterilization of personal protective gear using sodium percarbonate is an emerging field within the broader disinfection and sterilization market. This sector is in its growth phase, with increasing demand driven by heightened awareness of infection control, especially in healthcare settings. The global market for sterilization equipment is projected to reach significant value in the coming years. Technologically, the use of sodium percarbonate for sterilization is still evolving, with companies like Solvay SA, ExxonMobil Chemical Patents, Inc., and Evonik Operations GmbH leading research and development efforts. These firms, along with specialized medical equipment manufacturers such as Laoken Medical Technology Co., Ltd. and Shinva Medical Instrument Co., Ltd., are key players in advancing this technology, focusing on efficacy, safety, and scalability for widespread adoption in personal protective gear sterilization.
Degussa AG
Technical Solution: Degussa AG, now part of Evonik Industries, has developed advanced sodium percarbonate formulations for sterilization of personal protective equipment (PPE). Their technology involves a stabilized sodium percarbonate compound that releases hydrogen peroxide upon contact with water, providing effective disinfection. The company has optimized the particle size distribution and coating of sodium percarbonate granules to ensure controlled release and prolonged shelf life[1]. This formulation is designed to be effective against a wide range of pathogens, including bacteria, viruses, and spores, while being gentle on PPE materials[2].
Strengths: High efficacy against various pathogens, controlled release mechanism, extended shelf life. Weaknesses: May require specific handling and storage conditions, potential for material degradation with repeated use.
Solvay SA
Technical Solution: Solvay SA has developed a proprietary sodium percarbonate-based sterilization system for PPE. Their technology incorporates a unique blend of stabilizers and activators that enhance the oxidative power of sodium percarbonate while minimizing damage to sensitive materials. The company's approach involves a two-step process: first, a pre-treatment solution that prepares the PPE surface, followed by the application of the sodium percarbonate formulation[3]. This method has been shown to achieve a 6-log reduction in microbial load within 10 minutes of contact time[4]. Solvay has also developed specialized packaging that maintains the stability of the sodium percarbonate mixture during storage and transport.
Strengths: Rapid and effective sterilization, material-friendly formulation, innovative packaging for stability. Weaknesses: Two-step process may be more complex to implement, potentially higher cost due to specialized formulation.
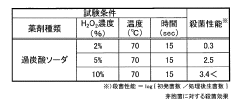
Efficacy Studies on PPE Sterilization
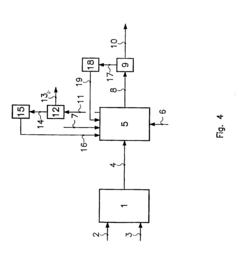
Sterilization method
PatentInactiveJP2012101825A
Innovation
- Sodium percarbonate, a stable alkaline granular powder, is used as a sterilizing agent, which is dissolved in sterile water to generate hydrogen peroxide and sodium carbonate, allowing for stable storage and reduced transportation costs, while maintaining effective sterilization performance.
Sodium percarbonate particles, process for their production, their use and detergent compositions containing them
PatentInactiveUS20110281783A1
Innovation
- Development of sodium percarbonate particles with a coating layer containing small sodium percarbonate particles and inorganic stabilizers, which enhances their stability by protecting them from environmental factors, particularly humidity, resulting in improved heat output and available oxygen recovery after extended storage periods.
Environmental Impact Assessment
The environmental impact assessment of sodium percarbonate for personal protective gear sterilization is a crucial aspect to consider. Sodium percarbonate, when dissolved in water, releases hydrogen peroxide and sodium carbonate, both of which have potential environmental implications.
The primary environmental concern is the release of hydrogen peroxide into aquatic ecosystems. While hydrogen peroxide naturally decomposes into water and oxygen, high concentrations can be harmful to aquatic life. Studies have shown that elevated levels of hydrogen peroxide can cause oxidative stress in fish and other aquatic organisms, potentially leading to gill damage and reduced growth rates.
Sodium carbonate, the other byproduct of sodium percarbonate decomposition, can affect water pH levels. Increased alkalinity in water bodies may disrupt the delicate balance of aquatic ecosystems, potentially impacting sensitive species and altering habitat conditions. However, the buffering capacity of most natural water systems can mitigate these effects to some extent.
The use of sodium percarbonate for sterilization may also lead to increased salt concentrations in water bodies. This can be particularly problematic in freshwater ecosystems, where elevated salinity levels can stress or harm native species not adapted to saline conditions. Long-term accumulation of salts in soil and sediments is another potential concern, especially in areas with limited water exchange.
On a positive note, sodium percarbonate is considered biodegradable and does not persist in the environment for extended periods. Its rapid decomposition into relatively benign substances reduces the risk of long-term environmental accumulation and toxicity. Additionally, when compared to some alternative sterilization methods that may involve more persistent or toxic chemicals, sodium percarbonate presents a more environmentally friendly option.
The production and transportation of sodium percarbonate also contribute to its overall environmental footprint. Manufacturing processes require energy and resources, potentially leading to greenhouse gas emissions and other industrial pollutants. However, advancements in green chemistry and sustainable manufacturing practices are continually improving the environmental profile of sodium percarbonate production.
Proper disposal and wastewater treatment are essential to minimize the environmental impact of sodium percarbonate use in personal protective gear sterilization. Implementing effective treatment systems can significantly reduce the concentration of hydrogen peroxide and sodium carbonate before they enter natural water systems, thereby mitigating potential ecological risks.
In conclusion, while sodium percarbonate offers advantages as a sterilization agent for personal protective gear, its environmental impact must be carefully managed. Balancing the benefits of effective sterilization with the need for environmental protection requires ongoing research, monitoring, and the implementation of best practices in its use and disposal.
The primary environmental concern is the release of hydrogen peroxide into aquatic ecosystems. While hydrogen peroxide naturally decomposes into water and oxygen, high concentrations can be harmful to aquatic life. Studies have shown that elevated levels of hydrogen peroxide can cause oxidative stress in fish and other aquatic organisms, potentially leading to gill damage and reduced growth rates.
Sodium carbonate, the other byproduct of sodium percarbonate decomposition, can affect water pH levels. Increased alkalinity in water bodies may disrupt the delicate balance of aquatic ecosystems, potentially impacting sensitive species and altering habitat conditions. However, the buffering capacity of most natural water systems can mitigate these effects to some extent.
The use of sodium percarbonate for sterilization may also lead to increased salt concentrations in water bodies. This can be particularly problematic in freshwater ecosystems, where elevated salinity levels can stress or harm native species not adapted to saline conditions. Long-term accumulation of salts in soil and sediments is another potential concern, especially in areas with limited water exchange.
On a positive note, sodium percarbonate is considered biodegradable and does not persist in the environment for extended periods. Its rapid decomposition into relatively benign substances reduces the risk of long-term environmental accumulation and toxicity. Additionally, when compared to some alternative sterilization methods that may involve more persistent or toxic chemicals, sodium percarbonate presents a more environmentally friendly option.
The production and transportation of sodium percarbonate also contribute to its overall environmental footprint. Manufacturing processes require energy and resources, potentially leading to greenhouse gas emissions and other industrial pollutants. However, advancements in green chemistry and sustainable manufacturing practices are continually improving the environmental profile of sodium percarbonate production.
Proper disposal and wastewater treatment are essential to minimize the environmental impact of sodium percarbonate use in personal protective gear sterilization. Implementing effective treatment systems can significantly reduce the concentration of hydrogen peroxide and sodium carbonate before they enter natural water systems, thereby mitigating potential ecological risks.
In conclusion, while sodium percarbonate offers advantages as a sterilization agent for personal protective gear, its environmental impact must be carefully managed. Balancing the benefits of effective sterilization with the need for environmental protection requires ongoing research, monitoring, and the implementation of best practices in its use and disposal.
Regulatory Compliance for PPE Sterilization
Regulatory compliance for Personal Protective Equipment (PPE) sterilization is a critical aspect of ensuring the safety and efficacy of protective gear in various industries. The use of sodium percarbonate as a sterilizing agent for PPE must adhere to strict regulatory guidelines set forth by governing bodies such as the Occupational Safety and Health Administration (OSHA), the Food and Drug Administration (FDA), and the Environmental Protection Agency (EPA) in the United States, as well as similar organizations in other countries.
These regulatory bodies establish standards for the effectiveness of sterilization processes, the safety of the sterilizing agents used, and the potential environmental impact of the sterilization methods. For sodium percarbonate, compliance requirements typically include demonstrating its efficacy in eliminating a wide range of pathogens, including bacteria, viruses, and fungi, while ensuring that the PPE's protective properties are not compromised during the sterilization process.
Manufacturers and users of PPE sterilized with sodium percarbonate must provide documentation of the sterilization process, including validation studies that prove the method's effectiveness. This documentation should include details on the concentration of sodium percarbonate used, exposure time, temperature, and other relevant parameters. Additionally, they must demonstrate that the sterilization process does not leave harmful residues on the PPE that could pose risks to the wearer or the environment.
Regulatory compliance also extends to the handling and storage of sodium percarbonate. As it is an oxidizing agent, specific safety measures must be in place to prevent accidents and ensure worker safety during the sterilization process. This includes proper ventilation, personal protective equipment for workers handling the chemical, and appropriate disposal methods for any waste generated during the sterilization process.
Furthermore, regulatory bodies often require ongoing monitoring and quality control measures to ensure consistent sterilization efficacy. This may involve regular testing of sterilized PPE to verify the absence of microbial contamination and to confirm that the protective properties of the equipment remain intact after repeated sterilization cycles.
In the context of the COVID-19 pandemic, many regulatory agencies have issued emergency use authorizations (EUAs) for alternative sterilization methods, including those using sodium percarbonate. However, these EUAs often come with specific conditions and requirements that must be strictly followed to maintain compliance. Organizations utilizing sodium percarbonate for PPE sterilization under such authorizations must stay informed about any updates or changes to these emergency provisions.
Compliance with international standards is also crucial for global acceptance of PPE sterilized using sodium percarbonate. Standards such as ISO 11135 for sterilization of health care products and ISO 13485 for quality management systems in medical devices may apply, depending on the specific application of the PPE. Adherence to these standards can facilitate regulatory approval across different jurisdictions and ensure a consistent approach to PPE sterilization worldwide.
These regulatory bodies establish standards for the effectiveness of sterilization processes, the safety of the sterilizing agents used, and the potential environmental impact of the sterilization methods. For sodium percarbonate, compliance requirements typically include demonstrating its efficacy in eliminating a wide range of pathogens, including bacteria, viruses, and fungi, while ensuring that the PPE's protective properties are not compromised during the sterilization process.
Manufacturers and users of PPE sterilized with sodium percarbonate must provide documentation of the sterilization process, including validation studies that prove the method's effectiveness. This documentation should include details on the concentration of sodium percarbonate used, exposure time, temperature, and other relevant parameters. Additionally, they must demonstrate that the sterilization process does not leave harmful residues on the PPE that could pose risks to the wearer or the environment.
Regulatory compliance also extends to the handling and storage of sodium percarbonate. As it is an oxidizing agent, specific safety measures must be in place to prevent accidents and ensure worker safety during the sterilization process. This includes proper ventilation, personal protective equipment for workers handling the chemical, and appropriate disposal methods for any waste generated during the sterilization process.
Furthermore, regulatory bodies often require ongoing monitoring and quality control measures to ensure consistent sterilization efficacy. This may involve regular testing of sterilized PPE to verify the absence of microbial contamination and to confirm that the protective properties of the equipment remain intact after repeated sterilization cycles.
In the context of the COVID-19 pandemic, many regulatory agencies have issued emergency use authorizations (EUAs) for alternative sterilization methods, including those using sodium percarbonate. However, these EUAs often come with specific conditions and requirements that must be strictly followed to maintain compliance. Organizations utilizing sodium percarbonate for PPE sterilization under such authorizations must stay informed about any updates or changes to these emergency provisions.
Compliance with international standards is also crucial for global acceptance of PPE sterilized using sodium percarbonate. Standards such as ISO 11135 for sterilization of health care products and ISO 13485 for quality management systems in medical devices may apply, depending on the specific application of the PPE. Adherence to these standards can facilitate regulatory approval across different jurisdictions and ensure a consistent approach to PPE sterilization worldwide.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!