Comparison of Sodium Percarbonate and Sodium Carbonate in Stain Removal
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Stain Removal Evolution
The evolution of stain removal techniques has been a journey marked by significant advancements in chemistry and technology. In the early 20th century, basic soaps and manual scrubbing were the primary methods for removing stains. The 1930s saw the introduction of synthetic detergents, which revolutionized the cleaning industry by offering improved cleaning power and versatility.
The 1950s and 1960s brought about the development of enzyme-based detergents, which could break down protein-based stains more effectively. This period also saw the rise of bleach as a powerful stain remover, particularly for white fabrics. However, the harsh nature of bleach led to the search for gentler alternatives.
In the 1970s and 1980s, the focus shifted towards developing more environmentally friendly cleaning agents. This era saw the introduction of phosphate-free detergents and the increased use of oxygen-based bleaches, such as sodium percarbonate. These innovations addressed growing concerns about water pollution and the impact of cleaning products on aquatic ecosystems.
The 1990s and early 2000s witnessed a surge in the development of specialized stain removal products. Companies began producing targeted solutions for specific types of stains, such as grass, wine, or grease. This period also saw the rise of pre-treatment products designed to be applied directly to stains before washing.
In recent years, the stain removal industry has been influenced by the growing demand for eco-friendly and natural cleaning solutions. This has led to the development of plant-based enzymes and biodegradable surfactants. Additionally, there has been increased research into the use of nanotechnology in stain removal, with the potential to create more effective and efficient cleaning agents at the molecular level.
The comparison between sodium percarbonate and sodium carbonate in stain removal represents a significant milestone in this evolution. Sodium percarbonate, which releases hydrogen peroxide when dissolved in water, offers superior stain-removing capabilities compared to traditional sodium carbonate. This shift towards more effective oxygen-based bleaches has been driven by the need for powerful yet environmentally friendly cleaning solutions.
As we look to the future, the stain removal industry continues to evolve. Current research focuses on developing smart fabrics that resist staining, as well as advanced cleaning technologies that use less water and energy. The ongoing challenge is to balance effective stain removal with environmental sustainability and fabric care, driving innovation in this ever-evolving field.
The 1950s and 1960s brought about the development of enzyme-based detergents, which could break down protein-based stains more effectively. This period also saw the rise of bleach as a powerful stain remover, particularly for white fabrics. However, the harsh nature of bleach led to the search for gentler alternatives.
In the 1970s and 1980s, the focus shifted towards developing more environmentally friendly cleaning agents. This era saw the introduction of phosphate-free detergents and the increased use of oxygen-based bleaches, such as sodium percarbonate. These innovations addressed growing concerns about water pollution and the impact of cleaning products on aquatic ecosystems.
The 1990s and early 2000s witnessed a surge in the development of specialized stain removal products. Companies began producing targeted solutions for specific types of stains, such as grass, wine, or grease. This period also saw the rise of pre-treatment products designed to be applied directly to stains before washing.
In recent years, the stain removal industry has been influenced by the growing demand for eco-friendly and natural cleaning solutions. This has led to the development of plant-based enzymes and biodegradable surfactants. Additionally, there has been increased research into the use of nanotechnology in stain removal, with the potential to create more effective and efficient cleaning agents at the molecular level.
The comparison between sodium percarbonate and sodium carbonate in stain removal represents a significant milestone in this evolution. Sodium percarbonate, which releases hydrogen peroxide when dissolved in water, offers superior stain-removing capabilities compared to traditional sodium carbonate. This shift towards more effective oxygen-based bleaches has been driven by the need for powerful yet environmentally friendly cleaning solutions.
As we look to the future, the stain removal industry continues to evolve. Current research focuses on developing smart fabrics that resist staining, as well as advanced cleaning technologies that use less water and energy. The ongoing challenge is to balance effective stain removal with environmental sustainability and fabric care, driving innovation in this ever-evolving field.
Market Demand Analysis
The market demand for effective stain removal solutions continues to grow, driven by consumer desire for cleaner and more hygienic living environments. Both sodium percarbonate and sodium carbonate have established positions in this market, with distinct advantages and applications.
Sodium percarbonate, often marketed as "oxygen bleach," has seen increasing demand due to its eco-friendly nature and effectiveness against a wide range of stains. Its ability to release hydrogen peroxide when dissolved in water makes it particularly effective against organic stains such as food, wine, and grass. The global sodium percarbonate market is experiencing steady growth, with projections indicating a compound annual growth rate (CAGR) of around 3-4% over the next five years.
On the other hand, sodium carbonate, commonly known as washing soda, maintains a strong presence in the market due to its versatility and cost-effectiveness. It is widely used in laundry detergents and as a standalone cleaning agent. The sodium carbonate market is mature but stable, with a projected CAGR of 1-2% in the coming years.
Consumer trends are shifting towards more environmentally friendly and safer cleaning products, which has boosted the demand for sodium percarbonate. Its oxygen-based bleaching action is perceived as gentler on fabrics and safer for the environment compared to chlorine-based bleaches. This aligns with the growing consumer preference for sustainable and non-toxic household products.
However, sodium carbonate continues to hold a significant market share due to its lower cost and multi-purpose applications. It is particularly favored in regions where price sensitivity is a key factor in consumer purchasing decisions. The industrial cleaning sector also contributes significantly to the demand for sodium carbonate.
The COVID-19 pandemic has further intensified the focus on cleanliness and hygiene, leading to increased demand for both products. Consumers are now more conscious about the effectiveness of cleaning agents, particularly in removing stubborn stains and disinfecting surfaces.
In terms of regional demand, developed markets such as North America and Europe show a stronger preference for sodium percarbonate due to stricter environmental regulations and higher consumer awareness. Emerging markets in Asia-Pacific and Latin America present growth opportunities for both products, with sodium carbonate currently holding a larger market share due to its lower cost.
The future market dynamics will likely be influenced by factors such as technological advancements in stain removal formulations, evolving consumer preferences, and regulatory changes regarding chemical usage in household products. Manufacturers are expected to focus on developing more efficient and environmentally friendly stain removal solutions, potentially combining the strengths of both sodium percarbonate and sodium carbonate to meet diverse consumer needs.
Sodium percarbonate, often marketed as "oxygen bleach," has seen increasing demand due to its eco-friendly nature and effectiveness against a wide range of stains. Its ability to release hydrogen peroxide when dissolved in water makes it particularly effective against organic stains such as food, wine, and grass. The global sodium percarbonate market is experiencing steady growth, with projections indicating a compound annual growth rate (CAGR) of around 3-4% over the next five years.
On the other hand, sodium carbonate, commonly known as washing soda, maintains a strong presence in the market due to its versatility and cost-effectiveness. It is widely used in laundry detergents and as a standalone cleaning agent. The sodium carbonate market is mature but stable, with a projected CAGR of 1-2% in the coming years.
Consumer trends are shifting towards more environmentally friendly and safer cleaning products, which has boosted the demand for sodium percarbonate. Its oxygen-based bleaching action is perceived as gentler on fabrics and safer for the environment compared to chlorine-based bleaches. This aligns with the growing consumer preference for sustainable and non-toxic household products.
However, sodium carbonate continues to hold a significant market share due to its lower cost and multi-purpose applications. It is particularly favored in regions where price sensitivity is a key factor in consumer purchasing decisions. The industrial cleaning sector also contributes significantly to the demand for sodium carbonate.
The COVID-19 pandemic has further intensified the focus on cleanliness and hygiene, leading to increased demand for both products. Consumers are now more conscious about the effectiveness of cleaning agents, particularly in removing stubborn stains and disinfecting surfaces.
In terms of regional demand, developed markets such as North America and Europe show a stronger preference for sodium percarbonate due to stricter environmental regulations and higher consumer awareness. Emerging markets in Asia-Pacific and Latin America present growth opportunities for both products, with sodium carbonate currently holding a larger market share due to its lower cost.
The future market dynamics will likely be influenced by factors such as technological advancements in stain removal formulations, evolving consumer preferences, and regulatory changes regarding chemical usage in household products. Manufacturers are expected to focus on developing more efficient and environmentally friendly stain removal solutions, potentially combining the strengths of both sodium percarbonate and sodium carbonate to meet diverse consumer needs.
Current Challenges
The current challenges in comparing sodium percarbonate and sodium carbonate for stain removal are multifaceted and involve both technical and practical aspects. One of the primary challenges is the lack of standardized testing methods for evaluating the efficacy of these compounds across a wide range of stain types and fabric materials. This absence of uniform protocols makes it difficult to draw accurate comparisons and can lead to inconsistent results across different studies.
Another significant challenge lies in the complex chemistry of stain removal. Both sodium percarbonate and sodium carbonate interact differently with various types of stains, such as protein-based, tannin-based, or oil-based stains. Understanding and quantifying these interactions under diverse conditions, including water temperature, pH levels, and exposure time, presents a considerable technical hurdle. This complexity is further compounded by the varying compositions of fabric materials, which can significantly influence the stain removal process.
Environmental concerns also pose a challenge in this comparison. While both compounds are generally considered environmentally friendly, there is a growing need to assess their long-term ecological impact, particularly in terms of water treatment and biodegradability. The challenge lies in developing comprehensive life cycle assessments that account for production, use, and disposal of these compounds.
The stability and shelf life of sodium percarbonate present another challenge. As an unstable compound that readily decomposes into sodium carbonate and hydrogen peroxide, maintaining its efficacy over time, especially in liquid detergent formulations, is problematic. This instability can lead to inconsistent performance and makes it difficult to conduct long-term comparative studies with sodium carbonate.
Cost-effectiveness and scalability also present challenges in the comparison. While sodium percarbonate may show superior stain removal properties in some cases, its higher production costs compared to sodium carbonate can be a limiting factor for widespread adoption. Balancing performance with economic viability remains a key challenge for manufacturers and researchers alike.
Lastly, there is a challenge in addressing consumer perceptions and preferences. Many consumers have established habits and beliefs regarding laundry practices, which can influence their acceptance of new stain removal technologies. Overcoming these preconceptions and effectively communicating the benefits of either compound requires careful consideration of marketing strategies and consumer education.
Another significant challenge lies in the complex chemistry of stain removal. Both sodium percarbonate and sodium carbonate interact differently with various types of stains, such as protein-based, tannin-based, or oil-based stains. Understanding and quantifying these interactions under diverse conditions, including water temperature, pH levels, and exposure time, presents a considerable technical hurdle. This complexity is further compounded by the varying compositions of fabric materials, which can significantly influence the stain removal process.
Environmental concerns also pose a challenge in this comparison. While both compounds are generally considered environmentally friendly, there is a growing need to assess their long-term ecological impact, particularly in terms of water treatment and biodegradability. The challenge lies in developing comprehensive life cycle assessments that account for production, use, and disposal of these compounds.
The stability and shelf life of sodium percarbonate present another challenge. As an unstable compound that readily decomposes into sodium carbonate and hydrogen peroxide, maintaining its efficacy over time, especially in liquid detergent formulations, is problematic. This instability can lead to inconsistent performance and makes it difficult to conduct long-term comparative studies with sodium carbonate.
Cost-effectiveness and scalability also present challenges in the comparison. While sodium percarbonate may show superior stain removal properties in some cases, its higher production costs compared to sodium carbonate can be a limiting factor for widespread adoption. Balancing performance with economic viability remains a key challenge for manufacturers and researchers alike.
Lastly, there is a challenge in addressing consumer perceptions and preferences. Many consumers have established habits and beliefs regarding laundry practices, which can influence their acceptance of new stain removal technologies. Overcoming these preconceptions and effectively communicating the benefits of either compound requires careful consideration of marketing strategies and consumer education.
Existing Solutions
01 Combination of sodium percarbonate and sodium carbonate for stain removal
The combination of sodium percarbonate and sodium carbonate is effective for stain removal. Sodium percarbonate acts as a bleaching agent, releasing oxygen when dissolved in water, while sodium carbonate helps to maintain an alkaline pH, enhancing the cleaning power. This combination is particularly useful in laundry detergents and cleaning products for removing tough stains.- Combination of sodium percarbonate and sodium carbonate for stain removal: The combination of sodium percarbonate and sodium carbonate is effective for stain removal. Sodium percarbonate acts as a bleaching agent, releasing oxygen when dissolved in water, while sodium carbonate helps to maintain an alkaline pH, enhancing the cleaning power. This combination is particularly useful in laundry detergents and cleaning products for removing tough stains.
- Stabilization of sodium percarbonate: Stabilizing sodium percarbonate is crucial for maintaining its effectiveness in stain removal applications. Various methods and additives can be used to improve the stability of sodium percarbonate, including coating techniques and the addition of stabilizing agents. This enhances the shelf life and performance of cleaning products containing sodium percarbonate.
- Formulation of cleaning compositions with sodium percarbonate and sodium carbonate: Cleaning compositions containing sodium percarbonate and sodium carbonate can be formulated with additional ingredients to enhance their stain removal properties. These may include surfactants, enzymes, and other cleaning agents. The formulation process involves optimizing the ratios of ingredients to achieve maximum cleaning efficiency while maintaining product stability.
- Application methods for stain removal using sodium percarbonate and sodium carbonate: Various application methods can be employed for effective stain removal using sodium percarbonate and sodium carbonate. These may include pre-treating stains, soaking fabrics, or incorporating the compounds into washing machine cycles. The choice of application method depends on the type of stain and the material being treated.
- Environmental and safety considerations in stain removal formulations: When developing stain removal products using sodium percarbonate and sodium carbonate, environmental and safety aspects must be considered. This includes ensuring biodegradability, minimizing environmental impact, and addressing potential skin irritation concerns. Formulations may incorporate additional ingredients to improve safety and eco-friendliness while maintaining effective stain removal properties.
02 Stabilization of sodium percarbonate
Stabilizing sodium percarbonate is crucial for maintaining its effectiveness in stain removal applications. Various methods are employed to improve the stability of sodium percarbonate, including coating with inorganic materials, adding stabilizing agents, or controlling moisture content. These techniques help to prevent premature decomposition and ensure prolonged shelf life of cleaning products containing sodium percarbonate.Expand Specific Solutions03 Formulation of cleaning compositions with sodium percarbonate and sodium carbonate
Cleaning compositions containing sodium percarbonate and sodium carbonate are formulated to optimize stain removal performance. These formulations may include additional components such as surfactants, enzymes, or other cleaning agents to enhance the overall cleaning efficacy. The ratio of sodium percarbonate to sodium carbonate and other ingredients is carefully balanced to achieve optimal stain removal results.Expand Specific Solutions04 Application methods for sodium percarbonate and sodium carbonate in stain removal
Various application methods are used to maximize the stain removal effectiveness of sodium percarbonate and sodium carbonate. These may include pre-treating stains, soaking fabrics, or incorporating the compounds into washing machine cycles. The application method can be tailored to specific types of stains or fabric materials to achieve the best cleaning results.Expand Specific Solutions05 Environmental and safety considerations in using sodium percarbonate and sodium carbonate for stain removal
The use of sodium percarbonate and sodium carbonate for stain removal offers environmental benefits due to their biodegradability and low toxicity. However, safety precautions must be taken when handling these compounds, as they can be irritating to skin and eyes. Proper storage and handling procedures are essential to ensure safe use in household and industrial cleaning applications.Expand Specific Solutions
Key Industry Players
The competition landscape for "Comparison of Sodium Percarbonate and Sodium Carbonate in Stain Removal" is in a mature stage, with established players and ongoing research. The market size is significant, driven by the widespread use of these compounds in cleaning products. Technologically, the field is well-developed but still evolving. Companies like Solvay SA, Evonik Operations GmbH, and Henkel AG & Co. KGaA are leading players, with strong research capabilities and product portfolios. Emerging companies such as Zhejiang Jinke Daily Chemical Co. Ltd. and Hunan Jieyu New Technology Co., Ltd. are also contributing to innovation in this space, particularly in the Asian market.
Solvay SA
Technical Solution: Solvay SA has developed advanced sodium percarbonate (SPC) technology for stain removal applications. Their SPC formulation features enhanced stability and controlled oxygen release, optimizing its effectiveness in laundry detergents. Solvay's process involves coating SPC particles with sodium silicate and sodium carbonate, creating a protective layer that improves shelf life and performance[1]. This technology allows for a more concentrated and efficient stain removal solution, reducing the amount of product needed per wash cycle. Solvay has also implemented a continuous production method, increasing output and consistency while reducing energy consumption[2].
Strengths: Superior stability and controlled oxygen release, improved shelf life, and more efficient stain removal. Weaknesses: Potentially higher production costs due to coating process and specialized equipment requirements.
Evonik Operations GmbH
Technical Solution: Evonik has innovated in the field of sodium percarbonate (SPC) technology for stain removal. Their approach focuses on developing highly stable SPC formulations with enhanced solubility and rapid oxygen release. Evonik's proprietary process involves a unique crystallization technique that results in more uniform SPC particles with improved flow properties[3]. This technology allows for better dispersion in water and faster activation of the stain-removing properties. Additionally, Evonik has developed eco-friendly stabilizers that extend the shelf life of SPC without compromising its effectiveness or environmental profile[4].
Strengths: Enhanced solubility and rapid oxygen release, improved flow properties, and eco-friendly stabilization. Weaknesses: May require specialized production facilities and potentially higher raw material costs for eco-friendly additives.
Core Innovations
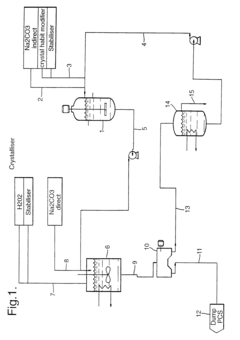
A method for preparing sodium percarbonate particles with improved stability
PatentInactiveEP0681557B2
Innovation
- Coating sodium percarbonate particles with a thin, impermeable layer of sodium bicarbonate formed by reacting with CO2 in the presence of moisture, which can be enhanced with additional conventional coating methods using sodium bicarbonate or sodium sulphate solutions.
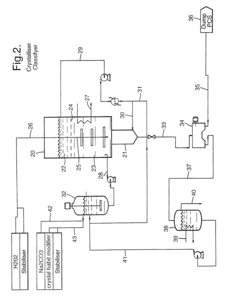
Sodium percarbonate and process for producing sodium percarbonate
PatentInactiveUS6482385B2
Innovation
- A continuous process that controls the concentration of sodium carbonate and temperature in the dissolution tank, and maintains a specific mole ratio of hydrogen peroxide to sodium carbonate, allowing for the production of sodium percarbonate without a salting-out agent, thereby minimizing hydrogen peroxide decomposition and improving product quality.
Environmental Impact
The environmental impact of sodium percarbonate and sodium carbonate in stain removal processes is a crucial consideration for both manufacturers and consumers. Sodium percarbonate, when dissolved in water, releases hydrogen peroxide and sodium carbonate, making it a more potent cleaning agent compared to sodium carbonate alone. This increased efficacy can lead to reduced water and energy consumption during washing processes, potentially lowering the overall environmental footprint.
Sodium percarbonate's decomposition products are generally considered environmentally friendly. The hydrogen peroxide breaks down into water and oxygen, while sodium carbonate is a naturally occurring mineral. This biodegradability minimizes the long-term impact on aquatic ecosystems when these compounds are released into wastewater systems. However, the production of sodium percarbonate requires more energy and resources compared to sodium carbonate, which may offset some of its environmental benefits.
Sodium carbonate, also known as washing soda, has been used in cleaning applications for centuries. Its production process is less energy-intensive than that of sodium percarbonate, resulting in lower carbon emissions during manufacturing. Additionally, sodium carbonate is mined from natural deposits or produced through the Solvay process, which utilizes readily available raw materials like salt and limestone.
Both compounds can affect water pH levels when released into the environment. Sodium carbonate, being alkaline, can raise the pH of water bodies, potentially impacting aquatic life. Sodium percarbonate, while also alkaline, may have a less pronounced effect due to its lower concentration in typical cleaning formulations. However, the release of oxygen from sodium percarbonate decomposition could potentially lead to localized oxygen oversaturation in water bodies, which may disrupt aquatic ecosystems.
The choice between sodium percarbonate and sodium carbonate for stain removal also impacts packaging and transportation. Sodium percarbonate's higher efficacy means that smaller quantities are required for the same cleaning power, potentially reducing packaging materials and transportation emissions. However, sodium percarbonate's reactivity necessitates more robust packaging to prevent moisture ingress and premature decomposition, which may increase overall packaging waste.
In terms of user safety and handling, both compounds pose minimal risks when used as directed. However, sodium percarbonate's oxidizing properties require more careful handling and storage to prevent accidental reactions or degradation. This aspect may influence consumer behavior and disposal practices, indirectly affecting environmental impact through improper use or disposal.
Sodium percarbonate's decomposition products are generally considered environmentally friendly. The hydrogen peroxide breaks down into water and oxygen, while sodium carbonate is a naturally occurring mineral. This biodegradability minimizes the long-term impact on aquatic ecosystems when these compounds are released into wastewater systems. However, the production of sodium percarbonate requires more energy and resources compared to sodium carbonate, which may offset some of its environmental benefits.
Sodium carbonate, also known as washing soda, has been used in cleaning applications for centuries. Its production process is less energy-intensive than that of sodium percarbonate, resulting in lower carbon emissions during manufacturing. Additionally, sodium carbonate is mined from natural deposits or produced through the Solvay process, which utilizes readily available raw materials like salt and limestone.
Both compounds can affect water pH levels when released into the environment. Sodium carbonate, being alkaline, can raise the pH of water bodies, potentially impacting aquatic life. Sodium percarbonate, while also alkaline, may have a less pronounced effect due to its lower concentration in typical cleaning formulations. However, the release of oxygen from sodium percarbonate decomposition could potentially lead to localized oxygen oversaturation in water bodies, which may disrupt aquatic ecosystems.
The choice between sodium percarbonate and sodium carbonate for stain removal also impacts packaging and transportation. Sodium percarbonate's higher efficacy means that smaller quantities are required for the same cleaning power, potentially reducing packaging materials and transportation emissions. However, sodium percarbonate's reactivity necessitates more robust packaging to prevent moisture ingress and premature decomposition, which may increase overall packaging waste.
In terms of user safety and handling, both compounds pose minimal risks when used as directed. However, sodium percarbonate's oxidizing properties require more careful handling and storage to prevent accidental reactions or degradation. This aspect may influence consumer behavior and disposal practices, indirectly affecting environmental impact through improper use or disposal.
Consumer Safety Aspects
When comparing sodium percarbonate and sodium carbonate in stain removal applications, consumer safety is a paramount consideration. Both compounds are widely used in household cleaning products, but they possess different chemical properties that impact their safety profiles.
Sodium percarbonate, also known as sodium carbonate peroxide, is a more potent oxidizing agent compared to sodium carbonate. It releases hydrogen peroxide when dissolved in water, which enhances its stain-removing capabilities. However, this property also necessitates additional safety precautions. The release of hydrogen peroxide can cause skin and eye irritation upon direct contact. Consumers should be advised to wear protective gloves and avoid prolonged skin exposure when using products containing sodium percarbonate.
In contrast, sodium carbonate, commonly known as washing soda, is generally considered less hazardous. It is an alkaline substance that does not release oxidizing agents. While it can still cause skin irritation, especially in concentrated forms, the risk is generally lower compared to sodium percarbonate. However, its alkaline nature can be corrosive to certain materials, and precautions should be taken to prevent accidental ingestion or eye contact.
Both compounds pose risks if ingested. Sodium percarbonate can cause gastrointestinal irritation and, in severe cases, internal bleeding due to the release of hydrogen peroxide. Sodium carbonate ingestion can lead to nausea, vomiting, and in extreme cases, gastrointestinal perforation due to its caustic nature. Proper storage and clear labeling are crucial to prevent accidental ingestion, especially in households with children.
Environmental considerations also factor into consumer safety. Sodium percarbonate breaks down into oxygen, water, and sodium carbonate, making it more environmentally friendly. Sodium carbonate, being a naturally occurring mineral, has minimal environmental impact when used in typical household quantities. However, both compounds can affect aquatic ecosystems if released in large amounts, emphasizing the importance of proper disposal instructions for consumers.
Product formulation plays a significant role in mitigating safety risks. Manufacturers often include buffering agents and stabilizers to reduce the potential for irritation and to maintain product efficacy. Clear usage instructions and appropriate warning labels are essential for ensuring consumer safety. These should include guidance on proper dilution, recommended protective equipment, and first aid measures in case of accidental exposure.
In conclusion, while both sodium percarbonate and sodium carbonate are effective stain removers, their distinct chemical properties necessitate different safety considerations. Manufacturers and consumers alike must be aware of these differences to ensure safe and effective use in household cleaning applications.
Sodium percarbonate, also known as sodium carbonate peroxide, is a more potent oxidizing agent compared to sodium carbonate. It releases hydrogen peroxide when dissolved in water, which enhances its stain-removing capabilities. However, this property also necessitates additional safety precautions. The release of hydrogen peroxide can cause skin and eye irritation upon direct contact. Consumers should be advised to wear protective gloves and avoid prolonged skin exposure when using products containing sodium percarbonate.
In contrast, sodium carbonate, commonly known as washing soda, is generally considered less hazardous. It is an alkaline substance that does not release oxidizing agents. While it can still cause skin irritation, especially in concentrated forms, the risk is generally lower compared to sodium percarbonate. However, its alkaline nature can be corrosive to certain materials, and precautions should be taken to prevent accidental ingestion or eye contact.
Both compounds pose risks if ingested. Sodium percarbonate can cause gastrointestinal irritation and, in severe cases, internal bleeding due to the release of hydrogen peroxide. Sodium carbonate ingestion can lead to nausea, vomiting, and in extreme cases, gastrointestinal perforation due to its caustic nature. Proper storage and clear labeling are crucial to prevent accidental ingestion, especially in households with children.
Environmental considerations also factor into consumer safety. Sodium percarbonate breaks down into oxygen, water, and sodium carbonate, making it more environmentally friendly. Sodium carbonate, being a naturally occurring mineral, has minimal environmental impact when used in typical household quantities. However, both compounds can affect aquatic ecosystems if released in large amounts, emphasizing the importance of proper disposal instructions for consumers.
Product formulation plays a significant role in mitigating safety risks. Manufacturers often include buffering agents and stabilizers to reduce the potential for irritation and to maintain product efficacy. Clear usage instructions and appropriate warning labels are essential for ensuring consumer safety. These should include guidance on proper dilution, recommended protective equipment, and first aid measures in case of accidental exposure.
In conclusion, while both sodium percarbonate and sodium carbonate are effective stain removers, their distinct chemical properties necessitate different safety considerations. Manufacturers and consumers alike must be aware of these differences to ensure safe and effective use in household cleaning applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!